Abstract
Heterogeneous photocatalysis is one of the most studied and promising techniques for degradation of contaminants of emerging concern, especially pharmaceuticals, and it represents a potential application in wastewater treatment of recalcitrant pollutants, such as fluoroquinolones, which are almost not abated by standard WWTPs. Although photodegradation partially contributes to alleviate their accumulation into the aquatic systems, heterogeneous photocatalysis assures complete sequestration and mineralization of FQs and their photoproducts and offers many advantages with respect to the other advanced oxidation processes (AOPs). The present brief review summarizes the most recent studies regarding the development and application of novel photocatalytic materials to the removal of FQs from contaminated waters. The collected data are arranged relating the mechanistic aspects to specific catalysts’ properties, such as adsorption capacity, easy recovery, and reusability, especially under actual conditions.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The ubiquitous presence of pharmaceuticals in the environment has now been recognized as a subject of global concern. Due to their continuous input and persistence, even at low concentrations, they are considered emerging contaminants (EC) [1, 2]. They have been detected worldwide in surface waters, drinking waters, wastewater effluents, and soils. Their concentrations range from a few tens of nanograms per liter in surface and drinking water [1, 3], to some hundreds in wastewater effluents from highly urbanized and industrialized areas [4]. Such low concentrations are enough to generate collateral effects on environmental organisms and human health [2], while the potential chronic effects of long-term low-level exposure to pharmaceuticals and their degradation products are still largely unknown.
Among the different groups of pharmaceuticals, antibiotics are the most severe threat since they stimulate bacterial resistance [2]. They are regularly administered in large amounts for therapeutic purposes and, in the past, as feed additives to support growth in livestock. Once administered, most of them reach the urban wastewater treatment plants (WWTPs) in their pharmaceutically active or partially metabolized form with the wastewater, because the WWTPs are generally unable to remove such chemically stable molecules [3]. Therefore, several compounds persist in the treated water and contaminate surface and groundwater at low but potentially toxic concentrations. Veterinary antibacterials can directly reach soil and groundwater through the standard recycling of manure from animal husbandry or sewage sludge from WWTPs as fertilizers [2]. As recently reported in the European directive and in the literature [2, 5,6,7], antibiotics enhance bacterial resistance, and reducing their consumption seems not enough to mitigate this effect that can lead to an ecological disaster [8].
Fluoroquinolones (FQs) are beneficial antibacterial agents and form the last class of antibiotics of extensive use, particularly in highly developed countries. In the 1980s, they were synthesized in Europe and the USA for humans and about 10 years later as veterinary medicines. They show both a broad activity spectrum against Gram bacteria and good oral absorption. Human and veterinary fluoroquinolones are used mainly in highly urbanized areas and swine and cattle farms, such as those in the Lombardy valley [3, 4, 9, 10]. Their occurrence in the environment has been widely assessed for the past decade. FQs have been found in aquatic [9] and soil [10] compartments at concentrations in the ranges of ng L−1–μg L−1 and µg kg−1–mg kg−1, respectively. There are different reasons for the presence of FQs in the environment. FQs are excreted in their active form with no modification of the heterocyclic ring, and their excellent chemical stability and relatively high solubility favour environmental diffusion, especially in surface waters, before environmental mechanisms intervene in their transformation and removal. On this account, FQs may be defined as “pseudo-persistent” contaminants.
Alongside with the increasing environmental concern, the interest in the photobiological effect of fluoroquinolones (generally 7-dialkylamino-6-fluoroquinol-4-one-3-carboxylic acids FQs (1 in Scheme 1), stimulated many photochemical investigations [11,12,13]. They absorb in the UV-A region, and they are photochemically reactive. The photochemical reactions of these drugs can cause toxic effects on skin and eyes and, in some cases, mutagenesis and carcinogenesis [14, 15]. Furthermore, as mentioned above, these drugs possess a high chemical stability, and this characteristic makes them persistent pollutants. Solar irradiation may be one of the few effective paths for the decontamination of surface water and soil [5, 16, 17]. Finally, such compounds have shown an interesting and, in part, unexpected photoreactivity among heteroaromatics [18, 19]. Their photochemistry has been deeply investigated and the photoreaction mechanism and the structure of the photoproducts are well-known. For this reason, they are particularly suitable to supply information on the oxidative degradation paths in environmental conditions and, when photocatalytic methods are applied to remove these drugs from water and soil, it is possible to compare and distinguish the catalyst effect from the base photochemical reaction.
2 Photoreaction mechanism of fluoroquinolones
FQs photochemistry is mainly due to the population of the first triplet excited state by a very efficient ISC [20]. FQs, as in general polynuclear (hetero)aromatics, are not expected to photorearrange [21], nor abstract hydrogen [22]. The occurrence of reactions after photoexcitation is due to processes involving a substituent. Various types of photochemically labile molecular sites have been identified, among which the carbon–fluorine bond is particularly important. A fluorine atom is a quite ubiquitous presence in the scaffold of commercially available fluoroquinolones due to its beneficial effects on the antibacterial activity of these drugs. For a large number of these compounds, the main photoprocess is the heterolytic defluorination, an unusual reaction with fluoroaromatics due to the strength of the aromatic C–F bond (dissociation energy ca. 120 kcal mol−1). However, in the case of FQs different mechanisms that are characterized by very different quantum yield (reported values from 0.55 to < 0.001) are involved, which outcome is strongly influenced by the position of fluorine atom on the heteroaromatic moiety [20]. In particular monofluoroderivatives show a lower quantum yield with respect to polyfluorinated derivatives.
With 6-monofluoroderivatives (X=H), such as enoxacin, norfloxacin and ciprofloxacin, the primary process is photosubstitution of the fluorine atom by a hydroxyl group via a SN2Ar process (Φ ca. 0.1). The reaction proceeds from the triplet state and involves a long-lived intermediate (t = 3.6 ms, λmax > 650 nm), the cyclohexadienyl anion 2− (Scheme1, path a) [18, 19, 23,24,25].
FQs bearing substituents that heavily modify the electronic structure (e.g. X=OR, SR) may undergo different chemistry, as observed for ofloxacin, rufloxacin, and marbofloxacin, and only inefficient processes occur (Φ ≤ 0.01), such as decarboxylation and degradation of the alkylamino side-chain (or of the N1 side-chain) (Scheme 2), probably via a radical path [26,27,28].
The presence of a second halogen in position 8 makes the molecule much more sensitive. When X=F, Cl photoheterolytic cleavage takes place selectively from that position through an efficient SN1 process (Φ ca. 0.5), with the formation of the aryl cation 3+ by defluorination in position 8 [10] (Scheme 1 path b). Such reactivity has been observed with lomefloxacin, fleroxacin, orbifloxacin, sparfloxacin, and related derivatives [29,30,31,32,33,34].
3 Photocatalytic degradation of fluoroquinolones
Although photodegradation is the main FQs abiotic removal pathway, at least for some, and it might alleviate their accumulation into the aquatic systems [35, 36], researchers are continuously developing new strategies to abate such persistent contaminants.
As largely demonstrated, photocatalysis assures FQs disappearance and that of their photoproducts and offers many advantages with respect to the other advanced oxidation processes (AOPs): (i) sunlight often exceeds UV light; (ii) additional reagents are not necessary; (iii) catalysts can be quickly recovered and eventually reused. For these reasons, nowadays it is still the most investigated AOP [37].
In the following, we reported only the most recent applications for the sake of shortness. Table 1 summarizes the most recent photocatalytic applications of different catalysts to remove some FQs under ideal and actual conditions. The collected data are arranged highlighting the mechanistic and environmental aspects, and the experimental working conditions. On the other hand, the synthesis and physical characterization of the reported materials, and their detoxification efficacy (inactivation of the active principles, evaluation of acute and chronic effects against microorganisms), are not explored here.
3.1 Types of catalysts
Titanium dioxide has been the most investigated catalyst in photocatalysis, and photochemistry in general. Due to its excellent properties it was also tested for water remediation in the last two decades, especially to remove pharmaceuticals and personal care products [37]. The generally accepted mechanism of TiO2 photocatalysis is initiated by the absorption of a photon with energy greater than the bandgap of the semiconductor (~ 3.2 eV), leading to the generation of an excitation couple with positive holes (h+) in the valence band and free electrons (e−) in the conduction band. When the charge separation on the catalyst surface overcome electron cb/hole vb recombination (occurring in the ps to ns range), the charge carriers can migrate on the catalyst surface, eventually reacting within the semiconductor/adsorbed molecule complex.
The most recent studies pushed the boundaries and explored new materials based on metallic nanoalloy, metal oxides, g-C3N4, and allotropic carbon structures, e.g. MWCNs (multi walled carbon nanotubes) and graphene (see Table 1).
All these materials share a common general mechanism, which can be summarized into four main steps: FQs adsorption on the catalyst surface, light absorption by the catalyst leading to the generation of the reactive oxidizing species, pollutant degradation and eventually mineralization, and catalyst regeneration. Starting from the most studied catalyst, P25 TiO2, a lot of efforts in producing new catalysts have been devoted to improving one or more of the above steps.
All the new catalysts presented in this review have adsorption times between 10 min and a few hours, with only a few exceptions, which require longer adsorption times up to several hours [38, 39]. A direct comparison between different catalyst is often difficult and hampered by the fact that each study has been conducted under very different experimental conditions, i.e. FQ concentration, catalyst load, water matrix, light source (see Table 1). The catalyst load varies from 75 mg L−1 [40], increasing up to 6 g where a fixed bed geometry has been evaluated [41] for the more applicative studies. Other two important aspects that qualify a catalyst for practical use are the turn-over number (TO), i.e. the number of times the catalyst can be regenerated and re-employed without losing its activity, and the resistance to the poisoning effect of the matrix constituents. All the new material presented hereafter showed a good TO number, retaining more than 90% activity up to 11 catalytic cycles [42] in the case of TiO2 and to 6 cycles for the higher performer among the new presented materials, viz. ZnBiOI bimetallic nanoalloy [43]. Nonetheless, all the explored catalysts demonstrated very high efficiency in FQs removal above 80% [44,45,46], often above 90% and in the most favourable cases (high catalyst/FQ ratio, longer irradiation times, DI water) it rises up to almost 100% [40, 41, 47,48,49].
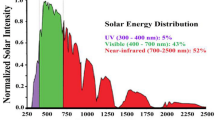
The strongest driver in exploring new materials, apart from TiO2, in the last decades, has been the attempt to modify the semiconductor band gap; TiO2, with a band gap of 3.2 eV (~ 400 nm) is not able to absorb efficiently in the visible spectrum of the solar light, limiting its utilization in practical environmental applications. Most of the new catalyst have a more efficient UV–Vis spectrum absorption, increasing the amount of sunlight available to the photocatalytic reaction: if N-doped TiO2, historically one the first approach to this problem, allows reducing the bandgap down to 2.9 eV [47], is with the new materials that scientists have really been able to access the visible part of the sunlight emission, reaching band gaps below 2.5 eV (~ 500 nm; see Table 1). If from one side this has been an improvement in the catalyst photophysical efficiency, from the other side it required a sharp and accurate fine-tuning of semiconductors valence and conduction bands energies, to keep their photochemical activity as powerful oxidants.
In the case of TiO2, when photogenerated holes (h+) reach the organic molecules adsorbed on the catalyst surface, an electron transfer occurs at a rate from 108 to 1011 M−1 s−1 [37], initiating a cascade complex reactions which may eventually end in the complete mineralization of the organic compound (Fig. 1). At the same time, photogenerated holes can also react with the water medium generating the OH radical, a powerful and rather non-selective oxidant, whose contribution to the photocatalyst driven mineralization process has been widely recognized in literature. Furthermore, the photogenerated electrons (e−), in the presence of dissolved oxygen, can take part in the mineralization process through the formation of the superoxide anion and other powerful oxidant species, like the singlet oxygen. The relative importance of each of these ROS in the FQ degradation strongly depends on the physico/chemical characteristics of each catalyst. Despite the differences in the studied catalysts, all the authors reported the contribution of both the direct (h+)-FQ electron transfer mechanism and the OH radical initiated oxidation while the contribution of superoxide and singlet oxygen are way more debated and influenced by the presence of water matrix constituents, such as nitrate, sulphates, carbonates, metal ions and dissolved organic matter (DOM).
Mechanism of TiO2 photocatalysis (ECs—contaminants of emerging concern, TPs—transformation products, VB—valence band, CB—conduction band) [37]
3.2 Mechanistic pathways
Differently from the direct photolysis, the degradation mechanism of FQs in the presence of a photocatalyst is almost independent of the drug molecular structure and proceeds through the oxidation of the molecule backbone, starting from the more labile sites.
This privileged reaction path massively increases the number of photoproducts and the degree of degradation of the pollutant: most of the authors [38,39,40, 42, 44, 46, 48,49,50,51,52] agree in identifying the electron-rich piperazine side-chain as the first site of attack of the photogenerated OH radicals, followed by the carboxylic acid in position 3 [38, 42, 44, 46, 51, 52], while in some cases also the alkyl side-chains may be interested in the oxidative reaction as reported in Ref. [40, 42, 48]. Examples of this type of products are shown in Fig. 2 for ciprofloxacin and ofloxacin.
The degradation pathway of CIP and OFL molecules over CuBi/c-CNTs photocatalyst under visible light irradiation [50]
Secondary pathways involve either the direct hydroxylation of the aromatic ring [40, 50, 51] or the fluorine substitution [40, 42, 46]; this latter mechanism has already been identified also in the direct photolysis of 6 mono fluoroquinolones and can be ascribed to the population of the excited triplet of FQ via energy transfer from the catalyst (Fig. 3) [53].
Proposed excitation of 3.0–3.2 eV OFL by hole transfer or triple–triplet energy transfer [53]
Once initiated, the degradation process proceeds through a cascade of subsequent reactions, leading to FQ ring-opening [38, 40, 46, 51] and, eventually, to complete mineralization and pollutant removal, with only water and carbon dioxide being released as by-products (Fig. 4). The different nature of the photocatalyst has little/no effect on the degradation process outcome and all the authors found end products distributions similar to those previously reported in the literature [35], confirming one more time that the photocatalytic reaction always proceed through a robust mechanism involving strong and non-selective oxidizing species, i.e. (h+), OH radicals, superoxide, and singlet oxygen.
Photolytic and photocatalytic degradation paths of fluoroquinolones [37]
3.3 Effects of the environmental conditions
The most recent catalysts represent important advances in materials synthesis and design: they can combine high adsorption capacity and photocatalytic activity for sequestering and mineralizing one or more FQs, are easily recoverable and reusable at the end of the treatment, and are based on inexpensive reagents. As shown in Table 1, all of them ensure quantitative FQs removal, despite the different working conditions. The photodegradation of FQs occurred mainly through a pseudo-first-order kinetic law, except in ref. [54], with comparable kinetic degradation constant values (kdeg, same order of magnitude 10–2 s−1) independent of the catalyst’s nature and working conditions, but dependent on the common oxidative process. Despite the satisfactory FQs abatement mainly obtained in a pH range 6–8 close to environmental waters, the photocatalytic efficiency is still investigated under non-representative conditions, i.e. in ultrapure water. Instead, the characterization of the adsorbent and photocatalytic performances under environmental conditions can give relevant indications about the implementation of the water remediation processes. Only some papers explore the effects of the main matrix constituents on the photocatalytic process by adding salts and standard reference materials, such as humic acids, to distilled water samples [42, 44, 51, 55, 56], or spiking samples collected from rivers, lakes, and wastewater effluents [41, 44, 49, 51, 55, 57, 58] with proper amounts of FQs. The latter route provides more representative information than the single contribution of each anion/cation on the photocatalytic efficiency.
Tap water is chosen as a solvent because of its invariant and similar composition to surface waters. As expected and previously demonstrated [17, 35], slight differences in kdeg were observed between tap and distilled water [41]. A similar trend has been observed in surface water samples (tap, river, lake and effluent) [42, 51, 55], because of the opposite effects of HA and anions.
On the contrary, kdeg decreased up to one order of magnitude in very complex matrices, such as WWTPs effluent containing a high amount of suspended solids (8.4 mg L−1) and total organic carbon (TOC, 14.3 mg L−1) [58]. Something different occurs in the presence of biodegradable organic matter, such as grease, fats, and proteins, high amounts of phosphate and nitrate ions of a simulated poultry farm [57]. This behaviour highlights the different applicability of two types of graphene-based materials, i.e. the polyacrylic acid- grafted-carboxylic graphene/titanium nanotube composite compared with the biopolymer-supported nanocomposite graphene oxide film.
The most added ions are HCO3−, Cl−, NO3− SO42−, Na+, K+, Mg2+, Ca2+, Mn2+, and Fe3+, rarely NO2−, PO43−, Cu2+, HCO3−, and Ca2+ ions are surely the predominant species in the pH range found in most waters, and their role in the environmental photochemistry is well ascertained [59]. In general, HCO3− and Cl− are OH radical scavengers, NO3− is a photosensitizer, while cations may give stable complexes. As for FQs, a slight [49, 55] or a moderate inhibiting effect from anions was observed depending on the concentration added [44, 51, 55].
Instead, antibiotic degradation was significantly reduced in the presence of divalent ions that give stable complexes with FQs [51]. Interesting results showed that the divalent transition metal, such as Cu2+, drastically reduced the FQs removal with a magnetic TiO2-MIP [56] and a mesoporous g-C3N4 [51].
The ubiquitous HAs exerted a double inhibiting effect towards FQs, competing with reactive species [42, 51, 56] and absorbing solar radiation [51].
4 Conclusions
Heterogeneous photocatalysis is one of the most studied techniques for degradation of contaminants of emerging concern, especially pharmaceuticals, and it represents a potential application in wastewater treatment also in the case of photosensitive molecules, such as fluoroquinolones. To this end, many research efforts are being made to develop and test innovative materials.
The review incorporates a brief part focusing on the photochemistry of fluoroquinolones and summarizes the most recent photocatalytic applications for FQs removal. In particular, the mechanistic aspects were related to specific catalysts’ properties, such as high surface area, high stability in the aqueous medium, easy recovery, and reusability. It also evaluated the catalysts’ applicability under actual conditions, highlighting the effects of the matrix constituents. Effects of NOM, ions, pH, and ionic strength are mandatory to elucidate the photodegradation mechanism, overcome the limitation of the technique and improve the efficiency of the degradation process. Although information reported here can help igniting future research, the photocatalytic degradation of fluoroquinolones in actual conditions is still an ongoing challenge which keeps open the door to further studies and improvement, especially to what regards the wider application on a larger scale of this technology, in real, full-size WWTPs.
References
Sousa, J. C. G., Ribeiro, A. R., Barbosa, M. O., Pereira, M. F. R., & Silva, A. M. T. (2018). A review on environmental monitoring of water organic pollutants identified by EU guidelines. Journal of Hazardous Materials, 344, 146–162.
E. Commission, Communication from the Commission to the European Parliament, the Council, and the European Economic and Social Committee: European Union Strategic Approach to Pharmaceuticals in the Environment, EU Commission, 2019, 128, 13.
Castiglioni, S., Davoli, E., Riva, F., Palmiotto, M., Camporini, P., Manenti, A., & Zuccato, E. (2018). Data on occurrence and fate of emerging contaminants in a urbanised area. Data in Brief, 17, 533–543.
Riva, F., Castiglioni, S., Fattore, E., Manenti, A., Davoli, E., & Zuccato, E. (2018). Monitoring emerging contaminants in the drinking water of Milan and assessment of the human risk. International Journal of Hygiene and Environmental Health, 221, 451–457.
Kummerer, K. (2004). Resistance in the environment. Journal of Antimicrobial Chemotherapy, 54, 311–320.
Menz, J., Baginska, E., Arrhenius, Å., Haiß, A., Backhaus, T., & Kümmerer, K. (2017). Antimicrobial activity of pharmaceutical cocktails in sewage treatment plant effluent—An experimental and predictive approach to mixture risk assessment. Environmental Pollution, 231, 1507–1517.
Lindberg, R. H., Björklund, K., Rendahl, P., Johansson, M. I., Tysklind, M., & Andersson, B. A. V. (2007). Environmental risk assessment of antibiotics in the Swedish environment with emphasis on sewage treatment plants. Water Research, 41, 613–619.
Merlin, C. (2020). Reducing the consumption of antibiotics: would that be enough to slow down the dissemination of resistances in the downstream environment? Frontiers in Microbiology. https://doi.org/10.3389/fmicb.2020.00033
Sturini, M., Speltini, A., Pretali, L., Fasani, E., & Profumo, A. (2009). Solid-phase extraction and HPLC determination of fluoroquinolones in surface waters. Journal of Separation Science, 32, 3020–3028.
Sturini, M., Speltini, A., Maraschi, F., Profumo, A., Pretali, L., Fasani, E., & Albini, A. (2012). Sunlight-induced degradation of soil-adsorbed veterinary antimicrobials marbofloxacin and enrofloxacin. Chemosphere, 86, 130–137.
Albini, A., & Monti, S. (2003). Photophysics and photochemistry of fluoroquinolones. Chemical Society Reviews, 32, 238.
Lhiaubet-Vallet, V., Bosca, F., & Miranda, M. A. (2009). Photosensitized DNA damage: the case of fluoroquinolones. Photochemistry and Photobiology, 85, 861–868.
Lorenzo, F., Navaratnam, S., Edge, R., & Allen, N. S. (2009). Primary photoprocesses in a fluoroquinolone antibiotic sarafloxacin. Photochemistry and Photobiology, 85, 886–894.
Marrot, L., Belaïdi, J. P., Chaubo, C., Meunier, J. R., Perez, P., & Agapakis-Causse, C. (2001). Fluoroquinolones as chemical tools to define a strategy for photogenotoxicity in vitro assessment. Toxicology in Vitro, 15, 131–142.
Marrot, L., Belaïdi, J. P., Jones, C., Perez, P., Meunier, J. R., Riou, L., & Sarasin, A. (2003). Molecular responses to photogenotoxic stress induced by the antibiotic lomefloxacin in human skin cells: from DNA damage to apoptosis. Journal of Investigative Dermatology, 121, 596–606.
Nakata, H., Kannan, K., Jones, P. D., & Giesy, J. P. (2005). Determination of fluoroquinolone antibiotics in wastewater effluents by liquid chromatography–mass spectrometry and fluorescence detection. Chemosphere, 58, 759–766.
Sturini, M., Speltini, A., Maraschi, F., Profumo, A., Pretali, L., Fasani, E., & Albini, A. (2010). Photochemical degradation of marbofloxacin and enrofloxacin in natural waters. Environmental Science & Technology, 44, 4564–4569.
Monti, S., Sortino, S., Fasani, E., & Albini, A. (2001). Multifaceted photoreactivity of 6-fluoro-7-aminoquinolones from the lowest excited states in aqueous media: A study by nanosecond and picosecond spectroscopic techniques. Chemistry, 7, 2185–2196.
Fasani, E., Barberis Negra, F. F., Mella, M., Monti, S., & Albini, A. (1999). Photoinduced C-F bond cleavage in some fluorinated 7-amino-4-quinolone-3-carboxylic acids. The Journal of Organic Chemistry, 64, 5388–5395.
Freccero, M., Fasani, E., Mella, M., Manet, I., Monti, S., & Albini, A. (2008). Modeling the photochemistry of the reference phototoxic drug lomefloxacin by steady-state and time-resolved experiments, and DFT and post-HF calculations. Chemistry—A European Journal, 14, 653–663.
Lablache-Combier, 1995. In P. S. S. W. M. Horspool (ed) CRC handbook of organic photochemistry and photobiology (pp. 1063–1120). CRC Press.
Whitten, D. G. (1976). Photoreduction and photoaddition reaction of heterocyclic compounds. Wiley.
Sortino, S., De Guidi, G., Giuffrida, S., Monti, S., & Velardita, A. (2008). pH effects on the spectroscopic and photochemical behavior of enoxacin: A steady-state and time-resolved study. Photochemistry and Photobiology, 67, 167–173.
Bilski, P., Martinez, L. J., Koker, E. B., & Chignell, C. F. (1998). Influence of solvent polarity and proticity on the photochemical properties of norfloxacin. Photochemistry and Photobiology, 68, 20–24.
Mella, M., Fasani, E., & Albini, A. (2001). Photochemistry of 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(piperazin-1-yl)quinoline-3-carboxylic acid (=ciprofloxacin) in aqueous solutions. Helvetica Chimica Acta, 84, 2508.
Condorelli, G., De Guidi, G., Giuffrida, S., Sortino, S., Chillemi, R., & Sciuto, S. (1999). Molecular mechanisms of photosensitization induced by drugs XII photochemistry and photosensitization of rufloxacin: an unusual photodegradation path for the antibacterials containing a fluoroquinolone-like chromophore. Photochemistry and Photobiology, 70, 280–286.
Navaratnam, S., & Claridge, J. (2000). Primary photophysical properties of ofloxacin. Photochemistry and Photobiology, 72, 283.
Pretali, L., Fasani, E., Dondi, D., Mella, M., & Albini, A. (2010). The unexpected photochemistry of marbofloxacin. Tetrahedron Letters, 51, 4696–4698.
Fasani, E., Mella, M., Caccia, D., Tassi, S., Fagnoni, M., & Albini, A. 1997. The photochemistry of lomefloxacin. An aromatic carbene as the key intermediate in photodecomposition. Chemical Communications, 1329–1330.
Martinez, L. J., Li, G., & Chignell, C. F. (1997). Photogeneration of fluoride by the fluoroquinolone antimicrobial agents lomefloxacin and fleroxacin. Photochemistry and Photobiology, 65, 599–602.
Morimura, T., Nobuhara, Y., & Matsukura, H. (1997). Photodegradation products of a new antibacterial fluoroquinolone derivative, orbifloxacin, in aqueous solution. Chemical and Pharmaceutical Bulletin, 45, 373–377.
Morimura, T., Kohno, K., Nobuhara, Y., & Matsukura, H. (1997). Photoreaction and active oxygen generation by photosensitization of a new antibacterial fluoroquinolone derivative, orbifloxacin, in the presence of chloride ion. Chemical and Pharmaceutical Bulletin, 45, 1828–1832.
Engler, M., Rüsing, G., Sörgel, F., & Holzgrabe, U. (1998). Defluorinated sparfloxacin as a new photoproduct identified by liquid chromatography coupled with UV detection and tandem mass spectrometry. Antimicrobial Agents and Chemotherapy, 42, 1151–1159.
Robertson, D. G., Epling, G. A., Kiely, J. S., Bailey, D. L., & Song, B. (1991). Mechanistic studies of the phototoxic potential of PD 117596, a quinolone antibacterial compound. Toxicology and Applied Pharmacology, 111, 221–232.
Sturini, M., Speltini, A., Maraschi, F., Profumo, A., Pretali, L., Irastorza, E. A., Fasani, E., & Albini, A. (2012). Photolytic and photocatalytic degradation of fluoroquinolones in untreated river water under natural sunlight. Applied Catalysis B: Environmental, 119–120, 32–39.
Klementová, Š, Poncarová, M., Langhansová, H., Lieskovská, J., Kahoun, D., & Fojtíková, P. (2021). Photodegradation of fluoroquinolones in aqueous solution under light conditions relevant to surface waters, toxicity assessment of photoproduct mixtures. Environmental Science and Pollution Research. https://doi.org/10.1007/s11356-021-16182-6
Pretali, L., Maraschi, F., Cantalupi, A., Albini, A., & Sturini, M. (2020). Water depollution and photo-detoxification by means of TiO2: fluoroquinolone antibiotics as a case study. Catalysts, 10, 628.
Wang, Y., Wang, F., Feng, Y., Xie, Z., Zhang, Q., Jin, X., Liu, H., Liu, Y., Lv, W., & Liu, G. (2018). Facile synthesis of carbon quantum dots loaded with mesoporous g-C3N4 for synergistic absorption and visible light photodegradation of fluoroquinolone antibiotics. Dalton Transactions, 47, 1284–1293.
Kamagate, M., Pasturel, M., Brigante, M., & Hanna, K. (2020). Mineralization enhancement of pharmaceutical contaminants by radical-based oxidation promoted by oxide-bound metal ions. Environmental Science & Technology, 54, 476–485.
Sharma, S., Ibhadon, A. O., Francesconi, M. G., Mehta, S. K., Elumalai, S., Kansal, S. K., Umar, A., & Baskoutas, S. (2020). Bi2WO6/C-dots/TiO2: a novel Z-scheme photocatalyst for the degradation of fluoroquinolone levofloxacin from aqueous medium. Nanomaterials, 10, 910.
Karoui, S., Ben Arfi, R., Ghorbal, A., Amrane, A., & Assadi, A. A. (2021). Innovative sequential combination of fixed bed adsorption/desorption and photocatalysis cost-effective process to remove antibiotics in solution. Progress in Organic Coatings, 151, 106014.
Wei, D., Li, S., Fang, L., & Zhang, Y. (2018). Effect of environmental factors on enhanced adsorption and photocatalytic regeneration of molecular imprinted TiO2 polymers for fluoroquinolones. Environmental Science and Pollution Research, 25, 6729–6738.
Zhang, Q., Zhu, Z., Zhao, X., Xiao, X., Zuo, X., & Nan, J. (2021). Efficient and effective removal of emerging contaminants through the parallel coupling of rapid adsorption and photocatalytic degradation: A case study of fluoroquinolones. Chemosphere, 280, 130770.
Zhao, C., Li, Y., Chu, H., Pan, X., Ling, L., Wang, P., Fu, H., Wang, C.-C., & Wang, Z. (2021). Construction of direct Z-scheme Bi5O7I/UiO-66-NH2 heterojunction photocatalysts for enhanced degradation of ciprofloxacin: Mechanism insight, pathway analysis and toxicity evaluation. Journal of Hazardous Materials, 419, 126466.
Heidari, S., Haghighi, M., & Shabani, M. (2020). Sunlight-activated BiOCl/BiOBr–Bi24O31Br 10 photocatalyst for the removal of pharmaceutical compounds. Journal of Cleaner Production, 259, 120679.
Huang, J., Li, D., Li, R., Zhang, Q., Chen, T., Liu, H., Liu, Y., Lv, W., & Liu, G. (2019). An efficient metal-free phosphorus and oxygen co-doped g-C3N4 photocatalyst with enhanced visible light photocatalytic activity for the degradation of fluoroquinolone antibiotics. Chemical Engineering Journal, 374, 242–253.
Venancio, W. A. L., Rodrigues-Silva, C., Spina, M., & Guimarães, J. R. (2020). Removal of the antimicrobial activity from fortified effluents with fluoroquinolones by photocatalytic processes: a comparative study of differently synthesized TiO2-N. Water Science and Technology. https://doi.org/10.2166/wst.2020.340
Zhu, F., Lv, Y., Li, J., Ding, J., Xia, X., Wei, L., Jiang, J., Zhang, G., & Zhao, Q. (2020). Enhanced visible light photocatalytic performance with metal-doped Bi2WO6 for typical fluoroquinolones degradation: Efficiencies, pathways and mechanisms. Chemosphere, 252, 126577.
Chakraborty, J., Nath, I., Jabbour, C., Aljammal, N., Song, S., Kao, C.-M., Heynderickx, P. M., & Verpoort, F. (2020). Novel rapid room temperature synthesis of conjugated microporous polymer for metal-free photocatalytic degradation of fluoroquinolones. Journal of Hazardous Materials, 398, 122928.
Khazaee, Z., Mahjoub, A. R., & Cheshme Khavar, A. H. (2021). One-pot synthesis of CuBi bimetallic alloy nanosheets-supported functionalized multiwalled carbon nanotubes as efficient photocatalyst for oxidation of fluoroquinolones. Applied Catalysis B: Environmental, 297, 120480.
Wang, F., Feng, Y., Chen, P., Wang, Y., Su, Y., Zhang, Q., Zeng, Y., Xie, Z., Liu, H., Liu, Y., Lv, W., & Liu, G. (2018). Photocatalytic degradation of fluoroquinolone antibiotics using ordered mesoporous g-C3N4 under simulated sunlight irradiation: Kinetics, mechanism, and antibacterial activity elimination. Applied Catalysis B: Environmental, 227, 114–122.
He, W., Li, Z., Lv, S., Niu, M., Zhou, W., Li, J., Lu, R., Gao, H., Pan, C., & Zhang, S. (2021). Facile synthesis of Fe3O4@MIL-100(Fe) towards enhancing photo-Fenton like degradation of levofloxacin via a synergistic effect between Fe3O4 and MIL-100(Fe). Chemical Engineering Journal, 409, 128274.
Sturini, M., Speltini, A., Maraschi, F., Vinci, G., Profumo, A., Pretali, L., Albini, A., & Malavasi, L. (2017). g-C3N4-promoted degradation of ofloxacin antibiotic in natural waters under simulated sunlight. Environmental Science and Pollution Research, 24, 4153–4161.
Huang, X., Wu, S., Tang, S., Huang, L., Zhu, D., & Hu, Q. (2020). Photocatalytic hydrogel layer supported on alkali modified straw fibers for ciprofloxacin removal from water. Journal of Molecular Liquids, 317, 113961.
Du, R., Chen, P., Zhang, Q., & Yu, G. (2021). The degradation of enrofloxacin by a non-metallic heptazine-based OCN polymer: Kinetics, mechanism and effect of water constituents. Chemosphere, 273, 128435.
Fang, L., Miao, Y., Wei, D., Zhang, Y., & Zhou, Y. (2021). Efficient removal of norfloxacin in water using magnetic molecularly imprinted polymer. Chemosphere, 262, 128032.
Anirudhan, T. S., Shainy, F., & Christa, J. (2017). Synthesis and characterization of polyacrylic acid- grafted-carboxylic graphene/titanium nanotube composite for the effective removal of enrofloxacin from aqueous solutions: Adsorption and photocatalytic degradation studies. Journal of Hazardous Materials, 324, 117–130.
Malesic Eleftheriadou, N., Ofrydopoulou, A., Papageorgiou, M., & Lambropoulou, D. (2020). Development of novel polymer supported nanocomposite GO/TiO2 films, based on poly(L-lactic acid) for photocatalytic applications. Applied Sciences, 10, 2368.
Vione, D., & Scozzaro, A. (2019). Photochemistry of surface fresh waters in the framework of climate change. Environmental Science & Technology, 53, 7945–7963.
Imam, S. S., Adnan, R., & Mohd Kaus, N. H. (2020). Room-temperature synthesis of flower-like BiOBr/Bi2S3 composites for the catalytic degradation of fluoroquinolones using indoor fluorescent light illumination. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 585, 124069.
Yang, Z., Tong, X., Feng, J., He, S., Fu, M., Niu, X., Zhang, T., Liang, H., Ding, A., & Feng, X. (2019). Flower-like BiOBr/UiO-66-NH2 nanosphere with improved photocatalytic property for norfloxacin removal. Chemosphere, 220, 98–106.
Chen, Z., Chen, X., Di, J., Liu, Y., Yin, S., Xia, J., & Li, H. (2017). Graphene-like boron nitride modified bismuth phosphate materials for boosting photocatalytic degradation of enrofloxacin. Journal of Colloid and Interface Science, 492, 51–60.
Funding
Open access funding provided by Università degli Studi di Pavia within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
We dedicate this review to Professor Angelo Albini, Emeritus Professor of Chemistry at the University of Pavia, with gratitude, admiration, and profound esteem.
The original online version of this article was revised: The funding note was missing
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pretali, L., Fasani, E. & Sturini, M. Current advances on the photocatalytic degradation of fluoroquinolones: photoreaction mechanism and environmental application. Photochem Photobiol Sci 21, 899–912 (2022). https://doi.org/10.1007/s43630-022-00217-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43630-022-00217-z