Abstract
Sacred sites like monasteries harbour a higher diversity of flora and fauna than nearby unprotected areas and sometimes even higher than protected areas. However, anthropogenic disturbance puts woodlands in these monasteries and churches under significant threat. To study the floristic composition and forest degradation of Waldiba monastery woodland in Tigrai, Ethiopia, 30 plots were established along transect lines. We recorded 39 native woody species (33 trees, 5 shrubs and 1 liana) belonging to 19 families. The woodland had a Shannon diversity index of 3.10, Simpson similarity index of 0.94, evenness of 0.58 and dominance of 0.06. The density of woody species was 922 stems ha−1 with a total basal area of 49.27 m2 ha−1. The tree species with higher importance value index (IVI) value were Dichrostachys cinerea (86.04%), Combretum fragrans (71.87%), Combretum hartmannianum (60.30%) and Combretum sp. (50.15%). The population structure of the woody species exhibited various patterns; bell shape, irregular shape, interrupted J shape, and J shape. Plant regeneration was inadequate for most of the tree species. Vachellia venosa is listed as endangered while C. hartmannianum as vulnerable in the IUCN red list species category. Waldiba dry woodland has underwent significant cover changes. Waldiba has lost much (65%) of its dense forest and open forest (44%) mainly due to anthropogenic disturbance. The woodland with its biodiversity is under threat. In effect, the species with low IVI, species listed in the IUCN red list and those species with none to poor regeneration status should be prioritized for in situ and ex situ conservation intervention to avert the threat. Research on reproductive ecology of the threatened indigenous species is imperative, as the woodland should be managed for ecosystem services and biodiversity conservation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Tropical dry forests are forests in dryland areas that are distinguished by their marked dry season and low water availability. They have a ratio of potential evapotranspiration to precipitation between one and four and typically experience three to eight months of drought [1]. Dry tropical forests cover more than 40% of the global tropical forest area [2,3,4]. The largest proportion of dry forest is found in Africa [1, 3], which has an area of 17.3 million km2 [5]. Dry forests and woodlands are the largest remaining forest resources and cover 55 million ha in Ethiopia [4, 6, 7].
Dry tropical forests contain a wealth of unique biodiversity of immense local, regional and international significance [2, 8]; are home to half a billion people [2, 4]; directly support the livelihood of one billion people worldwide [1] and approximately 52 million rural households in the Horn of Africa [9]. For instance, tapping and grading of frankincense and natural gums in the drylands create seasonal employment opportunities for 20,000 to 30,000 people in Ethiopia [10]. The unique feature of dry forests and woodlands of Ethiopia is their richness with major gum and resin bearing species of Acacia, Boswellia and Commiphora [2, 4] that have high cultural, religious, economic and ecological significance [2, 7]. Despite this fact, dry forests remain among the world’s most threatened and least protected forests [4, 7, 8]. Until recently, very little attention has been given to developing, protecting and sustainably managing these valuable resources. However, Ethiopian dry forests are caught in a spiral of deforestation due to competing land uses [2]. For example, in Tigrai, one of the largest frankincense-producing areas in Ethiopia, about 170,000 ha of dry forest has been deforested and converted to croplands since the 1970s [3, 4].
In Tigrai, forest disturbance has a 3000-year history [11, 12]. As a result, large forests have been converted into arable land that the last remaining refugia for native woody plant species are found around churches and monasteries [13,14,15]. As such, church/monastery forests and woodlands are considered biodiversity hotspots, the last natural seed banks for native tree species, reference areas for local endemism and buffers against depletion and last corner stones for species distribution [13].
Waldiba monastery woodland is one of the natural vegetation types found in Tigrai. Founded around 490 AD, the Waldiba monastery is one of the oldest monasteries found in Africa. The monastery is situated in a remote area surrounded by mountains, rivers, deep gorges, and valleys. As a sacred place of worship, its resources are, traditionally, considered to be under God’s protection and thus off limits for resource extraction [38]. Thus, it has conserved patches of many indigenous trees and shrubs in situ [39]. However, like other tropical dry forests, land degradation is rife due to overgrazing, illegal logging and overexploitation of the woodlands, farming of its fragile lands, gold mining, settlement, burning (wildfire) and all of which resulted in clearing of Waldiba monastery woodland [16]. Problems associated with woodland degradation in Tigrai have been reported in many studies [3, 17,18,19,20,21]. However, most of these studies emphasis were on the population structure and focused on only single woody species, such as Boswellia papyrifera [3, 18, 21, 22], Adansonia digitata [17] and Oxytenanthera abyssinica [19]. To the best of our knowledge, studies on the floristic composition and forest degradation of the Waldiba Monastery's dry woodland using the Analytical Hierarchy Process (AHP) are scanty. AHP is a method of measurement through pairwise comparisons [23]. It is one of the most widely used multiple criteria decision-making tools in the areas of conservation evaluation and environmental management [18, 24,25,26] to address land-use problems [27]. It assists decision makers to construct their preferences via criteria weighting and scoring [28].
Understanding floristic composition and forest health is important for biodiversity conservation and management. However, majority of the floristic composition studies [29,30,31,32,33,34] in Ethiopia were done in the mountain chain highlands. Only a few studies [18, 35,36,37] have been conducted in the lowland dry woodland areas. Therefore, this study was conducted to (1) assess the diversity of woody species in the woodland (2) determine the population structure and woody species regeneration and (3) identify the drivers of deforestation and forest degradation using a multi-criteria decision model—the AHP in the sacred Waldiba monastery dry woodland.
2 Materials and methods
2.1 Study area
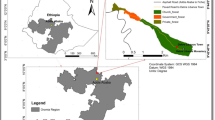
The sacred Waldiba Monastery woodlandFootnote 1 is situated within the Tselemti district in the north-western zone of Tigrai, Ethiopia (Fig. 1). Geographically, it lies between 13° 30′ to 14° 00′ North and 37° 45′ to 38°15′East in the western lowland (elevation below 1250 m) of Tigrai. It is about 450 km from Mekelle, the capital of Tigrai. It is bounded by three regional districts (Asgede Tsimbla to the North, Welqait to the West and Tsegede to the South) and by the National Regional State of Amhara to the South. Waldiba monastery woodland forest is the largest state forest with an area of almost 950 km2 next to Desa’a forest (1541 km2). The woodland is bordered by the Tekeze, Zarema, Tsotit and Insiya rivers. The monastery forest is surrounded by villages with 47,000 inhabitants. Waldiba receives more than 1000 mm of rainfall annually. Besides, the mean annual temperature is around 26 ºC and varies from 40.8 to 49.4 ºC for the maximum and from 12.3 to 21 ºC for the minimum temperature. The hottest months are March to June. The woodland is a kind of broad-leaved deciduous Combretum-Terminalia woodland in physiognomy with varying proportions of woody vegetation and perennial grasses [38].
2.2 Vegetation survey
2.2.1 Vegetation sampling
A systematic samplingFootnote 2 method was employed for the inventory of woody species [36]. In the woodland, we laid parallel line transects in the south-north direction. Along the line transects, sample plots measuring 20 m × 20 m were established at approximately 1 km intervals to measure the trees and shrubs’ attributes [39]. Overall, 30 representative sample plots were established in the woodland. Inside the plots, nested subplots of 3 m × 3 m were established to measure sapling/seedling attributes and herb cover.
2.2.2 Vegetation data collection
In each sample plot, vegetation related components such as species composition, density (seedling, sapling, shrub and tree), frequency, diameter at breast height (DBH) for trees, diameter at stump height (DSH) for shrubs and some—saplings and tree height were recorded. Furthermore, environmental and disturbance variables such as topography, illegal logging, livestock pressure and fire incidence were recorded for each sample plot. Diameter tape and Suunto hypsometer were used to measure DBH and height, respectively. All woody species were identified by their local and/or scientific names. Species identification was performed directly in the field using relevant reference materials [40, 41] as well as with the help of local people familiar with the flora [42, 43]. Furthermore, woody species that occurred outside the plots were also recorded to produce a more complete list of the woody plants in the study area. Finally, the woody species were classified into different growth forms based on their height and diameter: Height > 1.5 m and DBH ≥ 2.5 cm were considered trees, a height ≥ 0.50 m and < 1.5 m or DBH/DSH < 2.5 cm as saplings/shrubs and a height < 0.50 m was considered a seedling [44].
2.2.3 Vegetation data analysis
Diversity was analysed using the Shannon diversity index (H) and evenness or equitability index (E) [33, 45]. The Shannon diversity index takes into account species richness and evenness [46] and it usually lies between 1.5 and 3.5 and rarely exceeds 4.5 [47]. A score ≥ 3 is considered high, medium (2–3), low (1–2) and very low when it is ≤ 1.0 [29]. The community types were identified with the multivariate hierarchical clustering (dendrogram) method using Ward’s algorithm and based on the Bray–Curtis similarity index [48]. Diversity analysis and hierarchical clustering were performed with PAST software version 3.06 [49]. Likewise, vegetation structure was computed based on density, frequency, DBH and basal area and the importance value index (IVI) using Microsoft Excel [33, 39, 42, 43, 45, 46]. To describe population structure, individuals of the recorded tree species were grouped into 10 diameter classes. The resulting frequency histograms were subsequently interpreted as proxies for regeneration status via frequency histograms. Only tree species with high IVI and one economical flagship tree were considered for analyses of population structure. The IVI and basal area of each tree/shrub species were calculated using the following equations:
where RD is the relative density of a species and Ni is the number of individuals of the ith species,
where RF is relative frequency of a species and Fi is the number of occurrences of the ith species;
where RDO is relative dominance of a species and Bi is the total basal area of the ith species
where, BA is basal area, d is the diameter of the tree or shrub
2.3 Focus group discussion and key informant interviews
To substantiate the analysis of floristic composition and to identify the major driving forces of deforestation and forest degradation and other relevant nonvisual information, focus group discussion (FGD) and key informant interviews (KIIs) were undertaken.
The KII participant were selected using the snow-ballFootnote 3 method, while the individuals for FGD were selected with the help of a facilitator. A total of 12 individuals representing various stakeholders (NGOs, forest experts, forest guards, monks, herders and gold miners) were selected for the FGD. Then, they were informed about the purpose of the study and were invited to a two-day workshop in Maitsebri town, Tselemti district. In the workshop, the FGD group members were encouraged to talk freely to identify the drivers of deforestation and select the criteria and alternatives for the AHP. To do so, an unstructured questionnaire (a check list) was developed for both the KI interview and the FGD.
The data obtained from FGDs were used to weight and rank the major drivers of cover change using AHP technique as per the procedures outlined in Gidey et al. [25], Gidey et al. [18], and Marques [50]. AHP is one of the best known and most widely used multi-criteria decision making (MCDM) approaches [18, 23, 25, 50]. It allows users to assess the relative weight of multiple criteria or multiple options against given criteria in an intuitive manner. In case quantitative ratings are not available, nature conservationists can still recognize whether one criterion is more important than another [23].
The overall objective of AHP was to identify and select the driving force for forest degradation using the criteria proximate and underlying causes and their respective alternatives. In light of this, we used the proximate and underlying causes of forest degradation as criteria. The alternative proximate causes included: Overgrazing, cultivation (irrigation development, dam construction and settlement), mining, illegal logging and over exploitation (fuelwood, charcoal making, and house construction, poaching, over tapping,Footnote 4 wildfire), biotic factors (insect attack) and abiotic factors (drought-induced tree dieback). The alternative underlying causes included population pressure, poverty, policy (institutional) issues and lack of awareness.
In addition, the floristic composition and AHP results of the woodland were further substantiated by the analysis of land use and land cover (LULC) change for the years 1972, 1986 and 2018.
3 Results
3.1 Vegetation survey
3.1.1 Floristic composition
In our study, we recorded a total of 39 (33 trees, 5 shrubs and 1 liana) indigenous woody species (Table 1) belonging to 19 families. The Combretaceae and the Fabaceae were the dominant families, contributing seven species and six species to the total woody species composition, respectively. The remaining, 17 families, were represented by either two or a single species. This finding does not include the gigantic tree Adansonia digitata (Bombacaceae) that was present in the woodland but was not recorded in the sample plots. In the IUCN red list, C. hartmannianum is considered as vulnerable while V. venosa is considered endangered (https://www.iucnredlist.org/).
3.1.2 Plant community types
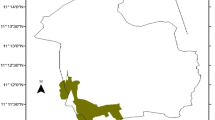
Three plant community types were identified (Fig. 2); Combretum–Terminalia, Boswellia– Ziziphus and Vachellia–Ximenia. The Combretum–Terminalia community occurred at flat plains to hilly slopes between 958 and 1201 m above sea level. The main species in this community were C. fragrans, C. hartmannianum, Combretum sp., C. molle, Terminalia sp. T. brownie, G. ferruginea and G. flavescens. The Boswellia–Ziziphus community type occurred at altitude of 835–1201 m in hilly slopes with shallow soils. The dominant species include B. papyrifera, Z. abyssinica, Z. spina—christi, Pterocarpus lucens, Stereospermum kunthianum, Otostegia fruticosa and Lannea fruticosa. The Vachellia–Ximenia community type occurred at the altitudinal range of 999–1174 at gentle slopes and in the flat plain with deep soil and also around riverbanks. The main species of this community type include V. polyacantha, V. sieberiana, V. etbaica, Ximenia americana, Tamarindus indica, Sterculata setegra, Dovyalis abyssinica and Diospyros mespiliformis.
3.1.3 Woody species diversity
In our study, we found Shannon diversity index, Simpson similarity index, evenness and dominance values of 3.10, 0.94, 0.58 and 0.06, respectively. This indicates that the woodland is relatively high in species diversity, with almost even representation of each species and with low dominance of species.
3.1.4 Frequency, density and dominance
Dichrostachys cinerea was the most frequent species, occurring in 70% of the sampled plots, followed by C. fragrans (56.67%), C. hartmannianum (40%), Combretum sp. (36.67%), and Z. abyssinica (30%) (Table 1). This indicates that the woodland is being dominated by thorny species that are less palatable to cattle (Table 1 and Fig. 3). This variation in frequency of species is attributed to their habitat preferences, regeneration ability and disturbance degree.
Population structure of selected dominant woody species recorded at Waldiba monastery woodland. Stem diameter classes are (in cm): (1) DBH < 2, (2) ≥ 2 DBH < 4, (3) ≥ 4 DBH < 6, (4) ≥ 6 DBH < 8, (5) ≥ 8 DBH < 10, (6) ≥ 10 DBH < 20, (7) ≥ 20 DBH < 30, (8) ≥ 30 DBH < 40, (9) ≥ 40 DBH < 50, and (10) DBH > 50
The five most important woody species in terms of their density were C. hartmannianum, Dichrostachys cinerea, C. fragrans, V. polyacantha and Terminalia sp. (Table 1), with densities ranging from 47 to 107 stems ha−1. The relative density (RD), which is similar to the density (stems ha−1), also followed the same trend as of the density (Table 1). In terms of dominance (Table 1), the six most dominant tree species contributing to more than 50% of the basal cover were B. papyrifera, Combretum sp., C. fragrans, C. hartmannianum, Terminalia brownii and V. polyacantha. Mostly of these species were found in mature stands with large diameters (up to 50 cm).
3.1.5 Basal area
The total basal area of all the woody species was 49.27 m2 ha−1. The woody species with the largest contribution to the basal area can be considered the most important [45, 51]. The species with the largest basal area was B. papyrifera [52]. The other five species with the largest basal area contribution were C. fragrans, Combretum sp., C. hartmannianum, Terminalia brownie and V. polyacantha. Thus, these are considered the most ecologically important species and the difference in basal area could be due to the presence of old growth and large diameter trees.
3.1.6 Importance value index (IVI)
The most ecologically important species in the woodland (Table 1) with IVI value of more than 50 were D. cinerea, C. fragrans, Combretum sp., and C. hartmannianum. The three economically important tree species in the woodland, B. papyrifera, V. polyacantha and Sterculia africana, had IVI values > 10.77. Around 80% of the species in the forest have IVI of more than 5, indicating that the woodland is composed of ecologically important woody species.
Tamarindus indica, Cissus petiolata, Commiphora africana, Diospyros mesphiliformis, G. flavescens, Z. spina‑christ and Otostegia fruticosa are species with IVI value of < 5%, and therefore need immediate conservation intervention. Moreover, there are indigenous plants that are listed in the IUCN red list that need special attention and conservation action.
3.1.7 Population structure
The selected woody species demonstrated various patterns of population structure (Fig. 3), implying different population dynamics of each species. Only D. cinerea demonstrated a normal (inverted J shape) population growth pattern with good reproduction and recruitment capacity. The others showed either bell-shaped, J-shaped or irregular growth patterns with missing diameter classes in either the lower, upper or middle diameters. For instance, B. papyrifera showed a hampered seedling, sapling and shrub recruitment and a very low stocking in the upper diameter class (2 stems/ha), implying the high level of a threat to the species due to its limited recruitment capacity.
3.1.8 Regeneration status
The distribution of seedlings, saplings and mature trees/shrubs in Waldiba dry woodland showed three distinct patterns. The first group (of good regeneration) included species represented by all three stages and included V. polyacantha, C. hartmannianum and D. cinerea. The second group (poor regeneration) is represented by seedling and mature trees/shrubs but not by saplings and includes D. melanoxylon, D. abyssinica, T. brownii and X. americana. The third group (of fair regeneration) was represented by sapling and mature trees/shrub but not seedlings, and comprised G. ferruginea and J. floribundum.
3.2 Drivers of deforestation and forest degradation in Waldiba dry woodland
The informants noticed that the forest cover dwindled and declared that both the proximate (87.50%) and underlying (12.5%) causes were the main reasons for the forest cover change (Figs. 4a and 5). Uncontrolled grazing, illegal logging, cropland expansion, mining, biotic and abiotic factors were the main proximate drivers of forest cover change (Figs. 4b and 5). Whereas, population increase, poverty, lack of awareness and institutional (policy) issues with their respective weighted value of 7.43%, 2.57%, 1.46% and 1.04%, respectively, were the main underlying causes for cover change.
Drivers of forest cover change in Waldiba a uncontrolled grazing, b a tree cut for goat browse, c tree dieback, d a dead B. papyrifera tree because of attack by the long horn beetle, e newly cut logs of Combretum sp. f wood cut for fuelwood and construction poles, and g grassland burnt for expansion of land for cultivation
Uncontrolled/free livestock grazing is a proximate driver that needs much attention, as it could also be the underlying cause of other forms of degradation. Cattle from nearby villages in Asgede Tsimbla, Tselemti, Sheraro and Tsegede districts of the woodland and distant districts, such as Inticho (~ 235 km away) and previously Mendefera in Eritrea, are brought to Waldiba for grazing. During the rainy season, the livestock browse in the woodland. However, during the dry season the herders cut tree branches to feed their livestock. In most cases, they do not do proper lopping, but rather they cut the trees at some point above the ground that might be difficult for the tree to rejuvenate or regrow. The mass movement of livestock in certain locations has also impacted the regeneration of the flagship tree species—B. papyrifera and A. digitata. Furthermore, herders cut for firewood trees that have good calorific value and less smoke. There is less tendency of using dead trees that are present in the woodland. When the herders fall short of ranching areas, they also burn the woodland to obtain space for their animals and to construct dwellings for themselves.
The other major stakeholders who are drivers of forest degradation (land use change) are gold miners, monks of the monastery, tappers and poachers. For traditional gold mining, vast area of the woodland has been destroyed. Moreover, the gold miners and the tappers use wood and wood products for making utensils, digging and tapping material, shelter construction, cooking and heating. Likewise, due to lack of awareness, monks are also involved in logging of trees for fuelwood and construction poles. Worse still, they increasingly cut quality stands of rare and valuable trees like Combretum sp. to construct shelter. Wood of the species is hardy in nature and resistant to termite attack. The poachers are involved in biodiversity loss through the hunting of wildlife apart from the use of fuel wood and poles, all of which was further affirmed by the KII. As a result of poaching for bush meat, many wildlife such as Minilik bushbuck and other antelope that used to occur in the woodland have been lost or are greatly reduced in numbers.
4 Discussion
4.1 Floristic composition, diversity and community types
Floristic composition of a vegetation can be described in terms of species richness, abundance, dominance, and frequency [48, 53]. In this study, a total of 39 indigenous woods species including trees, shrubs and lianas were recorded. The Combretaceae and the Fabaceae were the dominant families. The dominance of the Combretaceae and the Fabaceae in the present study is in line with what has been reported for Dambos ecosystems in northern Mozambique [54] and Eastern Ghats of Andhra Pradesh [55]. Similarly, the Combretaceae and the Fabaceae were reported to be the most species rich families in the woodlands of Hirmi [37], Kafta-Sheraro National Park [35], Metema [48], Xobe, Island Safari and Shorobe [43], Xobe and Shorobe [42] and Mopane [46].
In Ethiopia, the family Fabaceae is the most species rich family with 678 species [35, 56] while Combretaceae is common woodland species [35, 37, 42, 43, 46, 48, 57]. The dominance of these two families could be attributed to their efficient pollination and successful seed dispersal mechanisms [35]. Furthermore, the leguminous species contains drought tolerant, deciduous, nitrogen fixing and spiny species that are well adapted to the prevailing drought conditions [29, 35] of dry woodlands.
Patterns of species community are associated with environmental factors like climate, topography (slope, aspect, altitude), soil as well as anthropogenic and livestock disturbances [37]. In the present study, three plant community types were identified from the agglomerative hierarchical cluster analysis. This is far lower than those reported for Hirmi [37] due to the low altitudinal variation in the present study.
Factors like anthropogenic and environmental influences have a strong impact on species richness, forest structure and composition [53]. The species richness of the woodland is comparable to that reported for Metema [48], Xobe and Shorobe [42, 43], and Mopane [46] but slightly lower than that reported for Kafta-Sheraro National Park [35] and Hirmi woodland [37], which have the same ecological setting.
Species diversity is the most often used depiction of ecological diversity [58]. The relatively high diversity and evenness indices indicate the need to conserve the floristic diversity of the study site and protect the woody vegetation from human disturbances [52]. The Shannon diversity values recorded in the present study were higher than those reported for Gera woodland in Abergele [18], South Nandi forest in Kenya [32], Kafta Sheraro National Park [35], Mopane woodlands [46], islands of Lake Ziway [36], Tara Gedam and Abebaye forests [52], Xobe and Shorobe [42], Island Safari, Shorobe and Xobe [43], Kumorakata Reserve Forest and the Hojai Reserve Forest [59] and lower than those reported for Hirmi [37], Metema [48], Ryukyu Archipelago [60] and Eastern Ghats [55]. Likewise, the evenness is comparable with that reported in other studies conducted on other dry woodlands [42, 43, 46].
The most frequent species were D. cinerea, followed by C. fragrans, then C. hartmannianum, Combretum sp., and Z. abyssinica. The relative frequency of a species reflects the pattern of distribution and provides an approximate indication of the heterogeneity of a stand [36, 48, 52]. Thus, the high frequency of the species could be the result of a wide adaptability range and dispersal mechanisms [51].
Basal area is used to explain the crowdedness of a stand of natural vegetation (Table 1). A stand of large trees is more crowded than one with the same number of trees of smaller diameter [29]. The total basal area of the forest in the present study was 49.27 m2 ha−1 which is greater than the expected range of basal area of 23–37 m2 ha−1 for virgin tropical forests. The species with the largest basal area was B. papyrifera, possibly due to its big size [52], followed by C. fragrans, Combretum sp., C. hartmannianum, Terminalia brownie and V. polyacantha. Thus, these species are considered the most ecologically important species and the difference in basal area could be due to the presence of old growth and big diameter trees. The basal area of the woodland was greater than that of Hirmi [37], Metema [48], South Nandi forest [32], Zengena Forest [61] and Eastern Ghats of Andhra Pradesh [55], comparable with that of Ishigaki forest [60], Abebaye forest [52], and less than that of Okinawa forest [60], Tara Gedam [52], Kumorakata Reserve Forest [59] and islands of Lake Ziway [36].
The total density of mature woody plants in the present study was 922 stems ha−1. This value is higher than those reported for other similar woodlands like Hirmi [37], Galilla, Debre Sina, Tullu Guddo, Funduro and Tsedacha [36] Eastern Ghats of Andhra Pradesh [55] and Metema [48] and lower than those reported for Tara Gedam and Abebaye [52]. These differences in density could be attributed to the variation in natural regeneration and dispersal patterns of the species, the inability/ability of individuals to withstand the harsh environmental conditions (low rainfall regime, high temperature), disturbance degree, livestock trampling and grazing, other biotic and abiotic factors [48].
Species with the highest dominance indices might be considered the most important species in the studied woodland [48]. The IVI is an important parameter that expresses the structural importance or ecological significance of species in a given ecosystem [32, 45, 48, 52, 62]. Species with high IVI values are considered more important than those with low IVI values. Likewise, IVI can be used to prioritize species for conservation. Species with lower IVI values need high conservation efforts while those with higher IVI values need monitoring [45] and less conservation effort [52]. The IVI value of the present study is higher than those of other similar woodlands like Hirmi [37], Metema [48], islands of Lake Ziway [36], Tara Gedam [52], Komto forest [45], Island Safari, Shorobe and Xobe [43] and Shorobe [42]; comparable with those of Xobe [42] and Mopane woodland [46]; and lower than those of Abebaye forest [52].
In the absence of long-term demographic data of the species populations, the use of diameter class distributions of woody species from a single survey has been shown to be a potential and reliable tool for revealing population dynamics and recruitment of woody species [42, 45]. It is also used to predict responses of the a species to disturbance, to its environment and to the resultant changes in population structure [42, 45, 48]. In the present study, the population structure exhibited various patterns. To this end, several researchers have argued that species like B. papyrifera are facing extinction [63, 64], and have proposed that they should be included in the “vulnerable” threat category of the IUCN red list [65]. Thus, the species that show irregular population growth patterns should be considered for conservation intervention [51]. Similar findings were reported for other woodlands [36, 37, 42, 43, 48, 51, 52].
Regeneration is a central component of tropical forest ecosystem dynamics and management, conservation and restoration of degraded woodlands. Sustainable forest utilization is possible only if adequate information on regeneration dynamics and the factors influencing important canopy tree species is available [66]. The composition, distribution and density of seedlings and saplings are indicators of the future regeneration status of any woodland [45]. Such deforestation and forest degradation in church forests and woodlands were reported elsewhere in Ethiopia [13, 14, 67]. Consistent with the findings of present study, the impact of transhumance on vegetation has been acknowledged in Ethiopia [68, 69], and other parts of the world [70, 71].
5 Conclusion
Floristic composition results revealed that the study site harboured 39 woody species with high levels of nativity, species richness and diversity with more or less even distribution and less dominance. The regeneration status was not promising for the vast majority of the species. Species with low IVI values, hampered recruitment, vulnerable species, flagship species, rare, threatened and endangered species should be prioritized for in situ and ex situ conservation intervention. Whereas in the study area, both proximate (87.50%) and underlying (12.50%) causes played a pivotal role in driving the forest cover dynamics with more than 76% of the cover change occurring due to overgrazing-induced illegal logging and farmland expansion. As such, introducing participatory forest management is crucial for reducing anthropogenic pressure and ensuring the woodland’s conservation, management, and sustainable use. Furthermore, as the woodland should be managed for ecosystem service and biodiversity conservation, research on the soil seed bank, regeneration, population dynamics, biology and reproductive ecology (pollination, seed production, dispersal and germination) of native and endemic species is imperative.
Data availability
The data used to support the findings of this study are included in the manuscript.
Notes
Woodlands are open stands of trees of at least 8 m tall with a canopy cover of 40 percent or more, but never with interlocking crowns and usually with a field layer of heliophilous (‘sun-loving’) grasses. Woodlands have similar height as forests, but woodlands never have densely interlocking crowns (although the crowns can be in contact) [38]
Systematic sampling is a type of probability sampling method in which sample members from a larger population are selected according to a random starting point but with a fixed, periodic interval [72].
Snowball is a method of selecting key informants based on individuals (happen to meet by chance) suggestions.
Over tapping refers to frankincense production without resting time [18]
References
Abiyu A, Bongers F, Eshete A, Gebrehiwot K, Kindu M, Lemenih M, Moges Y, Ogbazghi W, Sterck FJ. Incense woodlands in Ethiopia and Eritrea: Regeneration problems and restoration possibilities. In: Bongers F, Tennigkeit T, editors. Degraded forests in eastern Africa. Milton Park: Routledge; 2010. p. 133–52.
Lemenih M, Arts B, Wiersum KF, Bongers F. Modelling the future of Boswellia papyrifera population and its frankincense production. J Arid Environ. 2014;105:33–40. https://doi.org/10.1016/j.jaridenv.2014.02.006.
Gebrehiwot K, Muys B, Haile M, Mitloehner R. Introducing Boswellia papyrifera (Del.) Hochst and its non-timber forest product, Frankincense. Int For Rev. 2003;5:348–53. https://doi.org/10.1505/IFOR.5.4.348.22661.
Dejene T, Lemenih M, Bongers F. Manage or convert Boswellia woodlands? Can frankincense production payoff? J Arid Environ. 2013;89:77–83. https://doi.org/10.1016/j.jaridenv.2012.09.010.
Chidumayo EN, Gumbo DJ. the dry forests and woodlands of Africa: managing for products and services. Milton Park: Routledge; 2010.
Haile G, Gebrehiwot K, Lemenih M, Bongers F. Time of collection and cutting sizes affect vegetative propagation of Boswellia papyrifera (Del.) Hochst through leafless branch cuttings. J Arid Environ. 2011;75:873–7. https://doi.org/10.1016/j.jaridenv.2011.04.007.
Mokria M, Tolera M, Sterck FJ, Gebrekirstos A, Bongers F, Decuyper M, Sass-klaassen U. The frankincense tree Boswellia neglecta reveals high potential for restoration of woodlands in the horn of Africa. For Ecol Manage. 2017;385:16–24. https://doi.org/10.1016/j.foreco.2016.11.020.
Teshome M, Eshete A, Bongers F. Uniquely regenerating frankincense tree populations in western Ethiopia. For Ecol Manage. 2017;389:127–35. https://doi.org/10.1016/j.foreco.2016.12.033.
Hassan BA, Glover EK, Luukkanen O, Kanninen M, Jamnadass R. Boswellia and Commiphora species as a resource base for rural livelihood security in the horn of Africa: a systematic review. Forests. 2019;10:1–15. https://doi.org/10.3390/f10070551.
Tilahun M, Maertens M, Deckers J, Muys B, Mathijs E. Impact of membership in frankincense cooperative firms on rural income and poverty in Tigray, Northern Ethiopia. For Policy Econ. 2015;62:95–108. https://doi.org/10.1016/j.forpol.2015.08.009.
Bard KA, Coltorti M, Diblasi MC, Dramis F, Fattovich R. The environmental history of Tigray (Northern Ethiopia) in the middle and Late Holocene: a preliminary outline. Afr Archaeol Rev. 2000;17:65–86.
Darbyshire I, Lamb H, Umer M. Forest clearance and regrowth in Northern Ethiopia during the last 3000 years. Holocene. 2003;13(4):537–46.
Kassahun T, Bender S. Saving the last endemic-church forests in Ethiopia : the case of Lake Tana Biosphere Reserve. In: Filho WL, editor. Handbook of climate change and biodiversity, climate change management. Berlin: Springer International Publishing; 2019. p. 195–210.
Aerts R, Van Overtveld K, November E, Wassie A, Abiyu A, Demissew S, Daye DD, Giday K, Haile M, Tewoldeberhan S, et al. Conservation of the Ethiopian church forests: threats, opportunities and implications for their management. Sci Total Environ. 2016;551–552:404–14. https://doi.org/10.1016/j.scitotenv.2016.02.034.
Abiyu A, Teketay D, Glatzel G, Gratzer G. Seed production, seed dispersal and seedling establishment of two Afromontane tree species in and around a church forest: implications for forest restoration. For Ecosyst. 2016;3:1–10. https://doi.org/10.1186/s40663-016-0076-5.
WeForest and GIZ Exploratory Forest Resources Survey in Tigray; Mekelle, 2019.
Birhane E, Tadesse K, Tadesse T, Asgedom KT, Tadesse T, Hishe H, Abrha H, Noulèkoun F. Vulnerability of baobab (Adansonia digitata L.) to human disturbances and climate change in western Tigray, Ethiopia: conservation concerns and priorities. GlobEcol Conserv. 2020;22:1–14. https://doi.org/10.1016/j.gecco.2020.e00943.
Gidey T, Hagos D, Mohammedseid H, Solomon N, Juhar HM, Solomon N, Negussie A, Crous-Duran J, Oliveira TS, Abiyu A, et al. Population status of Boswellia papyrifera woodland and prioritizing its conservation interventions using multi-criteria decision model in northern Ethiopia. Heliyon. 2020;6:1–8. https://doi.org/10.1016/j.heliyon.2020.e05139.
Gebrewahid Y, Abrehe S, Meresa E, Eyasu G, Abay K, Gebreab G, Kidanemariam K, Adissu G, Abreha G, Darcha G. Current and future predicting potential areas of Oxytenanthera Abyssinica (A. Richard) using MaxEnt model under climate change in Northern Ethiopia. Ecol Process. 2020. https://doi.org/10.1186/s13717-019-0210-8.
Negussie A, Gebrehiwot K, Yohannes M, Aynekulu E, Manjur B, Norgrove L. An exploratory survey of long horn beetle damage on the dryland flagship tree species Boswellia papyrifera (Del.) Hochst. J Arid Environ. 2018;152:6–11. https://doi.org/10.1016/j.jaridenv.2018.01.011.
Eshete A, Sterck F, Bongers F. Diversity and production of Ethiopian dry woodlands explained by climate- and soil-stress gradients. For Ecol Manage. 2011;261:1499–509. https://doi.org/10.1016/j.foreco.2011.01.021.
Tilahun M, Muys B, Mathijs E, Kleinn C, Olschewski R, Gebrehiwot K. Frankincense yield assessment and modeling in closed and grazed Boswellia papyrifera woodlands of Tigray, Northern Ethiopia. J Arid Environ. 2011;75:695–702. https://doi.org/10.1016/j.jaridenv.2011.03.005.
Saaty TL. Decision making with the analytic hierarchy process. Sci Iran. 2002;9:215–29. https://doi.org/10.1504/ijssci.2008.017590.
Ananda J, Herath G. A critical review of multi-criteria decision making methods with special reference to forest management and planning. Ecol Econ. 2009;68:2535–48. https://doi.org/10.1016/j.ecolecon.2009.05.010.
Gidey T, Birhane E, Manaye A, Kassa H, Atsbha T, Solomon N, Hishe H, Negussie A, Madera P, Borges JG. Prioritizing forest conservation strategies using a multi-attribute decision model to address concerns with the survival of the endangered dragon tree (Dracaena Ombet Kotschy and Peyr.). J Nat Conserv. 2023;73:126404. https://doi.org/10.1016/j.jnc.2023.126404.
Russo RDFSM, Camanho R. Criteria in AHP: a systematic review of literature. Proc Computer Sci. 2015;55:1123–32. https://doi.org/10.1016/j.procs.2015.07.081.
Gebre SL, Cattrysse D, Alemayehu E, Van Orshoven J. Multi-criteria decision making methods to address rural land allocation problems: a systematic review. Int Soil Water Conserv Res. 2021;9:490–501. https://doi.org/10.1016/j.iswcr.2021.04.005.
Renwick A, Dynes R, Johnstone P, King W, Holt L, Penelope J. Challenges and opportunities for land use transformation: insights from the Central Plains Water Scheme in New Zealand. Sustainability. 2019. https://doi.org/10.3390/su11184912.
Atsbha T, Desta AB, Zewdu T. Woody species diversity, population structure, and regeneration status in the Grat-Kahsu natural, vegetation southern Tigray of Ethiopia. Heliyon. 2019;5: e01120. https://doi.org/10.1016/j.heliyon.2019.e01120.
Kidane L, Nemomissa S, Woldu Z. The effects of disturbance on the population structure and regeneration potential of five dominant woody species-in Hugumburda-Gratkhassu National Forest Priority Area, North-Eastern Ethiopia. Afr J Ecol. 2016. https://doi.org/10.1111/aje.12254.
Aynekulu E, Aerts R, Negussie A, Friis I, Demissew S, Juergen H. Plant diversity and regeneration in a disturbed isolated dry Afromontane forest in northern Ethiopia. Folia Geobot. 2016;51:115–27. https://doi.org/10.1007/s12224-016-9247-y.
Odhiambo J, Mugatsiatsingalia H, Cheboiwo J, Odee D. Population structure and regeneration status of woody species in a remnant tropical forest: a case study of south. Glob Ecol Conserv. 2020;21: e00820. https://doi.org/10.1016/j.gecco.2019.e00820.
Yineger H, Kelbessa E, Bekele T, Lulekal E. Floristic composition and structure of the dry Afromontane Forest at Bale Mountains National Park Ethiopia. SINET Ethiop J Sci. 2008;31:103–20.
Gebrehiwot K, Demissew S, Woldu Z, Fekadu M, Desalegn T, Teferi E. Elevational changes in vascular plants richness, diversity, and distribution pattern in Abune Yosef Mountain Range, Northern Ethiopia. Plant Divers. 2019;41:220–8. https://doi.org/10.1016/j.pld.2019.06.005.
Temesgen F, Warkineh B, Asefa A. Human disturbance, plant species composition, diversity and community types of Kafta-Sheraro National Park, Tigray Region, Ethiopia. 2020.
Zegeye H, Teketay D, Kelbessa E. Diversity, regeneration status and socio-economic importance of the vegetation in the islands of Lake Ziway, South-Central Ethiopia. Flora. 2006;201:483–98. https://doi.org/10.1016/j.flora.2005.10.006.
Girmay M, Bekele T, Demissew S, Lulekal E. Ecological and floristic study of Hirmi woodland vegetation in Tigray Region, North Ethiopia. Ecol Process. 2020;9:1–19.
van Breugel P, Kindt R, Lillesø J-PB, Kalema J, Mulumba J, Namaganda M, Malinga M, Esegu JFO, Jamnadass R, Graudal L. Potential Natural Vegetation of Eastern Africa. Atlas and Tree Species Composition for Uganda; Vol 11. 2014.
Kebede B, Soromessa T, Kelbessa E. Floristic composition and community types of Gedo dry evergreen montane forest, west Shewa, Ethiopia. Acta Ecol Sin. 2016;36:392–400. https://doi.org/10.1016/j.chnaes.2016.07.001.
Azene BT. Useful trees and shrubs of Ethiopia: identification, propagation and management for 17 Agroclimatic Zones. In: Tengnäs B, Kelbesa E, Demissew S, Maundu P, editors. RELMA in ICRAF Project: Nairobi, 2007.
Bein E, Habte B, Jaber A, Birnie A, Tengnas B. Useful Trees and Shrubs in Eritrea: Identification, Propagation and Management for Agricultual and Pastoral Communites; Regional Soil Conservation Unit: Nairobi, 1996.
Neelo J, Teketay D, Masamba W, Kashe K. Diversity, population structure and regeneration status of woody species in dry woodlands adjacent to Molapo farms in northern Botswana. Open J For. 2013;3:138–51.
Neelo J, Teketay D, Kashe K, Masamba W. Stand structure, diversity and regeneration status of woody species in open and exclosed dry woodland sites around Molapo farming areas of the Okavango Delta Northeastern Botswana. Open J For. 2015;5:313–28.
Brhan A, Emiru G, Girmay B, Kiros G, Hadgu M, Norgrove L. Woody species diversity and carbon stock under different land use types at Gergera watershed in Eastern Tigray, Ethiopia. Agrofor Syst. 2018. https://doi.org/10.1007/s10457-018-0226-6.
Gurmessa F, Soromessa T, Kelbessa E. Structure and regeneration status of komto Afromontane moist forest, East Wollega Zone, west Ethiopia. J For Res. 2012;23:205–16. https://doi.org/10.1007/s11676-012-0242-8.
Teketay D, Kashe K, Madome J, Kabelo M, Neelo J, Mmusi M. Enhancement of diversity, stand structure and regeneration of woody species through area exclosure: the case of a mopane woodland in northern Botswana. Ecol Process. 2018;7:1–15. https://doi.org/10.1186/s13717-018-0116-x.
Gebrehiwot K, Woldu Z, Fekadu M, Teferi E, Desalegn T, Demissew S. Classification and ordination of plant communities in Abune Yosef mountain range, Ethiopia. Acta Ecol Sin. 2019. https://doi.org/10.1016/j.chnaes.2019.12.001.
Wale HA, Tamrat W, Gemedo B, Bekele T, Dalle G. Floristic diversity, regeneration status, and vegetation structure of woodlands in Metema Area, Amhara National Regional State, North-Western Ethiopia. J For Res. 2012;23:391–8. https://doi.org/10.1007/s11676-012-0275-z.
Hammer Ø, Harper DAT, Ryan PD. PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron. 2001;41:9.
Marques MMG. An expert model approach to assess the potential of non-wood forest products for forest owners. Universidade de Lisboa. 2016.
Awoke H, Mewded B. Changes in woody species composition and structure of Denkoro dry evergreen Afromontane forest over 16 years (2001–2017), South Wollo, Ethiopia. For Ecol Manag. 2019;441:71–9. https://doi.org/10.1016/j.foreco.2019.03.039.
Zegeye H, Teketay D, Kelbessa E. Diversity and regeneration status of woody species in Tara Gedam and Abebaye forests, northwestern Ethiopia. J For Res. 2011;22:315–28. https://doi.org/10.1007/s11676-011-0176-6.
Melese SM, Ayele B. Woody plant diversity, structure and regeneration in the ambo state forest, south Gondar zone, northwest Ethiopia. J For Res. 2017;28:133–44. https://doi.org/10.1007/s11676-016-0280-8.
Rivaes R, Ribeiro-barros AI, So N. Vegetation structure and effects of human use of the dambos ecosystem in northern Mozambique. Glob Ecol Conserv. 2019;20:1–24. https://doi.org/10.1016/j.gecco.2019.e00704.
Naidu MT, Kumar OA. Tree diversity, stand structure, and community composition of tropical forests in Eastern Ghats of Andhra Pradesh, India. J Asia-Pac Biodivers. 2016;9:328–34. https://doi.org/10.1016/j.japb.2016.03.019.
Kelbessa E, Demissew S. Diversity of vascular plant taxa of the flora of Ethiopia and Eritrea. Ethiop J Biol Sci. 2014;13:37–45.
Sêssi E, Assédé P, Fortuné A, Johannes C, Wanangwa P, Sorotori S, Biaou H. Sudanian versus Zambezian woodlands of Africa: composition, ecology biogeography and use. Acta Oecol. 2020;107:103599. https://doi.org/10.1016/j.actao.2020.103599.
Ameja LG, Ribeiro N, Sitoe AA, Guillot B. Regeneration and restoration status of Miombo Woodland following land use land cover changes at the buffer zone of Gile National Park, Central Mozambique. Trees For People. 2022;9:100290. https://doi.org/10.1016/j.tfp.2022.100290.
Dutta G, Devi A. Plant diversity, population structure, and regeneration status in disturbed tropical forests in Assam, northeast India. J For Res. 2013;24:715–20. https://doi.org/10.1007/s11676-013-0409-y.
Feroz SM, Kabir E, Hagihara A. Species composition, diversity and stratification in subtropical evergreen broadleaf forests along a latitudinal thermal gradient in the Ryukyu Archipelago, Japan. Glob Ecol Conserv. 2015;4:63–72. https://doi.org/10.1016/j.gecco.2015.05.002.
Tadele D, Lulekal E, Damtie D, Assefa A. Floristic diversity and regeneration status of woody plants in Zengena Forest, a remnant montane forest patch in northwestern Ethiopia. J For Res. 2014;25:329–36. https://doi.org/10.1007/s11676-013-0420-3.
Abunie AA, Dalle G. Woody species diversity, structure, and regeneration status of Yemrehane Kirstos Church Forest of Lasta Woreda, North Wollo Zone, Amhara Region, Ethiopia. Int J For Res. 2018;2018:1–9.
Muys B. Frankincense facing extinction. Nat Sustain. 2019;2:665–6. https://doi.org/10.1038/s41893-019-0355-6.
Yaynemsa KG. Introduction. In: Plant biodiversity conservation in Ethiopia. Springer International Publishing AG. 2022; pp. 1–308.
Bongers F, Groenendijk P, Bekele T, Birhane E, Damtew A, Decuyper M, Eshete A, Gezahgne A, Girma A, Khamis MA, et al. Frankincense in peril. Nat Sustain. 2019;2:1–9. https://doi.org/10.1038/s41893-019-0322-2.
Tesfaye G, Teketay D, Fetene M, Beck E. Regeneration of seven indigenous tree species in a dry Afromontane forest, southern Ethiopia. Flora. 2009;205:135–43. https://doi.org/10.1016/j.flora.2008.12.006.
Cardelus CL, Woods CL, Mekonnen AB, Dexter S, Scull P, Tsegay BA. Human disturbance impacts the integrity of sacred church forests, Ethiopia. PLoS ONE. 2019;14:1–14.
Nyssen J, Descheemaeker K, Zenebe A, Poesen J, Deckers J, Haile M. Transhumance in the Tigray highlands (Ethiopia). Mt Res Dev. 2009;29:255–64. https://doi.org/10.1659/mrd.00033.
Tesfamariam Z, Nyssen J, Ghebreyohannes T, Tafere K, Zenebe A, Poesen J, Deckers S, Van Eetvelde V. Transhumance as a driving force of landscape change in the marginal grabens of northern Ethiopia. Singap J Trop Geogr. 2019;40:476–95.
Michler LM, Kaczensky P, Ploechl JF, Batsukh D, Baumgartner SA, Battogtokh B, Treydte AC. Moving toward the greener side: environmental aspects guiding pastoral mobility and impacting vegetation in the Dzungarian Gobi, Mongolia. Rangel Ecol Manag. 2022;83:149–60. https://doi.org/10.1016/j.rama.2022.03.006.
Thomson A. Transhumance and vegetation. In: Thompson DBA, Price MF, Galbraith CA, editors. Mountains of northern Europe: conservation, management, people and nature. Edinburgh: TSO Scotland; 2005. p. 179.
Köhl M, Magnussen S, Marchetti M. Sampling methods, remote sensing and GIS multiresource forest inventory. Berlin: Springer; 2006.
Acknowledgements
The authors are grateful to WeForest and GIZ-BFP for provision of data for the study and extend their appreciation to the local communities, forest guards, forest experts, data collectors, people who were involved in FGD and in KII and people in the security.
Author information
Authors and Affiliations
Contributions
DBH & AN: Conception and design of the research, data collection, data analysis and interpretation, drafting and revising the manuscript. EB, ANR, AST, TG, KMH & KG: data analysis and interpretation, drafting and revising the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Berhe, D.H., Retta, A.N., Tefera, A.S. et al. Ecological study and forest degradation of the Waldiba Monastery woodland in Tigrai, Ethiopia. Discov Sustain 5, 57 (2024). https://doi.org/10.1007/s43621-024-00223-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43621-024-00223-4