Abstract
Carotenoids have the potential to improve the human health which leads to an increasing consumer demand for carotenoid-rich vegetables. The implementation of new, less energy-consuming vegetable production systems using artificial light such as light-emitting diodes (LEDs) is essential. In the present study, pak choi (Brassica rapa ssp. chinensis ‘Black Behi’) sprouts were grown under a combination of blue and white LEDs, red and white LEDs or only white LEDs for 7 days. Total carotenoid levels of ~ 700 ng/mg DM were measured under white LEDs. The combination of blue and white LEDs increased the carotenoid levels by ~ 15% in comparison to only white LEDs, while red and white LEDs reduced them. The transcript levels of important carotenoid metabolism-related genes were enhanced under blue and white LEDs. Phytoene measurement after Norflurazon-treatment, a phytoene desaturase inhibitor, revealed that phytoene increased by 38% (37.5 µM Norflurazon) and by 56% (50.0 µM Norflurazon) after growth under blue and white LEDs in comparison to only white LEDs suggesting an up-regulation of the upper carotenoid biosynthetic pathway. Thus, the transcript levels and the enhanced phytoene levels correlated well with the higher accumulation of carotenoids under blue and white LEDs. Furthermore, a comparison to sprouts grown under blue LEDs without additional white LEDs showed that blue light alone does not increase the phytoene levels after Norflurazon-treatment. Overall, this study demonstrated a beneficial effect of a higher blue light percentage in growing carotenoid-rich pak choi sprouts, and implies that an increased biosynthesis within the upper carotenoid biosynthetic pathway is responsible for the enhanced carotenoid accumulation.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The health-promoting properties of many plant secondary metabolites increase the demand for fresh vegetables throughout the whole year. A year-round delivery of vegetables can only be guaranteed by a vegetable production in greenhouses or enclosed facilities with limited natural light. In addition, indoor farming is getting more and more attention due to its advantages including saving land and water resources, the reduction of herbicide and pesticide application, and avoidance of crop losses caused by extreme weather changes created by climate change. For above-mentioned vegetable production systems, artificial lighting is essential.
To avoid impaired vegetable growth [1] and to optimize quantum yield of photosynthesis [2], LEDs emitting specific light wavelengths are the preferred light source. In addition, the vegetable quality can be enhanced using specific wavelengths. In particular, secondary plant compounds determining the flavour, colour and health-promoting properties of vegetables can be influenced using different light qualities [3]. For instance, there are indications that blue light induces the accumulation of phenolic compounds in leafy vegetables [4, 5].
Carotenoids are secondary plant compounds that colour leaves, flowers, fruits, and vegetables yellow, orange or red [6]. There are indications that carotenoids protect against cardiovascular diseases, certain types of cancer, eye-related diseases, and light-induced skin damage [7], thereby making them of key interest to consumers. However, although the steps of carotenoid biosynthesis and degradation are well known, only limited knowledge about their regulation is available for vegetables. Yet, a better understanding of the carotenoid metabolism would support the implementation of suitable production strategies as well as the enrichment of plant secondary metabolites.
In green tissues, carotenoids play a crucial role in photosynthesis [6]. Concomitantly, a manipulation of the light exposure during vegetable growth can lead to changed levels. However, to date, the understanding of light effects on carotenoid turnover is still limited. De-etiolation is the greening process of a dark-grown seedling initiated by light due to chloroplast formation and chlorophyll accumulation. During this process, an increase in phytoene synthase (PSY) transcripts (see carotenoid biosynthesis pathway Fig. 1) and in carotenoid levels was observed in Arabidopsis thaliana, white mustard (Sinapis alba), and in tobacco seedlings (Nicotiana tabacum ‘Samsun’) [8, 9]. But also the transcript levels of the carotenoid biosynthesis genes β-cyclase (βLCY), ε-cyclase (εLCY), β-carotene hydroxylase (βOHASE), zeaxanthin epoxidase (ZEP) and violaxanthin de-epoxidase (VDE) were shown to be increased by light during de-etiolation [8]. However, although the de-etiolation process is initiated by light, the induced changes might be regulated differently after the de-etiolation process. After the de-etiolation process, in lettuce (Lactuca sativa ‘Banchu Red Fire’ or ‘Red Cross’), blue light was shown to enhance the carotenoid levels [4, 10]. In another study, total carotenoid levels were highest under blue fluorescent lamps in spinach (Spinacia oleracea ‘Okame’), while they were highest under white and red fluorescent lamps in lettuce (Lactuca sativa ‘Redfire’) and under white fluorescent lamps in komatsuna (Brassica campestris ‘Komatsuna’) [11]. Thus, the influence of different light qualities on carotenoid concentrations seems to be species-dependent.
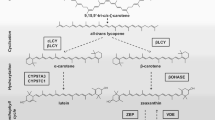
Carotenoid biosynthesis pathway in plants (arrow with dashed line: more than 1 reaction; arrow with continuous line: 1 reaction; CYP97A3: cytochrome P450 97A3; CYP97C1: cytochrome P450 97C1; DMAPP: dimethylallyl diphosphate; GGPP: geranylgeranyl diphosphate; IPP: isopentenyl diphosphate; βLCY: β-cyclase; εLCY: ε-cyclase; β-OHASE: β-carotene hydroxylase; PDS: phytoene desaturase; PSY: phytoene synthase; VDE: violaxanthin de-epoxidase; ZEP: zeaxanthin epoxidase)
However, only a few studies are dealing with the underlying mechanisms via which specific wavelengths regulate the carotenoid biosynthesis pathway in green tissues. In higher plants, light can influence the metabolism by different mechanisms. Plant responses can be mediated by photoreceptors such as cryptochromes and phototropins for blue light perception and phytochromes for red light perception [12], by light-modulated processes such as shade avoidance and circadian rhythm [13], and by the ability of the light environment to influence the photosynthesis activity, thereby changing the redox state of components related to the electron transport chain of photosynthesis and activating a retrograde signalling process from the chloroplast to the nucleus [14, 15]. The transcription of PSY during the de-etiolation process seems to be phytochrome-mediated in Sinapis alba and Arabidopsis thaliana [9]. In the dark, phytochrome interacting factor 1 (PIF1) was shown to bind to the PSY promoter, thereby repressing PSY transcription, whereas light triggers the degradation of PIF1 mediated by photo-activated phytochromes leading to an increased PSY transcription [16]. In addition, the transcription factor elongated hypocotyl 5 (HY5), which is an integration point downstream of all photoreceptors, positively regulates the PSY transcription [16]. In regard to the circadian clock, the regulation of the carotenoid biosynthesis pathway has hardly been explored to date [17,18,19]. As to redox-controlled responses, they can be activated by specific wavelengths which lead to an imbalance in the excitation of the two photosystems. The imbalance impacts the expression of genes under redox control with the goal to adjust the photosystems, thereby antagonizing the imbalance in photosystem excitation [20,21,22]. To date, there is evidence that the transcription of βOHASE and ZEP are under redox control [8, 23].
In summary, the influence of light quality on the carotenoid metabolism is species-dependent, and the underlying mechanisms are not well known. If strategies for the production of vegetables rich in health-promoting carotenoids should be implemented, the effect of specific wavelengths during the growth on carotenoids has to be determined for vegetables of economic value. The aim of this study was to explore the effect of different light spectra on the carotenoid metabolism in pak choi (Brassica rapa ssp. chinensis ‘Black Behi’) sprouts, a green leafy vegetable with a high economic and nutritional relevance in East and Southeast Asia and growing importance in Europe and North America [24,25,26]. In addition to determining the wavelength most favourable for increasing carotenoid levels, the underlying mechanisms were also investigated by analysing transcriptional patterns of important carotenoid biosynthesis and degradation enzymes as well as by measuring phytoene as an indirect indicator for the metabolic activity of the upper carotenoid biosynthetic pathway.
2 Materials and methods
2.1 Plant material and growth conditions
The pak choi (Brassica rapa ssp. chinensis) cultivar ‘Black Behi’ was used in all experiments (Allied Botanical Corporation, Quezon City, Philippines). Aluminium foil trays (ø ~ 10 cm) were filled with wet perlite. One gram of seeds (∼370 seeds) was sown on a wet strip of fleece which was placed onto the perlite. The seeds were sprayed with water. The sprouts were grown on the moist fleece in the dark for 4 days (25 °C, 75% air humidity). Afterwards, the sprouts were transferred to a growth chamber (25 °C, 75% air humidity) equipped with blue and white, red and white or only white LEDs (LED stripes, 24 V/DC, Conrad, Berlin, Germany). The light spectrum of the white LEDs was adjusted with a filter to reduce the amount of blue light. The properties of the filter were measured with a Jasco spectrometer V670 (Jasco, Tokyo, Japan) at 3 points of the filter. The spectra of the LEDs were determined with an EPP2000 StellarNet spectrometer (StellarNet, Tampa, FL, USA). The presented spectra of the LEDs measured beneath the filter are the means of 3 measuring spots per LED type (Online Resource Fig. S1). The photoperiod in the growth chamber was 12 h from 06:00 h to 18:00 h. A photosynthetic active radiation of 90 μmol m−2 s−1 was confirmed for each light treatment (LI-250 Light Meter, LI-COR, Lincoln, NE, USA). Sprouts were grown for 7 days under the three different light spectra and were watered as needed. The trays were spatially rotated every day, in order to optimize the uniformity of light treatment. Throughout day 7 of the light treatment, the sprouts were harvested at 2 different time points (10:00 h and 18:00 h) with 4 biological replicates for each time point per treatment. In detail, the sprouts of one tray were cut above the perlite surface (cotyledons and hypocotyl) and pooled for 1 replicate. These samples were weighed for fresh mass determination, frozen immediately in liquid nitrogen and stored at − 80 °C until further analysis. Two independent experimental series (I and II) were performed. For further analysis, the frozen samples were ground to a fine powder under liquid nitrogen and stored at − 80 °C. Carotenoid analysis was carried out using freeze-dried material.
2.2 Norflurazon experiments
The Norflurazon treatment was performed as previously described [27]. In principle, the plants were grown as described above. After a period of 4 days in the dark, the sprouts grew in a growth chamber equipped with LEDs (photoperiod 12 h; in a first experiment: photosynthetic active radiation of 90 μmol m−2 s−1, blue and white LEDS, red and white LEDs or only white LEDs; in a second experiment: photosynthetic active radiation of 80 μmol m−2 s−1, blue LEDs, red LEDs or white LEDs). At the end of the dark period of day 6, the sprouts were cut directly above the perlite surface. The cut sprouts were transferred to a Norflurazon solution (37.5 µM Norflurazon in 0.125% 2-propanol). Control samples were incubated in the respective solutions without Norflurazon. After 2.5 h in the dark, the sprouts in the solutions were again incubated under LED light for 3 h. Afterwards, the sprouts were harvested and immediately frozen in liquid nitrogen. The experiment was repeated with 50 µM Norflurazon in 0.17% 2-propanol. The treatment with the higher Norflurazon concentration served as test if PDS was completely inhibited in the first experiment with 37.5 µM Norflurazon. If both Norflurazon concentrations result in the same phytoene levels, an already complete inhibition of PDS under the lower concentration is indicated.
2.3 Determination of the dry matter
The samples were dried in a drying oven at 103 °C until a constant mass was reached. The dry matter (DM) was determined as dry matter per fresh mass in percent.
2.4 Analysis of carotenoids and chlorophylls
About 15 mg freeze-dried plant material per sample were used for the analysis. Carotenoids and chlorophylls were extracted by adding 750 μL methanol/tetrahydrofuran (1: 1, v/v) (Th. Geyer, Berlin, Germany; VWR International, Radnor, PA, USA), shaking (5 min, 1400 rpm) followed by centrifugation for 5 min at 4000 g. The supernatant was transferred into a new 4-mL glass vial. The extraction step was repeated three times using 500 μL of methanol/tetrahydrofuran (1: 1, v/v). The combined supernatant was concentrated under N2 to dryness. Afterwards, it was re-dissolved in 100 μL of dichloromethane (Merck, Darmstadt, Germany) and 400 μL of 2-propanol (Merck, Darmstadt, Germany). The extracts were filtered (0.2 μm, PTFE) and kept at 10 °C in the autosampler. Carotenoids and chlorophylls were measured as previously described [28]. The substances were separated on a C30 column (YMC Co. Ltd, Kyoto, Japan, YMC C30, 100 × 2.1 mm, 3 μm) on an Agilent Technologies 1290 Infinity UHPLC. The column temperature was maintained at 20 °C. The mobile phases were (A) methanol/water (96: 4, v/v) and (B) methanol/tert-butyl methyl ether (Roth, Karlsruhe, Germany)/water (6: 90: 4, v/v/v). To increase the ionization, 20 mM ammonium acetate (Roth, Karlsruhe, Germany) was added to the mobile phases. The flow rate was 0.2 mL min−1. Elution was carried out with the following gradient: 100% A for 10 min, 100% A to 80% A in 7 min, 80% A for 28 min, 80% A to 0% A in 10 min and 0% A to 100% A in 2 min. Detection took place with an Agilent Technologies 6230 TOF LC/MS equipped with a multimode ion source in positive ionization mode. The gas temperature was set to 300 °C at a flow rate of 8 L min−1, the vaporizer was set to 200 °C and the nebulizer pressure was set to 35 psig. The voltage was adjusted to 3500 V and a fragmentor voltage of 175 V was applied at a corona current of 4 μA. Carotenoid (CaroteNature GmbH, Münsingen Switzerland) and chlorophyll (Sigma-Aldrich, St Louis, MO, USA) standards were used. Their concentrations were determined spectrophotometrically using the specific absorption coefficients [29]. Quantification was performed at a detection wavelength of 450 nm using an external calibration.
2.5 Quantification of phytoene
About 5 mg freeze-dried plant material per sample were used for the analysis. Phytoene was extracted by adding 200 μL ethanol (VWR International, Radnor, PA, USA) and 100 µL H2O. After vortexing and ultrasonication for 1 min, 500 µL hexane (Merck, Darmstadt, Germany) were added. The extract was shaken (5 min, 1400 rpm), followed by centrifugation for 5 min at 4000 g. The supernatant was transferred into a new 4 mL glass vial. The extraction step was repeated once using 500 µL hexane and twice using 300 μL hexane. The combined supernatant was concentrated under N2 to dryness. Afterwards, it was re-dissolved in 100 μL of dichloromethane (Merck, Darmstadt, Germany) and 400 μL of 2-propanol (Merck, Darmstadt, Germany). The extracts were filtered (0.2 μm, PTFE) and kept at 10 °C in the autosampler. The substances were separated on a C30 column (YMC Co. Ltd, Kyoto, Japan, YMC C30, 100 × 2.1 mm, 3 μm) on an Agilent Technologies 1290 Infinity UHPLC. The column temperature was maintained at 20 °C. The mobile phases were (A) methanol/water (96: 4, v/v) and (B) methanol/tert-butyl methyl ether (Roth, Karlsruhe, Germany)/water (6: 90: 4, v/v/v). To increase the ionization, 20 mM ammonium acetate (Roth, Karlsruhe, Germany) was added to the mobile phases. The flow rate was 0.2 mL min−1. Elution was carried out with the following gradient: 100% A for 10 min, 100% A to 80% A in 7 min, 80% A for 28 min, 80% A to 0% A in 10 min and 0% A to 100% A in 2 min. Detection took place with an Agilent Technologies 6546 LC/Q-TOF equipped with an APCI source in positive ionization mode. The gas temperature was set to 325 °C at a flow rate of 8 L min−1, the vaporizer was set to 350 °C and the nebulizer pressure was set to 35 psig. The voltage was adjusted to 3500 V and a fragmentor voltage of 175 V was applied at a corona current of 6.5 μA. A phytoene standard (CaroteNature GmbH, Münsingen Switzerland) was used for external quantification. Its concentration was determined spectrophotometrically using the specific absorption coefficient (A1%1 cm: 780; 286 nm).
2.6 RT-qPCR analysis
Total RNA extraction was performed from about 50 mg ground, frozen plant material using the RNeasy Plus Mini Kit (Qiagen, Hilden, Germany) as described by the manufacturer, including on-column DNase I digestion. RNA was quantified spectrophotometrically at 260 nm (Nanodrop ND1000, Thermo Fisher Scientific, Waltham, MA, USA). The purity was determined using the ratios of absorbance at 260/280 nm (desired value: ∼2.0) and 260/230 nm (desired value: ∼2.0–2.2). cDNA was synthesized from 1 μg total RNA using SuperScript III reverse transcriptase (Thermo Fisher Scientific, Waltham, MA, USA) with oligo (dT)12−18 primers according to the manufacturer’s instructions. The reaction mix per well consisted of 3 μL cDNA (diluted 1: 10), 5 μL 2 × SensiMix SYBR Low-ROX (Bioline, Luckenwalde, Germany) and 2 μL 2 μM primer (forward and reverse primer). The primers were designed based on the sequence information available in the Brassica database BRAD (http://brassicadb.org/brad/) (for primer pair sequences, see Online Resource Table S1). In cases when more than a single gene encoded a protein, the primers were designed to cover all of them. Transcript quantification was carried out in triplicate. A CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used for RT-qPCR. The thermal cycling conditions were 50 °C for 2 min, 95 °C for 10 min, 39 cycles of 95 °C for 15 s and 60 °C for 1 min, followed by a melting curve analysis. The amplification efficiency for each primer pair was determined using a cDNA dilution series. Gene expression was calculated as n-fold induction of the gene of interest in sprouts grown under blue and white or red and white LEDs in comparison to sprouts grown under only white LEDs by the ΔΔCq method using the geometric mean of three reference genes (actin 2, ubiquitin-conjugating enzyme E2 30 and elongation factor 1-alpha; M value < 0.5; coefficient variance < 0.25) [30, 31]. No-template controls were included.
2.7 Statistical analyses
For statistical analyses, SigmaPlot 14.0 (Systat, Erkrath, Germany) was used. Treatment differences were analysed using one-way ANOVA followed by Tukey HSD post hoc test. If the assumption of homogeneity of variances or the normality assumption were violated, the Kruskal–Wallis-test was applied. A p-value ≤ 0.05 was considered as statistically significant. Data are presented as means ± standard deviation.
3 Results
3.1 Changes in fresh mass and dry matter
The fresh mass and the dry matter of the pak choi sprouts differed under the different light qualities (Fig. 2). After growth under only white LEDs, a fresh mass of 9.6 ± 0.4 g at 10:00 h and of 9.0 ± 0.4 g at 18:00 h was measured in the first experiment (I). A combination of blue and white LEDs decreased the fresh mass to 8.1 ± 0.5 g (10:00 h) and to 8.1 ± 0.7 g (18:00 h). However, red and white LEDs tended to enhance the values to 10.2 ± 0.5 g (10:00 h) and to 9.9 ± 0.8 g (18:00 h). Concerning the DM in percent, the results were vice versa. Combining blue and white LEDs led to an increase of the DM to 7.0 ± 0.3% (10:00 h) and to 6.9 ± 0.4% (18:00 h) compared to values of 6.1 ± 0.3% (10:00 h) and 6.6 ± 0.2% (18:00 h) under only white LEDs. Red and white LEDs had only a slight tendency to reduce the DM in comparison to only white LEDs. The repetition experiment (II) was able to reproduce most effects. Absolute values of the DM showed the same tendencies as the FM (see Online Resource Fig. S2).
(a) Fresh mass and (b) dry mass in pak choi sprouts grown under three different light qualities. Sprouts were germinated in darkness for 4 days. Subsequently, the sprouts were grown under white LEDs, blue and white LEDs or red and white LEDs, and were harvested on day 7 of LED treatment. DM was determined as dry matter per fresh mass in percent. Values are presented as mean ± SD (n = 4). Significant differences (p ≤ 0.05) between different light qualities are indicated by different lowercase letters. (I: first experiment; II: repetition experiment; B/W: blue and white LEDs; DM: dry mass; FM: fresh mass; R/W: red and white LEDs; W: white LEDs)
3.2 Effect of different light qualities on carotenoid and chlorophyll profiles
The average total carotenoid and chlorophyll levels increased and decreased about 15% under blue and white LEDs and red and white LEDs respectively, when compared to the measured values of about 700 ng/mg DM carotenoids and about 4000 ng/mg DM chlorophylls under only white LEDs (Fig. 3). In detail, lutein, β-carotene, neoxanthin and violaxanthin as well as chlorophyll a and chlorophyll b were determined. The two main carotenoids were lutein and β-carotene, with lutein accounting for about half and β-carotene for about a quarter of the total carotenoid contents. Regarding the chlorophylls, chlorophyll a was the dominant compound contributing 80% to the total levels. The carotenoid and chlorophyll compounds also showed enhanced levels under a combination of blue and white LEDs and a decrease under red and white LEDs, reflecting the overall total levels (Fig. 4).
Total a carotenoid and b chlorophyll levels on a dry mass basis in pak choi sprouts grown under three different light qualities. Sprouts were germinated in darkness for 4 days. Subsequently, the sprouts were grown under white LEDs, blue and white LEDs or red and white LEDs, and were harvested on day 7 of LED treatment. Carotenoids and chlorophylls were measured by UHPLC-ToF–MS analysis. Values are presented as mean ± SD (n = 4). Significant differences (p ≤ 0.05) between different light qualities are indicated by different lowercase letters. (I: first experiment; II: repetition experiment; B/W: blue and white LEDs; R/W: red and white LEDs; TotCaro: total carotenoids; TotChl: total chlorophylls; W: white LEDs)
a Lutein, b β-carotene, c chlorophyll a and d chlorophyll b levels in pak choi sprouts grown under three different light qualities. Sprouts were germinated in darkness for 4 days. Subsequently, the sprouts were grown under white LEDs, blue and white LEDs or red and white LEDs, and were harvested on day 7 of LED treatment. Carotenoids and chlorophylls were measured by UHPLC-ToF–MS analysis. Values are presented as mean ± SD (n = 4). Significant differences (p ≤ 0.05) between different light qualities are indicated by different lowercase letters. (I: first experiment; II: repetition experiment; B/W: blue and white LEDs; βCar: β-carotene; Chl a: chlorophyll a; Chl b: chlorophyll b; Lut: lutein; R/W: red and white LEDs; W: white LEDs)
Since the light qualities also affected the dry matter content, the total carotenoid and chlorophyll levels were also calculated on a fresh mass basis (Fig. 5). For both carotenoids and chlorophylls, a combination of blue and white LEDs led to higher levels, while red and white LEDs decreased the contents. In detail, growth under only white LEDs delivered sprouts with more than 40 ng/mg FM total carotenoids and about 250 ng/mg FM total chlorophylls. Blue and white LEDs led to a rise of about 20–30% of the carotenoid and chlorophyll levels, while red and white LEDs decreased the values about 15–20%.
Total a carotenoid and b chlorophyll levels on a fresh mass basis in pak choi sprouts grown under three different light qualities. Sprouts were germinated in darkness for 4 days. Subsequently, the sprouts were grown under white LEDs, blue and white LEDs or red and white LEDs, and were harvested on day 7 of LED treatment. Carotenoids and chlorophylls were measured by UHPLC-ToF–MS analysis. Values are presented as mean ± SD (n = 4). Significant differences (p ≤ 0.05) between different light qualities are indicated by different lowercase letters. (I: first experiment; II: repetition experiment; B/W: blue and white LEDs; R/W: red and white LEDs; TotCaro: total carotenoids; TotChl: total chlorophylls; W: white LEDs)
3.3 RNA transcript levels of carotenoid biosynthesis and degradation genes under different light qualities
The transcript levels of key carotenoid biosynthesis and degradation genes under different light qualities were determined as n-fold expression of the expression in sprouts grown under only white LEDs at 10:00 h (Fig. 6). The four genes were chosen since they cover different steps of the carotenoid metabolism: the first biosynthesis step (PSY), cyclisation (βLCY), hydroxylation (βOHASE1) and degradation (carotenoid cleavage dioxygenase 4, CCD4). Additionally, it was previously shown that their transcripts react to different light qualities during growth [17]. The expression of PSY, the gene that encodes for the enzyme that forms phytoene from two geranylgeranyl diphosphates [32] (see Fig. 1), increased by 1.5 ± 0.1-fold after growth under a combination of blue and white LEDs compared to growth under only white LEDs at 10:00 h, while it was reduced to 0.8 ± 0.1-fold under red and white LEDs. Also at 18:00 h, highest transcript levels were measured under blue and white LEDs and lowest under red and white LEDs. The same observation was also made for βLCY, responsible for the cyclisation of lycopene and the formation of α-carotene and β-carotene [33], whose expression was enhanced by 1.3 ± 0.1-fold by blue and white LEDs and decreased to 0.8 ± 0.1-fold under red and white light at 10:00 h. At 18:00, again a combination of blue and white LEDs increased its expression. For βOHASE1, a hydroxylase that forms zeaxanthin from β-carotene [34], the expression also was highest under blue and white LEDs at 10:00 h (1.3 ± 0.1-fold). However, at 18:00 h, a combination of red and white light increased its transcript levels compared to only white LEDs. In regard to carotenoid cleavage dioxygenase 4 (CCD4), coding for a carotenoid degrading enzyme [35], the transcript levels were enhanced by 1.4 ± 0.1-fold by blue and white LEDs and reduced to 0.7 ± 0.1-fold by red and white LEDs at 10:00 h. At 18:00 h, there were no differences. All the results were confirmed by repeating the experiment (experiment II).
Transcript levels of a PSY, b βLCY, c βOHASE1 and d CCD4 in pak choi sprouts grown under three different light qualities. Sprouts were germinated in darkness for 4 days. Subsequently, the sprouts were grown under white LEDs, blue and white LEDs or red and white LEDs, and were harvested on day 7 of LED treatment. The RNA transcript levels were analysed by RT-qPCR. Gene expression was calculated as n-fold of the expression in sprouts grown under only white LEDs at 10:00 h by the ΔΔCq method. Values are presented as mean ± SD (n = 4). Significant differences (p ≤ 0.05) between different light qualities are indicated by different lowercase letters. (I: first experiment; II: repetition experiment; B/W: blue and white LEDs; CCD4: carotenoid cleavage dioxygenase 4; βLCY: β-cyclase; βOHASE1: β-carotene hydroxylase 1; PSY: phytoene synthase; R/W: red and white LEDs; W: white LEDs)
3.4 Accumulation of phytoene as indirect indicator of the metabolic activity of the upper carotenoid biosynthetic pathway
To get an idea of the flux through the carotenoid biosynthesis pathway, sprouts were treated with Norflurazon. Norflurazon is an herbicide that inhibits the enzyme phytoene desaturase (PDS) of the carotenoid biosynthesis pathway. PDS catalyses the desaturation of phytoene [36] (see Fig. 1). Thus, applying Norflurazon to plants leads to an accumulation of phytoene. The quantity of the phytoene levels is no direct flux measurement, but gives an idea about the metabolic activity of the upper carotenoid biosynthetic pathway.
In sprouts without Norflurazon treatment only traces of phytoene at the LOD were detected (Fig. 7). However, an incubation of the sprouts with the herbicide led to quantifiable phytoene concentrations. In this regard, it should be noted that both Norflurazon incubation concentrations of 37.5 µM and 50.0 µM caused comparable levels of phytoene. This indicates that when using 37.5 µM Norflurazon all PDS molecules were already inhibited and confirms that the used Norflurazon incubation concentrations were appropriate to perform this experiment. In a first experiment, sprouts were grown under either blue and white LEDs, red and white LEDs or only white LEDs. In a second series, the sprouts grew under only blue LEDs, only red LEDs or only white LEDs before Norflurazon incubation. The first experimental series showed that a combination of blue and white LEDs increased phytoene by 38% (37.5 µM Norflurazon) and by 56% (50.0 µM Norflurazon) in comparison to only white LEDs. Red and white LEDs decreased the phytoene levels slightly by 15% using 37.5 µM Norflurazon. After growth under red and white LEDs, 50 µM Norflurazon did not reduce the phytoene levels. Interestingly, the second experimental series with only blue or only red LEDs showed that the phytoene levels under only blue LEDs did not significantly change in comparison to only white LEDs. They increased only slightly by 11% (37.5 µM Norflurazon) and by 13% (50.0 µM Norflurazon). Thus, only a combination of blue LEDs with white LEDs led to a substantial phytoene enhancement.
Phytoene levels in pak choi sprouts grown under different light qualities after Norflurazon incubation. Sprouts were germinated in darkness for 4 days. Subsequently, the sprouts were grown under (a) white LEDs, blue and white LEDs and red and white LEDs or under (b) white LEDs, blue LEDs and red LEDs. At the end of the dark period of day 6, the sprouts were cut and incubated with 37.5 µM Norflurazon for 2.5 h in darkness, followed by 3 h under LED light before harvest. Phytoene was determined by UHPLC-QToF-MS analysis. The experiment was repeated with 50 µM Norflurazon. Values are presented as mean ± SD (n = 4). Significant differences (p ≤ 0.05) between different light qualities are indicated by different lowercase letters. (B: blue LEDs; B/W: blue and white LEDs; NFZ: Norflurazon; Phy: phytoene; R: red LEDs; R/W: red and white LEDs; tr.: traces; W: white LEDs)
4 Discussion
LEDs are considered the future of greenhouse lighting. However, to achieve a vegetable production that guarantees optimal growth and high contents of quality increasing compounds, the effects of specific wavelength on plant responses, in particular in vegetable plants, have to be investigated more thoroughly. Especially in regard to carotenoids, the effects and exact mechanisms by which specific wavelengths affect the carotenoid metabolism are not yet well understood.
In this context, the observation was made that the carotenoid metabolism is differently regulated in photosynthetically active and non-active tissues. In detail, while PSY is the limiting factor in non-active tissues like cassava (Manihot esculenta) roots [37], tomato (Solanum lycopersicum ‘Aisla Craig’) fruits [38] or rice (Oryza sativa) endosperm [39], it does not seem to be equally important in photosynthetically active tissues. In Arabidopsis thaliana seedlings, the overexpression of PSY did not affect the carotenoid concentrations in leaves, although in the calli and roots, higher carotenoid contents were determined [40]. Thus, the results of this present study were compared to studies conducted in leafy tissues.
This study showed that in regard to fresh mass production, a combination of red and white LEDs was favourable for sprout fresh mass in comparison to light treatments with a higher blue percentage. This observation has previously been made by other studies. It was shown that the growth of lettuce seedlings (Lactuca sativa ‘Banchu Red Fire’) under red LEDs increased the fresh mass in comparison to blue LEDs [4]. Also in the study of Li et al. [10], red LEDs tended to increase the fresh weight of lettuce plants (Lactuca sativa ‘Red Cross’), while blue LEDs tended to result in a decrease of fresh weight. This is in line with the observation that a higher blue percentage of photon flux during growth reduces the hypocotyl length of seedlings in a cryptochrome-dependent manner [41, 42] leading to smaller sprouts. Thus, in early developmental stage, red light seems to increase plant growth, although this can be different in other species or developmental stages [43]. This observation was confirmed by the absolute DM results. Regarding the relative DM, the presented results demonstrated that a higher percentage of blue light enhances the relative DM. Accordingly, Goins et al. [44] showed a rise of shoot DM in wheat plants grown under a combination of red and blue light in comparison to only red light 40 days after planting. In soybean seedlings, an application of only red LEDs reduced the plant dry mass in comparison to only blue LEDs or blue plus red LEDs [45].
Concerning the health value of the pak choi sprouts, the carotenoid levels have a high relevance. Under all three different light treatments, carotenoid concentrations of about 700 ng/mg DM were reached which are comparable to levels found in field-grown pak choi plants as shown in a study conducted in Taiwan during the dry and wet season [24]. This result confirms the observation of previous studies that demonstrated that LEDs as artificial light sources are sufficient to produce pak choi sprouts rich in carotenoids [17]. However, in the former mentioned study, there were no significant differences in the carotenoid levels among the different light treatments. In detail, only slightly higher carotenoid quantities were measured under only white LEDs in comparison to only blue LED or only red LEDs [17]. Whereas in the present study, in which the blue and red LEDs were combined with additional white LEDs, the combination of blue and white LEDs led to significant higher carotenoid levels. This is interesting since it shows that blue light has the potential to enhance carotenoid levels, but that blue light alone is not sufficient to increase them in comparison to white light with a relatively even distribution of light wavelengths over the visual spectrum. Thus, other wavelengths of the visual light spectrum in addition to blue light are essential for carotenoid enhancement. Our results also show that the combination of red and white LEDs, with a higher percentage of red light, was less efficient to produce carotenoid-rich sprouts in comparison to blue and white and only white LEDs. Also Naznin et al. [43] showed that, rather than monochromatic light, combining several wavelengths increases carotenoids. In detail, a higher blue light percentage when red and blue LEDs are combined affected the carotenoid levels more positively than red LEDs alone in lettuce (Lactuca sativa ‘Buttercrunch’), spinach (Spinacia oleracea ‘Unipack 151’), kale (Brassica oleracea var. sabellica ‘Vates Blue Curled’) and basil (Ocimum basilicum ‘G Lemon Basil’) [43]. In Brassica microgreens, amber light plus blue light increased total carotenoid levels in a dose-dependent manner when combined with decreasing red light, although it was not clear if amber, blue light or both wavelengths had a positive effect on the carotenoids [46]. Nevertheless, no general recommendation for the production of carotenoid-rich, green, leafy vegetables can be made, since other species or cultivars showed different results. For example, while also in some lettuce cultivars (Lactuca sativa ‘Banchu Red Fire’ or ‘Red Cross’) and spinach (Spinacia oleracea ‘Okame’), it was shown that blue light in combination with white light or after white light treatment enhances carotenoid levels [4, 10, 11], total carotenoid levels were highest under white and red fluorescent lamps in the lettuce cultivar ‘Redfire’ and under white fluorescent lamps in komatsuna (Brassica campestris ‘Komatsuna’) [11]. The present study also confirmed that chlorophyll levels concomitantly changed with carotenoid levels. Due to their central role as light-harvesting pigments and in photoprotection, carotenoids are essential for photosynthesis [6]. Thus, carotenoid and chlorophyll metabolism have to be coordinated which is reflected in the co-expression of carotenoid biosynthesis genes and photosynthesis-related genes [8, 19, 47].
To gain a better insight into the mechanisms that control the measured carotenoid levels, the RNA transcript levels of genes coding for three carotenoid biosynthesis and one degrading enzyme were determined. For all four genes, namely PSY, βLCY, βOHASE1 and CCD4, a combination of blue and white LEDs increased their transcript levels, while red and white LEDs reduced them, except for βOHASE1 at 18:00 h which showed higher transcript levels under red and white LEDs. The changes in transcripts of the carotenoid biosynthesis genes correlated well with the increase and decrease of carotenoids under blue and white LEDs and red and white LEDs, respectively. This indicates a higher biosynthesis of carotenoids using light sources with a high percentage of blue light. However, also the transcripts of CCD4 were induced, an enzyme most likely responsible for the carotenoid turnover in leaves. In Arabidopsis thaliana, β-carotene and total carotenoids of a CCD4 loss-of-function mutant were enhanced compared to the wild-type [48]. Another study conducted in Arabidopsis thaliana showed that overexpression of PSY led to an increase of β-carotene in roots and non-green calli, but not in leaves. Instead, higher levels of C13 apocarotenoid glycosides (AGs) were found which were reduced in a CCD4 mutant, indicating a role for CCD4 in AG formation [49]. Other studies also observed higher transcript levels of biosynthesis genes under blue light. Growth of pak choi sprouts under monochromatic blue LEDs increased the transcript levels of PSY, PDS, βLCY, εLCY, cytochrome P450 97A3 (CYP97A3), cytochrome P450 97C1 (CYP97C1), βOHASE1, ZEP, VDE and CCD4, while monochromatic red light reduced them in comparison to white LEDs [17]. However, as aforementioned, in the latter study, monochromatic blue light did not increase the carotenoid levels in comparison to white light indicating that blue light indeed enhanced transcript levels of carotenoid biosynthesis genes, but that another wavelength is more efficient in a post-transcriptional step leading to higher metabolite levels. In the study of Alrifai et al. [46], increasing amber and blue light enhanced the transcript levels of PSY and εLCY in Brassica microgreens, although a statement about the individual effects of both wavelengths cannot be made since the light intensities of both wavelengths were always adjusted together. The present study also showed an increase in transcript levels only for the gene βOHASE1 under red and white LEDs at 18:00 h. This result confirms previously made findings that demonstrated a possible redox control mechanism of the βOHASE gene transcription due to an imbalance in photosystem excitation under blue or red light, leading to an increased expression under red light [8, 17, 23].
The transcript level results indicated an increased carotenoid biosynthesis under a combination of blue and white LEDs. However, the steady-state carotenoid levels are the result of a balance between biosynthesis, storage and degradation processes. To confirm that an enhanced biosynthesis under blue and white light was responsible for the measured carotenoid levels, phytoene levels after Norflurazon-treatment were measured as an indirect indicator for the metabolic activity of the upper carotenoid biosynthesis pathway. In sprouts non-treated with Norflurazon, only traces of phytoene were detected. This result is in line with the observation that higher phytoene levels were only found in some high-carotenoid-containing fruits such as tomatoes, watermelon, apricots, cantaloupe, pink grapefruit, pumpkin, mango, papaya, peaches, prunes, and oranges, but not in green leafy vegetables [50, 51]. In plants naturally deficient in phytoene that were treated with Norflurazon, the determined phytoene levels provide information about the metabolic activity of the upper carotenoid biosynthesis pathway. The presented results showed an increase in phytoene under a combination of blue and white LEDs which correlates with the measured carotenoid levels. Thus, the results on transcriptional level were confirmed. The experiment also showed that under only blue LEDs without additional white LEDs, phytoene was not significantly enhanced, although transcript levels of biosynthetic genes under only blue LEDs are very similarly enhanced compared to growth under blue and white LEDs [17]. Thus, although blue light is more effective than other wavelengths to enhance transcript levels of carotenoid biosynthesis genes, an additional wavelength is required to convert the increased transcript levels into higher metabolite levels. It can be assumed that this wavelength is more efficient in inducing post-transcriptional processes such as translation, translocation or enzyme activity. In a study by Schofield and Paliyath conducted in tomato fruits, PSY enzyme activity was increased by red light, but not by red light followed by far-red light, while transcript levels did not differ between red and red/far-red light-treated fruits, thus indicating an involvement of phytochromes in the post-transcriptional regulation of PSY [52]. Since red light is more efficient in activating phytochromes than blue light [12], this might explain why a combination of blue and white light is the best option to enhance carotenoid levels, since the blue light seems more effective for increasing transcript levels of biosynthesis genes, while the addition of red light as part of the white LED spectrum could increase post-transcriptional processes leading to a higher accumulation of carotenoids. However, in this study, it was not clear if the red light portion of the white LEDs might be responsible for the increase of post-transcriptional processes leading to higher carotenoid levels under blue and white LEDs. It has to be studied in more detail which wavelengths were involved in activating the post-translational mechanisms that enhanced the carotenoid levels. Moreover, the study of Schofield and Paliyath was not conducted in photosynthetic active tissues which might show different regulatory mechanisms of the carotenoid metabolism.
The present study demonstrated that combining blue LEDs with white LEDs is more beneficial for the production of carotenoid-rich leafy vegetables than white LEDs alone. A higher blue percentage of the light source increased on the one hand the transcript levels of biosynthetic genes, and in addition potentially the metabolic activity of the upper carotenoid biosynthesis pathway, resulting in the accumulation of carotenoids. Although by measuring phytoene accumulation after Norflurazon-treatment under different LED treatments, it was shown that blue light alone is not sufficient to convert increased transcript levels to a higher PSY enzymatic activity, but that an additional wavelength of the visual spectrum is more efficient in the activation of post-transcriptional steps leading to the enzymatic activity of the biosynthetic pathway. The results of this study not only help to optimize the production of carotenoid-rich leafy vegetables, but also contribute to a better understanding of the underlying mechanisms which regulate the accumulation of secondary plant metabolites and have hardly been investigated in vegetables.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Mitchell, C. A., Both, A.-J., Bourget, C. M., Burr, J. F., Kubota, C., Lopez, R. G., et al. (2012). LEDs: The future of greenhouse lighting! Chronica Horticulturae, 52, 5–12.
McCree, K. J. (1972). Action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agricultural Meteorology, 9, 191–216. https://doi.org/10.1016/0002-1571(71)90022-7
Hasan, M. M., Bashir, T., Ghosh, R., Lee, S. K., & Bae, H. (2017). An overview of LEDs’ effects on the production of bioactive compounds and crop quality. Molecules, 22(9), E1420. https://doi.org/10.3390/molecules22091420
Johkan, M., Shoji, K., Goto, F., Hashida, S.-n., Yoshihara, T. (2010). Blue light-emitting diode light irradiation of seedlings improves seedling quality and growth after transplanting in red leaf lettuce. HortScience, 45(12):1809–1814. https://doi.org/10.21273/HORTSCI.45.12.1809
Bantis, F., Ouzounis, T., & Radoglou, K. (2016). Artificial LED lighting enhances growth characteristics and totalphenolic content of Ocimum basilicum, but variably affects transplant success. Scientia Horticulturae, 198, 277–283. https://doi.org/10.1016/j.scienta.2015.11.014
Cazzonelli, C. I. (2011). Goldacre review: Carotenoids in nature: Insights from plants and beyond. Functional Plant Biology, 38(11), 833–847. https://doi.org/10.1071/FP11192
Fiedor, J., & Burda, K. (2014). Potential role of carotenoids as antioxidants in human health and disease. Nutrients, 6(2), 466–488. https://doi.org/10.3390/nu6020466
Woitsch, S., & Römer, S. (2003). Expression of xanthophyll biosynthetic genes during light-dependent chloroplast differentiation. Plant Physiology, 132(3), 1508–1517. https://doi.org/10.1104/pp.102.019364
von Lintig, J., Welsch, R., Bonk, M., Giuliano, G., Batschauer, A., & Kleinig, H. (1997). Light-dependent regulation of carotenoid biosynthesis occurs at the level of phytoene synthase expression and is mediated by phytochrome in Sinapis alba and Arabidopsis thaliana seedlings. The Plant Journal, 12, 625–634. https://doi.org/10.1046/j.1365-313X.1997.00625.x
Li, Q., & Kubota, C. (2009). Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environmental and Experimental Botany, 67(1), 59–64. https://doi.org/10.1016/j.envexpbot.2009.06.011
Ohashi-Kaneko, K., Takase, M., Kon, N., Fujiwara, K., & Kurata, K. (2007). Effect of light quality on growth and vegetable quality in leaf lettuce, spinach and komatsuna. Environment Control in Biology, 45(3), 189–198. https://doi.org/10.2525/ecb.45.189
Kami, C., Lorrain, S., Hornitschek, P., & Fankhauser, C. (2010). Light-regulated plant growth and development. Current Topics in Developmental Biology, 91, 29–66. https://doi.org/10.1016/S0070-2153(10)91002-8
Jiao, Y., Lau, O. S., & Deng, X. W. (2007). Light-regulated transcriptional networks in higher plants. Nature Reviews: Genetics, 8(3), 217–230. https://doi.org/10.1038/nrg2049
Pfalz, J., Liebers, M., Hirth, M., Grübler, B., Holtzegel, U., Schröter, Y., et al. (2012). Environmental control of plant nuclear gene expression by chloroplast redox signals. Frontiers in Plant Science, 3:Article 257. https://doi.org/10.3389/fpls.2012.00257
Pfannschmidt, T., Bräutigam, K., Wagner, R., Dietzel, L., Schröter, Y., Steiner, S., et al. (2009). Potential regulation of gene expression in photosynthetic cells by redox and energy state: Approaches towards better understanding. Annals of Botany, 103(4), 599–607. https://doi.org/10.1093/aob/mcn081
Toledo-Ortiz, G., Johansson, H., Lee, K. P., Bou-Torrent, J., Stewart, K., Steel, G., et al. (2014). The HY5-PIF regulatory module coordinates light and temperature control of photosynthetic gene transcription. PLoS Genetics, 10(6), e1004416. https://doi.org/10.1371/journal.pgen.1004416
Frede, K., Schreiner, M., Zrenner, R., Graefe, J., & Baldermann, S. (2018). Carotenoid biosynthesis of pak choi (Brassica rapa ssp. chinensis) sprouts grown under different light-emitting diodes during the diurnal course. Photochemical and Photobiological Sciences, 17, 1289–1300. https://doi.org/10.1039/C8PP00136G
Loivamäki, M., Louis, S., Cinege, G., Zimmer, I., Fischbach, R. J., & Schnitzler, J. P. (2007). Circadian rhythms of isoprene biosynthesis in grey poplar leaves. Plant Physiology, 143(1), 540–551. https://doi.org/10.1104/pp.106.092759
Thompson, A. J., Jackson, A. C., Parker, R. A., Morpeth, D. R., Burbidge, A., & Taylor, I. B. (2000). Abscisic acid biosynthesis in tomato: Regulation of zeaxanthin epoxidase and 9-cis-epoxycarotenoid dioxygenase mRNAs by light/dark cycles, water stress and abscisic acid. Plant Molecular Biology, 42, 833–845. https://doi.org/10.1023/a:1006448428401
Chow, W. S., Melis, A., & Anderson, J. M. (1990). Adjustments of photosystem stoichiometry in chloroplasts improve the quantum efficiency of photosynthesis. National Academy of Sciences of the United States of America, 87, 7502–7506. https://doi.org/10.1073/pnas.87.19.7502
Kim, J. H., Glick, R. E., & Melis, A. (1993). Dynamics of photosystem stoichiometry adjustment by light quality in chloroplasts. Plant Physiology, 102, 181–190. https://doi.org/10.1104/pp.102.1.181
Moejes, F. W., Matuszynska, A., Adhikari, K., Bassi, R., Cariti, F., Cogne, G., et al. (2017). A systems-wide understanding of photosynthetic acclimation in algae and higher plants. Journal of Experimental Botany, 68(11), 2667–2681. https://doi.org/10.1093/jxb/erx137
Frede, K., Schreiner, M., & Baldermann, S. (2019). Light quality-induced changes of carotenoid composition in pak choi (Brassica rapa ssp. chinensis). Journal of Photochemistry and Photobiology B: Biology, 193, 18–30. https://doi.org/10.1016/j.jphotobiol.2019.02.001
Hanson, P., Yang, R.-y., Chang, L.-c., Ledesma, L., Ledesma, D. (2009). Contents of caroteniods, ascorbic acid, minerals and total glucosinolates in leafy brassica pak choi (Brassica rapa L. chinensis) as affected by season and variety. Journal of the Science of Food and Agriculture, 89(5):906–914. https://doi.org/10.1002/jsfa.3533
Nair, A., Irish, L. (2016). Commercial production of pak choi. Iowa State University Extension and Outreach. Retrieved April 9, 2018, from https://store.extension.iastate.edu/product/14611
Kallabis-Rippel, K. (2000). Untersuchungen zu Pak Choi (Brassica rapa L. ssp. chinensis (L.) Hanelt): Anbau und Genußeigenschaften. Munich, Germany: Herbert Utz Verlag.
Koschmieder, J., & Welsch, R. (2020). Quantification of carotenoid pathway flux in green and nongreen systems. In M. Rodríguez-Concepción & R. Welsch (Eds.), Plant and Food Carotenoids - Methods and Protocols (pp. 279–291). Humana.
Mageney, V., Baldermann, S., Albach, D. C. (2016). Intraspecific variation in carotenoids of Brassica oleracea var. sabellica. Journal of Agricultural and Food Chemistry, 64(16):3251–3257. https://doi.org/10.1021/acs.jafc.6b00268
Britton, G., Liaaen-Jensen, S., & Pfander, H. (2004). Carotenoids - Handbook. Birkhäuser Verlag.
Vandesompele, J., De Preter, K., Pattyn, F., Poppe, B., Van Roy, N., De Paepe, A., et al. (2002). Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biology, 3(7):research0034. https://doi.org/10.1186/gb-2002-3-7-research0034
Pfaffl, M. W. (2004). Quantification strategies in real-time PCR. In Bustin, S. A. (Ed.), A-Z of quantitative PCR (pp. 87–112) La Jolla, CA, USA: IUL Biotechnol. Ser.
Fraser, P. D., Schuch, W., & Bramley, P. M. (2000). Phytoene synthase from tomato (Lycopersicon esculentum) chloroplasts - partial purification and biochemical properties. Planta, 211, 361–369. https://doi.org/10.1007/s004250000293
Cunningham, F. X., Pogson, B., Sun, Z., McDonald, K. A., DellaPenna, D., & Gantt, E. (1996). Functional analysis of the beta and epsilon lycopene cyclase enzymes of Arabidopsis reveals a mechanism for control of cyclic carotenoid formation. The Plant Cell, 8(9), 1613–1626. https://doi.org/10.1105/tpc.8.9.1613
Kopsell, D. A., Kopsell, D. E., & Curran-Celentano, J. (2007). Carotenoid pigments in kale are influenced by nitrogen concentration and form. Journal of the Science of Food and Agriculture, 87(5), 900–907. https://doi.org/10.1002/jsfa.2807
Ahrazem, O., Gómez-Gómez, L., Rodrigo, M. J., Avalos, J., & Limón, M. C. (2016). Carotenoid cleavage oxygenases from microbes and photosynthetic organisms: Features and functions. International Journal of Molecular Sciences, 17(11), E1781. https://doi.org/10.3390/ijms17111781
Scolnik, P. A., & Bartley, G. E. (1993). Phytoene desaturase from Arabidopsis. Plant Physiology, 103(4), 1475. https://doi.org/10.1104/pp.103.4.1475
Welsch, R., Arango, J., Bar, C., Salazar, B., Al-Babili, S., Beltran, J., et al. (2010). Provitamin A accumulation in cassava (Manihot esculenta) roots driven by a single nucleotide polymorphism in a phytoene synthase gene. The Plant Cell, 22(10), 3348–3356. https://doi.org/10.1105/tpc.110.077560
Fraser, P. D., Enfissi, E. M., Halket, J. M., Truesdale, M. R., Yu, D., Gerrish, C., et al. (2007). Manipulation of phytoene levels in tomato fruit: Effects on isoprenoids, plastids, and intermediary metabolism. The Plant Cell, 19(10), 3194–3211. https://doi.org/10.1105/tpc.106.049817
Ye, X., Al-Babili, S., Klöti, A., Zhang, J., Lucca, P., Beyer, P., et al. (2000). Engineering the provitamin A (beta-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science, 287, 303–305. https://doi.org/10.1126/science.287.5451.303
Maass, D., Arango, J., Wust, F., Beyer, P., & Welsch, R. (2009). Carotenoid crystal formation in Arabidopsis and carrot roots caused by increased phytoene synthase protein levels. PLoS ONE, 4(7), e6373. https://doi.org/10.1371/journal.pone.0006373
Hernández-Verdeja, T., & Strand, A. (2018). Retrograde signals navigate the path to chloroplast development. Plant Physiology, 176(2), 967–976. https://doi.org/10.1104/pp.17.01299
Ahmad, M., Grancher, N., Heil, M., Black, R. C., Giovani, B., Galland, P., et al. (2002). Action spectrum for cryptochrome-dependent hypocotyl growth inhibition in Arabidopsis. Plant Physiology, 129(2), 774–785. https://doi.org/10.1104/pp.010969
Naznin, M. T., Lefsrud, M., Gravel, V., & Azad, M. O. K. (2019). Blue light added with red LEDs enhance growth characteristics, pigments content, and antioxidant capacity in lettuce, spinach, kale, basil, and sweet pepper in a controlled environment. Plants (Basel), 8(4), 93. https://doi.org/10.3390/plants8040093
Goins, G. D., Yorio, N. C., Sanwo, M. M., & Brown, C. S. (1997). Photomorphogenesis, photosynthesis, and seed yield of wheat plants grown under red light-emitting diodes (LEDs) with and without supplemental blue lighting. Journal of Experimental Botany, 48(312), 1407–1413. https://doi.org/10.1093/jxb/48.7.1407
Fang, L., Ma, Z., Wang, Q., Nian, H., Ma, Q., Huang, Q., et al. (2021). Plant growth and photosynthetic characteristics of soybean seedlings under different LED lighting quality conditions. Journal of Plant Growth Regulation, 40, 668–678. https://doi.org/10.1007/s00344-020-10131-2
Alrifai, O., Hao, X., Liu, R., Lu, Z., Marcone, M. F., & Tsao, R. (2021). LED-induced carotenoid synthesis and related gene expression in Brassica microgreens. Journal of Agricultural and Food Chemistry, 69(16), 4674–4685. https://doi.org/10.1021/acs.jafc.1c00200
Stange, C., Flores, C. (2012). Carotenoids and photosynthesis - regulation of carotenoid biosyntesis by photoreceptors. In Najafpour, M. (Ed.), Advances in photosynthesis – Fundamental aspects (pp. 77–96) Rijeka, Croatia: InTech.
Gonzalez-Jorge, S., Ha, S. H., Magallanes-Lundback, M., Gilliland, L. U., Zhou, A., Lipka, A. E., et al. (2013). Carotenoid cleavage dioxygenase4 is a negative regulator of beta-carotene content in Arabidopsis seeds. The Plant Cell, 25(12), 4812–4826. https://doi.org/10.1105/tpc.113.119677
Lätari, K., Wüst, F., Hübner, M., Schaub, P., Beisel, K. G., Matsubara, S., et al. (2015). Tissue-specific apocarotenoid glycosylation contributes to carotenoid homeostasis in Arabidopsis leaves. Plant Physiology, 168(4), 1550–1562. https://doi.org/10.1104/pp.15.00243
Khachik, F., Beecher, G. R., Goli, M. B., & Lusby, W. R. (1991). Separation, identification, and quantification of carotenoids in fruits, vegetables and human plasma by high performance liquid chromatography. Pure and Applied Chemistry, 63, 71–80. https://doi.org/10.1351/pac199163010071
Engelmann, N. J., Clinton, S. K., & Erdman, J. W. J. (2011). Nutritional aspects of phytoene and phytofluene, carotenoid precursors to lycopene. Advances in Nutrition, 2(1), 51–61. https://doi.org/10.3945/an.110.000075
Schofield, A., & Paliyath, G. (2005). Modulation of carotenoid biosynthesis during tomato fruit ripening through phytochrome regulation of phytoene synthase activity. Plant Physiology and Biochemistry, 43(12), 1052–1060. https://doi.org/10.1016/j.plaphy.2005.10.006
Acknowledgements
The authors would like to thank I. Hauschild for the LED module installations, and A. Maikath and A. Platalla for their excellent technical assistance.
Funding
Open Access funding enabled and organized by Projekt DEAL. No funds, grants, or other support was received.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by KF. All authors contributed to the interpretation of the data. The first draft of the manuscript was written by KF and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Frede, K., Baldermann, S. Accumulation of carotenoids in Brassica rapa ssp. chinensis by a high proportion of blue in the light spectrum. Photochem Photobiol Sci 21, 1947–1959 (2022). https://doi.org/10.1007/s43630-022-00270-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43630-022-00270-8