Abstract
According to the United Nations, Sustainable Development Goals (SDG) goal 6 and goal 14 seeks to ensure the sustainable management of water present over the earth for all. Urban cities saw a lot of expansion in terms of population and the number of industries established there. Water quality analysis becomes a huge requirement of today’s scenario due to the impurities present in water which harms the livelihood of society. Some of the hazardous impurities like heavy metals destruct the surroundings. In this study, the removal of heavy metals from wastewater with an efficient technique i.e. Bioremediation is represented with the analysis and evaluation of results recorded in the laboratory testing. Two samples were taken from two different sites which are being analyzed with the implementation methodology represented in the present article. The inclusion of the ANOVA model for the optimization of the outcome generated is evaluated and received the P-value and F-crit value. Two algae Chlorella Minutissima & Chlorella Singularis were evaluated based on their impurity removal efficiency as well an analysis of the biological treatment method over other chemical methods has been examined. The results were analyzed and represented in the form of a table as well and the variation in the value of WQP is shown in the form of graphs. The observation shows that the variation in WQP after the integration of algae lies under the permissible limit. Alkalinity is estimated in the range of 20–40 mg/l, Hardness lies in the range of 0–60mg/l, and pH comes approximately in a range of 6.5–8. The results of the ANOVA model is also depicted in graphical form highlighting the P-value and F-crit value of different result generated. Finally, the summary of the proposed work is illustrated with the challenges faced and future recommendations have been provided. Based on the evaluation, the framework is generated for the efficient technique used for heavy metal removal i.e. Bioremediation which provides a great advancement in the efficacy of removal of heavy metals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
United Nations stated the importance of the universal primary substance on the earth known as water. Due to its essential role in ensuring the survival of the numerous biota on the planet, water is considered the greatest gift of nature [1]. More recently, in September 2015, nearly all of the United Nations members vowed to work towards 17 Sustainable Development Goals (SDGs), which set clear objectives and deadlines for promoting inclusive well-being. United Nations 2030 agenda provides a framework of universal SDGs which targets the various challenges faced by the society [2]. SDG (Goal 6) especially addresses the water represents clean water and sanitation and is considered as the water goal & SDG (Goal 14) illustrates the life below the water. Before the establishment of SDGs one of the partnerships named Global Water Partnership (GWP) setbacks the water consideration globally. GWP emphasizes the need for synergies between long-term water resources management and the need for water and sanitation services [3].
The expanding issue of water shortage has a large and detrimental impact on global economic growth, human livelihoods, and environmental quality. Wastewater originates from various sources such as sewage, industries, agriculture & commercial waste which can be categorized by physical, chemical, and biological parameters [4]. The protection of water from contamination or developing a resource-efficient remediation method for its security has become a basic demand of the current situation. Over 1.1 billion people consume unsafe water worldwide on a global scale. Wastewater comprises 99.9% water and 0.1% solids as well as particulate matter in the sewage [5]. The technique for cleaning wastewater effluents coming out from industries as well as workplaces is typical and various existing methods are there for clearance of wastewater. The latest innovative green specialist procedure becoming more familiar with wastewater treatment [6]. The environmentally friendly method for wastewater treatment is considered the treatment of wastewater using microalgae which is utilized in the current investigation to expel the heavy metals from the wastewater [7]. Figure 1 illustrates various WQPs.
Some existing surveys and reviews related to the treatment of wastewater using microalgae have been studied and evaluated with the comparison. Reference [8] discussed the significance of microalgae in the wastewater treatment and removal of heavy metals from wastewater while exploring the advantages of this method where it concludes that the adoption of microalgae in the removal of contaminants from wastewater is considered as the environmental-friendly approach and helps in eliminating the nutrients and heavy metals biologically. Reference [9] has addressed the potential of microalgae for the treatment of wastewater as well as highlights the production of biofuel through microalgae and concluded that there are several advantages of using microalgae in wastewater treatment and after treatment remaining biomass can be utilized for creating biofuels. Reference [10] showed the inefficiency of conventional methods of wastewater treatment and considered microalgae to be used currently for bioremediation of wastewater and finally concluded the advantages of microalgae for the treatment of Pharmaceutical and Personal care products pollution from wastewater. Reference [11] evaluated that microalga is considered as a good source of oxygen in wastewater treatment explored the potential of microalgae for the removal of nitrogen and phosphorus from wastewater and reported the efficiency of oxygen generated for successful removal of ammonia from wastewater.
The survey papers previously stated have significantly advanced knowledge of the treatment of wastewater using microalgae for the successful removal of heavy metals. However, the literature on the removal of heavy metals from wastewater using microalgae is limited. There are very few articles that consider the potential of microalgae in the successful removal of heavy metals as well as an environmental-friendly approach towards sustainability. Based on the extensive evaluation, this study aims to highlight the potential of microalgae in the removal of heavy metals as well as enhance the quality of water by working over water quality parameters. With the motivation of these aspects, this study aims to evaluate two microalgae Chlorella Minutissima and Chlorella singularis for the treatment of wastewater. Along with this, the architecture of wastewater treatment using microalgae is proposed and stated in the present study. The variations of WQPs have been examined. The following are the key contributions of the study:
-
The study performs a comprehensive evaluation of the literature present across the globe on wastewater treatment using microalgae and the collaboration of SDGs for the achievement of sustainability.
-
The implementation methodology has been provided and the variation in WQP of wastewater and treated water is represented in tabulated form and shown graphically and the correlation with the standard value of WQP is also shown. The optimization of results has been evaluated using the ANOVA Model.
-
The sample collected from different sites shows different characteristics represented in the presented article and an evaluation of the algae was also discussed. The challenges faced in implementing the methodology over a large scale are illustrated and future recommendations have been provided accordingly.
The article is structured as follows: The first section provides a concise introduction to SDGs and wastewater treatment and their relation with necessity. The next step is to provide a literature review covered in Sect. 2. It is followed by Materials and methods highlighted in Sect. 3. Section 4 covers the Results and Discussion which is followed Summary of the proposed work elaborating challenges and future work in Sect. 5. The Last section concludes the article.
2 Review of literature
WQP shows the environment of a water body which comprises physical, chemical, and biological parameters. Physical parameters consist of Turbidity, Colour, Temperature, pH, Electrical Conductivity, and total Solids. Chemical parameters consist of Hardness, Chlorides, Alkalinity, Heavy metals, Dissolved Oxygen (DO), and biological Oxygen Demand (BOD). Biological Parameters consists of Bacteria, Virus, Protozoa, and fungi [12]. The comprehensive analysis of the typical cause of heavy metal contamination in aquatic habitats was conducted in 2012 and evaluated by the WQP. The evaluation runs for seven days at different metal loading rates as well as light schedules [13]. In 2013, the study was observed which demonstrates the use of micro-algae-based bioremediation and is considered as a viable method for the treatment of waste streams and lipid formation [14]. The potential of phyco-remediation using chlorella Minutissima was shown in 2015 to remove the load of primary treated wastewater of IARI & tertiary treated wastewater of CETP and after the conducted evaluation the remaining biomass was used for the production of bio-diesel [15]. The investigation of microalgae and calcined eggshells for the removal of heavy metal using the acid marine drainage (AMD) phenomenon was conducted in the year 2015. The result concludes that biomass productivity has increased 8.04 times the initial concentration and the removal of heavy metals was found to be 99.47% up to 100% [16].
In 2016, the evaluation of the discharge of inorganic and organic pollutants in the environment and the demonstration of various sources of water pollution were shown in the study. It also investigates the potential of techniques used for waste and sewage treatment [17]. Various technologies required for wastewater treatment have been integrated and the process of cultivation of microalgae for biomass production has been evaluated in 2017. The study also verifies that the pre-treatment of wastewater is very necessary before imparting the cultivation of microalgae and provides future suggestions regarding the generation growth of microalgae for separation out of biomass [18]. Different strategies to improve the scale of microalgae cultivation have been reported in 2017 and the biological mechanism for nutrients & contaminants was summed up in a study. Evaluation of the Phyco-remediation technique for the removal of nutrients and contaminants has been performed [19]. In 2019, a study found that chlorella sp. microalgae which is utilized for the removal of heavy metals and concluded with is considered an efficient cost-effective method for the removal of heavy metals. It demonstrates the biosorption technique using Box Behnken Design [20]. The evaluation in 2019 revealed that the activated sludge process and sludge thickening process made a significant impact on heavy metal distribution while anaerobic digestion and dehydration decreased its mobility. These methods help in reducing secondary contaminants with heavy metals and further reduce health risks [21].
In [22], the author described the environmental issues led by heavy metals in 2019 suggested the technique of biosorption, and evaluated the efficacy of the technique. They highlighted the mechanics and procedure for creating new algae biosorbents. [23] Revealed a synergistic potential of oleaginous microalgae chlorella sorokiniana for phyco-remediation and bio-diesel production in 2020. Evaluation of WQP has also indulged in the study after the completion of the remediation process and also highlights the comparison between generated lipid and Bold Bascal Media (BBM). The study demonstrated in [24] found the percentage removal & recovery of heavy metal interrelated with algal growth in 2020. The finding shows that the mathematical modeling of combined bio-assimilation & bio-accumulation was suggested as an efficient and robust tool for prediction and forecasting of heavy metal removal. In [25], the author reviewed various biomaterials-based bio-sorbents and evaluated several approaches toward the removal of contaminants from wastewater in 2021. They discussed effective technologies for the removal of contaminants and focussed on covering the gap between laboratory results and industrial use. The author of [26] outlines the symbiotic relationship between algae & bacteria and their application in wastewater treatment in 2021. They also made substantial studies to overcome the limitations of traditional methods of wastewater treatment.
The author of [27] reviewed the current state & prospects of bio-sorption and bio-accumulation for environmental bio-remediation as well as provided information regarding biological processes in 2022. They also evaluated the biotechnological strategies for the elimination of heavy metals from wastewater. In [28], the author demonstrated the modern methods for the removal of heavy metals from wastewater. They have made efforts to disseminate information about various microalgae strains utilized for the removal of heavy metals in 2022 and finally, the output comes that phyco-remediation plays a significant role in the remediation of heavy metals in a sustainable way with the realization of bio-economic potential. The author of [29] underscores the utility of indigenous microbial taxon in heavy metal removal with adsorption capacities and draws attention to the benefits of ecological engineering approaches over other alternatives. They investigated several heavy metals according to detoxification. In [8], the author presented an approach of microalgae in 2022 for the treatment of wastewater and removal of nutrients and heavy metals up to 100%. They also discussed the characteristics of wastewater and various conventional methods for treatment of wastewater.
These approaches provide complete and efficient knowledge regarding the procedures for wastewater treatment as well as heavy metal removal using various techniques, which are useful for the analysis of another technique of heavy metal removal which is cost efficient and uncompetitive method of wastewater treatment.
3 Materials and methods
In this section, various methods as well as proposed methods for the removal of heavy metals from wastewater have been presented. The whole process of treatment has been demonstrated.
3.1 Methods for heavy metal removal
Methods of removing heavy metals, nitrogen, phosphorus, and coliforms are either becoming inadequate to comply with the strict administrative regulatory restrictions in place today or are becoming more expensive. Therefore, smart, optional developments are in demand. Heavy metals were broken down by conventional evacuation techniques such as chemical precipitations, carbon adsorptions, ion exchange, dissipations, and membrane processes [17]. The selection of a particular therapy Procedure primarily depends on several factors, including waste kind and fixation, profluent heterogeneity, the required level of cleaning, as well as financial factors. Due to improved execution, accessibility, and low effort of crude substances, the use of natural materials, including living and non-living small-scale life forms, to remove and recover hazardous or precious metals from mechanical wastewater has gained popularity over time [30]. The prime objective of waste-water treatment is to prevent water sources from being contaminated and to protect public health by protecting water sources from spreading disease. A variety of treatment frameworks, including both on-site and off-site therapy frameworks were helpful [31]. Figure 2 depicts methods for heavy metal removal.
-
a.
Coagulation and flocculation These were important physico-chemical techniques used in the expulsion of turbid materials and organic particles from wastewater. Iron salt and hydrolytic aluminum were two prominent and well-known coagulants [32]. The feasible pH of Al(OH)3 uses 4.5 and 8 for Fe(OH)3.
$${\text{Al}}_{{2}} \left( {{\text{SO}}_{{4}} } \right)_{{3}} + {\text{6H}}_{{2}} {\text{O}} = {\text{2Al}}\left( {{\text{OH}}} \right) + {\text{3HS}}_{{2}} {\text{O}}_{{4}}$$$${\text{FeCl}}_{{3}} + {\text{6H}}_{{2}} {\text{O}} = {\text{Fe}}\left( {{\text{OH}}} \right)_{{3}} + {\text{3HCl}}$$Releasing a large amount of chemical sludge is a huge drawback. Additionally, the use of coagulants by aluminum causes an increase in the amount of residual aluminum fixation in cleaned water [33].
-
b.
Precipitation Chemical reactions with heavy metal particles during the precipitation process produce insoluble compounds, which water may then separate from sediments and filtrations [34]. It is extensively used to release metal ions, phosphoric compounds, and radioactive elements.
Due to their relative simplicity, low cost, and common pH control, hydroxide treatments are the most often used precipitation procedure. Utilize the compounds Ca(OH)2 and NaOH as a precipitant. By creating an additional circumstance, a device for heavy metals ejection by material precipitations might be established.
$${\text{M}}^{{{2} + }} + {2}\left( {{\text{OH}}} \right)^{ - } \leftrightarrow {\text{M}}\left( {{\text{OH}}} \right)_{{2}} \downarrow$$The main disadvantage of hydroxide precipitations is the production of large amounts of comparably low-density sludge, which causes problems with the dewatering process and removal [35].
-
c.
Ion exchange It refers to a technique that is often employed all over the world to remove heavy metals from squandered water. Major advantages of the ion exchange process include the potential for metal resurrection, a wide range of options, and little sludge volume [36]. The method's key premise is the exchange of ions between the liquid (electrolytic solution) and resin phases in an amount that is chemically equivalent, with no distinction made for the resin [37].
-
d.
Adsorption Adsorption is regarded as a profitable method for toxin expulsion from wastewater. Concentrations of molecules on the surfaces of sorbents allow a way to be identified [38]. Adsorption has several benefits, including lower cost, greater usability, increased productivity, flexibility in design and operation, and technique adaptability [39].
Due to its unique combination of large micropore and mesopore volumes and high surface area, activated carbon is one of the most often utilized sorbents and is typically employed for the removal of natural toxins from wastewater. Activated carbon can be categorized into one of four groups, depending on the manufacturing process: Powder activated carbon, activated carbon granules, activated carbon fibers, and activated carbon clothing, each with a particular use [40].
-
e.
Membrane Filtration Membrane filtration has drawn a lot of interest recently since it can remove contaminants from a variety of sources. An industrial operation that already uses membrane technology has the potential to lower costs and energy use overall. Ultra-filtration, nano-filtration, and reverse osmosis are all currently used membrane techniques [41].
-
i.
Ultra-filtration: A permeable membrane with pore sizes of 5 to 20 mm and an atomic load of separating substances between 1000 and 100,000 Da is used in the ultra-filtration process to separate heavy metals, including molecules and floating solids, from solutions [42]. High-weight molecules are retained by a force analogous to stress, but low-weight molecules escape through the membrane due to gradient concentration. This method is mainly employed in industries for purifying protein solutions.
-
ii.
Reverse osmosis: Reverse osmosis is a membrane-driven technique that uses pressure to push water through a membrane while trapping polluting metal ions. Metal ion evacuation from inorganic solutions through reverse osmosis is becoming more and more effective. The technique also works in a wide pH range of 3 to 11 and a pressure range of 4.5 to 15 [43]. Reverse osmosis further necessitates the employment of high-pressure pumps to drive the water to travel past the semi-permeable membranes, removing 95 to 99% of dissolved salts from the flow. The two main drawbacks of RO are the powerful utilization caused by the siphoning pressures and the potentially expensive layer rebuilding [44].
-
iii.
Nano-filtration: The procedure of nano-filtration, which sits between ultrafiltration and reverse osmosis, is appropriate for particles as small as 0.0001 to 0.001 µm in size. NF rejects excessive concentrations of divalent cations and multivalent particles while permitting monovalent ions to pass through the membrane. The method is used to remove color, microorganisms, and proteins as well as to desalt dyes, concentrate sugar, and soften water. Here are numerous details on the removal of heavy metals using a reverse osmosis membrane and nano-filtration [45].
-
i.
-
f.
Electrochemical treatment The electrochemical methods compete by using more physicochemical knowledge to provide solutions for many engineering needs, such as cleaning various types of wastewater. The electrochemical approach can effectively influence how metal revival is conducted, and its main advantage is determined by the usage of spotless electron reagents [46]. The adaptability of electro-chemical processes (electro-coagulation, electro-floatation, electro-oxidation, and electro-position) to be able to observe with low down ecological crash, simple practice and management, as well as be short of dangerous and poisonous scraps, are standards for technique [47].
Physical–chemical methods used to lessen the concentration of various contaminants in wastewater have benefits and drawbacks. Although there are conventional ways, they are carried out with the aid of synthetic chemicals, which severely pollute the environment and have an adverse effect on living things. One such approach can lead to minor water contamination and is also quite costly and ineffectual. The technique put out in the current study is uncompetitive and environmentally beneficial. The present study aimed at an environmentally friendly technique of wastewater treatment named “Bioremediation”. Bioremediation provides various advantages when compared with other treatment techniques such as: (1) It is biological remediation; no use of chemicals is required and helps to detoxify hazardous substances such as heavy metals from wastewater; (2) Environmentally friendly approach, eco-friendly method; (3) No use of chemical so it is cost-effective in comparing with other methods of wastewater treatment.
Bioremediation The concept of bioremediation involves detoxifying pollutants from soil and other surroundings primarily by using microbes, plants, or microbial or plant enzymes. A developing technology called bioremediation can be used in conjunction with existing physical and chemical treatment techniques to effectively handle a variety of environmental contaminants. There is a need for greater study in this area since it appears to be a sustainable strategy for managing environmental contamination. Bioremediation is a remediation technology that is widely used and is constantly developing. An inherently multidisciplinary technique is bioremediation. As a practical, sustainable, and affordable method of treating hazardous waste, bioremediation was created [48]. Site characterization, which determines the most appropriate and effective bioremediation strategies, is the most important phase in the successful bioremediation process (ex-situ or in-situ). Excavation and shipping costs add to the cost of the ex-situ bioremediation method, which makes it more expensive. According to the Federal Remediation Technologies However, due to its eco-friendly features, bioremediation has been demonstrated to be effective and dependable in the rehabilitation of many polluted areas [49].
3.1.1 Cost comparison
The magnitude of the research the particular toxins involved, and the site's location are just a few of the variables that can significantly affect the cost of bioremediation of wastewater. Comparing bioremediation to many other conventional techniques, it is typically less expensive. Table 1 illustrates the comparison between methods of wastewater treatment.
Considering all methods, bioremediation is frequently chosen due to its affordability and environmental sustainability. On the other hand, the selection of the treatment approach ought to be predicated on a careful analysis of the characteristics unique to the site, pollutants, and legal obligations. Furthermore, in certain circumstances, a treatment train comprising a variety of techniques may be the most successful strategy.
3.2 Implementation methodology
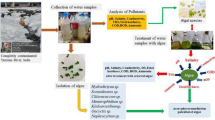
This section provides the implementation methodology of the experimental work performed. Discussion of various steps combining the framework of implementation methodology showed up in the form of a block diagram deployed in Fig. 3. The first stage of methodology is site selection from where the wastewater sample is collected then afterwards second stage comes where the preparation of BBM with the mixing of several chemicals is done. Now, the third stage arises where the cultivation of algae in BBM is carried out. Summing up, the fourth stage comes where the pre-testing of wastewater samples is carried out in the laboratory. Moving further, the fifth stage comes where the inclusion of Algae in a water sample is done. Then, the sixth stage goes through where the Recording of algal growth is carried out. Afterwards, the seventh stage arrives where the separation of biomass is done with the help of double filter paper. The last stage comes after the separation i.e. Post-Testing of a sample.
The various steps of the implementation methodology are as follows:
-
a)
Site selection The sample of effluent was collected from an Industry named Amber Enterprises India Ltd from Sidcul, Selaqui, Dehradun (30.358084°, 77.850414°) on 10th February 2023 at 11:50 AM. The other sample was collected from tons river (30.342774°, 77.953609°) near Uttaranchal University, Dehradun on 15th February 2023 at 09:40 AM. The industrial effluent comprises some amount of heavy metals & wastewater and river water comprises sewage & wastewater with bad quality parameters in both samples.
-
b)
BBM preparation A BBM is the standard control media in which the algae gets all the essential nutrients that are required for growth. The composition of chemicals used for BBM preparation is shown in Table 2.
-
c)
Cultivation of algae The strains of algae purchased from the market and introduced in the BBM sample prepared. Afterward, the sample is stored in a setup created for isolation for 14 Days. The growth of algae is checked into a UV Spectrophotometer at an optimum density of 730nm.
-
d)
Pre-testing of water sample The sample collected from the selected site is analyzed in the pre-testing of the water sample. The pre-testing consists of an evaluation of various WQPs which are been calculated in the laboratory and the analysis starts after the quality check of the wastewater sample. Pre-testing comprises checking of Physical Parameters i.e. Turbidity, Color, Temperature, pH & Total Solids as well as Chemical Parameters i.e. Hardness, Chlorides, Alkalinity, Heavy Metals, Dissolved Oxygen (DO), and biochemical Oxygen Demand (BOD).
-
e)
Inclusion of algae in wastewater sample: After the pre-testing stage, algae were introduced into the wastewater sample. The sample is split into 4 samplet in which the algae were introduced in a ratio of 1:5. Then afterward, each samplet is named Industrial Effluent (A) Chlorella Minutissima (X), Industrial Effluent (A) Chlorella Singularis (Y), River Water (B) Chlorella Minutissima (X) and River Water (B) Chlorella Singularis (Y).
-
f)
Recording of algal growth The growth of algae in wastewater samples is recorded in a UV Spectrophotometer at an optimum density of 730nm. Algal Growth is shown in Table 3.
-
g)
Separation of biomass: After the algal growth in the water sample, the sample is split into water and biomass. Separation of biomass is done using double filter paper in the laboratory.
-
h)
Post-testing of water sample The last stage of implementation methodology is the post-testing of the water sample in which the final evaluation of WQP is done. Post-testing is similar to pre-testing where all the WQPs are calculated once again for the comparison and checking of the efficiency of algae.
3.3 Optimization of HM removal and quality parameters
The optimal percentage concentration of pollutant removal was determined using response surface methodology (RSM). In the RSM, each parameter's significance is ascertained by a mix of statistical and mathematical techniques. Both industrial and river water were evaluated for statistical and mathematical significance using a one-way analysis of variance (ANOVA). By calculating the magnitude of the parameters and assessing their correlation, the ANOVA model makes it possible to quantify treatment approaches.
4 Results and discussion
In this section, the result and analysis of various quality parameters of the sample collected as well as comparison analysis of different samples and the quantitative comparison is represented in the form of graphs shown in Figs. 4 and 5.
4.1 Analysis of WQPs
In this section, the WQPs are analyzed with the variation in each parameter recorded under different conditions and sites. The variation in the parameters is tabulated below in Table 3. Table 3 describes the recorded result of WQPs shown below and shows the comparison between the algae present and the water of different sites used for analysis. In Site 1, the water is taken from the industry which comprises heavy metals as well as various impurities. The table demonstrates the value of WQP in the pre-test and after the post-test compared with the standard value. For the removal of heavy metals and comparative study, different types of water are considered. In Site 2, the water is taken from the river which comprises several impurities which is to be detoxified from it. A comprehensive analysis has been carried out for the comparison of different waters as well as the efficiency of different Chlorella Species.
4.1.1 Variation in physical WQP
Turbidity in both the sites selected is very high and crosses the standard value i.e. below 1 NTU. The recorded value of industrial effluent is 291NTU and the recorded value of river water is 691NTU. The variation comes in turbidity by the integration of algae chlorella Minutissima & chlorella Singularis which shows a substantial decrease in turbidity with the adsorption phenomenon of algae. Table 4 reveals the comparison of turbidity with the standard value provided by CPCB.
Color has shown the average values which link with the standard value allotted. In both the sites, the algae have substantially increased the quality of water. The value of color recorded in industrial water is 0.34 mg/l and river water is 0.28 mg/l estimated by the Spectro quant Picco meter. The observation shows that Chlorella Minutissima imparts light green color in Industrial effluent whereas yellow color in river water due to its oleaginous property and characteristics of water. Chlorella Singularis imparts yellow color in industrial effluent whereas dark green color in river water.
The standard value for the temperature of water is 0 °C but due to impurities and the presence of heavy metals the temperature recorded in industrial effluent is 10 °C & river water is 12 °C. After the inclusion of algae in the sample, the impurities were adsorbed by the algae present resulting in decrement in temperature.
Due to the presence of heavy metals in wastewater, the conductivity recorded is high in industrial water is 362uS & river water is 315uS estimated by the Conductivity meter. Algae inclusion made removal of heavy metals which leads to decrement of conductivity correlated with the standard value.
The presence of total solids is due to the suspended and dissolved solids in water which is calculated by the weighing phenomenon. The recorded value of total solids in industrial effluent is 180 mg/l whereas in river water is 60 mg/l.
4.1.2 Variation in chemical WQP
The hardness variation in the water sample is calculated by the titration process which has shown a high extent of hardness present in the water collected. Hardness recorded in industrial effluent is 65 mg/l whereas in river water is 62 mg/l. Interaction of water with algae leads to decrement in hardness which is tabulated below in Table 5. The standard range of hardness given by CPCB is 0 to 60 mg/l.
The chloride content in the water is recorded with the help of the titration process. The value depicted for industrial effluent is 8.33 mg/l whereas river water is 10 mg/l. The data series recorded for chloride content after the inclusion of algae leads to an increase in chloride content due to the presence of biological nutrients. Alkalinity shows a lower value in comparison with the standard which shows the acidity presence in water. The value recorded for Alkalinity in industrial effluent is 11.66 mg/l whereas in river water 10 mg/l. The evaluation shows the competitive method which is bioremediation considered for the conversion of acidic water into alkaline.
DO presence in wastewater is very less due to the presence of organic matter and nutrients. After the inclusion of algae in wastewater leads to the removal of organic matter present this gives rise to the DO. DO was recorded with a DO meter which was estimated in the morning and concluded that the attainment of value up to the standard range of 6.5 to 8 mg/l. BOD presence in wastewater is considered as the oxygen demand required by microbes. The inclusion of algae in wastewater removes the microbes by adsorption process and decreases the value of BOD recorded. The standard value for BOD is 1 to 2 mg/l.
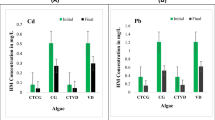
Heavy metal contamination in wastewater becomes hazardous for the livelihood of persons as well as the environment. Table 6 deploys the heavy metal comparison in the pre-test of the wastewater sample and after the post-test of treated water. Heavy metals estimated in the sample are Cd, Cu, Zn, Fe, Pb, Na+, and K+ and the removal of these heavy metals is conducted by the inclusion of Algae. These values of heavy metals are recorded by FAAS equipment at BBAU, Lucknow.
4.2 Optimized analysis of parameters
In this section, the WQPs are analyzed with the variation in each parameter and optimized by the ANOVA model. The variation of physical and chemical has been shown separately and stated as follows.
4.2.1 Analysis of physical parameters
ANOVA is a statistical model used to analyze the differences among group mean in a sample provided. The follow-up is the ANOVA Single factor applied over the estimated value of physical parameters among wastewater, Chlorella Minutissima, and Chlorella Singularis. The summary elaborates on the Groups, Count, Sum, Average, and Variance. Groups show various categories i.e. Wastewater, Chlorella Minutissima, and Chlorella Singularis. ANOVA section highlights the Source of Variation, SS (i.e. Sum of Squares), df (i.e. Degree of Freedom), MS (i.e. Mean Square), F (F-ratio), P-value, and F-crit (Critical Value) as demonstrated in Table 7.
The ANOVA tests the null hypothesis that there is no significant difference among the group means. The P-value (0.172956) in this instance is higher than the significance level (often 0.05), indicating that there is insufficient data to rule out the null hypothesis.
At the 0.05 significance level, the F-ratio is 1.851419 and the critical value is 3.284918. The notion that there are no appreciable variations in the group means is supported by the computed F-ratio, which is smaller than the critical value.
4.2.2 Analysis of chemical parameters
ANOVA is a statistical model used to analyze the differences among group mean in a sample provided. The follow-up is the ANOVA Single factor applied over the estimated value of chemical parameters among wastewater, Chlorella Minutissima, and Chlorella Singularis. The summary elaborates on the Groups, Count, Sum, Average, and Variance. Groups show various categories i.e. Wastewater, Chlorella Minutissima, and Chlorella Singularis. Variance is 518.2326 for Wastewater, 429.5306 for chlorella minutissima, and 532.4273 for chlorella singularis. ANOVA section highlights the Source of Variation, SS (i.e. Sum of Squares), df (i.e. Degree of Freedom), MS (i.e. Mean Square), F (F-ratio), P-value, and F-crit (Critical Value) as illustrated in Table 8.
The ANOVA tests the null hypothesis that there is no significant difference among the group means. The P-value (0.867487) in this instance is higher than the significance level (often 0.05), indicating that there is insufficient data to rule out the null hypothesis.
At the 0.05 significance level, the F-ratio is 0.142769 and the critical value is 3.284918. The notion that there are no appreciable variations in the group means is supported by the computed F-ratio, which is smaller than the critical value.
4.2.3 Analysis of heavy metals
-
a.
Pre-test
Result of ANOVA test for the heavy metals in different groups (Cd, Cu, Zn, Fe, Pb, Na+, and K+). The follow-up is the ANOVA Single factor applied over the estimated value of heavy metal in the pre-test among wastewater, Chlorella Minutissima, and Chlorella Singularis. The summary elaborates on the Groups, Count, Sum, Average, and Variance. ANOVA section highlights the Source of Variation, SS (i.e. Sum of Squares), df (i.e. Degree of Freedom), MS (i.e. Mean Square), F (F-ratio), P-value, and F-crit (Critical Value) as mentioned in Table 9.
The ANOVA tests the null hypothesis that there is no significant difference among the group means. The P-value (0.744976) in this instance is higher than the significance level (often 0.05), indicating that there is insufficient data to rule out the null hypothesis.
At the 0.05 significance level, the F-ratio is 0.569648 and the critical value is 3.865969. It is suggested that there are no appreciable variations in the average concentrations of the heavy metals in the pre-test since the computed F-ratio is below the threshold limit.
-
b.
Post test
Result of ANOVA test for the heavy metals in different groups (Cd, Cu, Zn, Fe, Pb, Na+, and K+). The follow-up is the ANOVA Single factor applied over the estimated value of heavy metal in the post-test among wastewater, Chlorella Minutissima, and Chlorella Singularis. The summary elaborates on the Groups, Count, Sum, Average, and Variance. ANOVA section highlights the Source of Variation, SS (i.e. Sum of Squares), df (i.e. Degree of Freedom), MS (i.e. Mean Square), F (F-ratio), P-value, and F-crit (Critical Value) as depicted in Table 10.
As in the last ANOVA table, there is insufficient evidence to reject the null hypothesis because the p-value (0.97106) is significantly higher than the significance threshold (typically 0.05).
At the 0.05 significance level, the F-ratio is 0.205882, and the critical value is 2.572712. It is suggested that there are no appreciable variations in the average concentrations of the heavy metals since the computed F-ratio is below the threshold limit.
4.3 Distribution of WQP under several parameters: quantitative analysis
Figure 4 illustrates the quantitative analysis of physical parameters between two types of water i.e. industrial effluent and river water showing the fluctuation in pre-test and post-test after the inclusion of algae. The variation in the WQP illustrated in the figure is due to various impurities present in the sample which is tackled by the inclusion of various algae like chlorella Minutissima & chlorella Singularis.
Graph (a) deploys turbidity comparison between wastewater and treated water recorded in pre-test and post-test. The value of turbidity recorded in industrial effluent is 291NTU whereas 691NTU in river water. The alteration in turbidity after the inclusion of both algae in different samples has been recorded. Chlorella Minutissima shows 100NTU in industrial effluent and 120 NTU in river water whereas Chlorella Singularis shows 94NTU in industrial effluent and 96.5NTU in river water. Graph (b) shows a color comparison between wastewater and treated water imparted by algae. Algae shows its oleaginous property in different water samples which is represented in the graph. The color alteration shown by algae in industrial effluent is recorded as 0.34 mg/l and in river water is recorded as 0.28 mg/l. When Chlorella Minutissima is included in the wastewater it shows 34.3 mg/l in both industrial effluent and river water whereas Chlorella Singularis shows 74.3 mg/l in industrial effluent and 84.3 mg/l in river water.
Graph (c) shows temperature variation between wastewater and treated water recorded in the pre-test and post-test. The temperature alteration is abrupt and shown graphically and a decrement in temperature after the post-test is recorded. The temperature estimated in pre-test for industrial water is 10 °C whereas river water is 12 °C due to the presence of microbes and heavy metals. After the inclusion of Chlorella Minutissima the temperature drops to 1 °C in both industrial effluent and river water whereas integration of Chlorella Singularis, the temperature drops to 2 °C in both industrial effluent and river water. Graph (d) shows the pH variation between wastewater and treated water. Algae show alteration in pH in different water samples. The pH recorded in the pre-test shows that the industrial effluent is acidic and its value is 5.1 whereas the river water is neutral and its value is 7.46. Now after the inclusion of Chlorella Minutissima, industrial effluent changes its nature from acidic to neutral and its value is 6.7 whereas the value of pH in river water estimated is at 6.8. Chlorella Singularis changes the industrial effluent into a neutral state and the value of pH recorded is 7.8 whereas the value of pH in river water is 7.5.
Graph (e) deploys a conductivity comparison between wastewater and treated water recorded in pre-test and post-test. The conductivity decreases in the water sample after the post-test due to the removal of nutrients from it. Due to the presence of heavy metal, the conductivity recorded in the pre-test of industrial effluent is 362uS and 315uS in river water. After the inclusion of chlorella Minutissima, the conductivity drops to 215uS in industrial effluent and 212uS in river water. Chlorella Singularis shows alteration and decrement in conductivity and the value estimated is 202uS in industrial effluent and 201uS in river water as shown in graph (e). Graph (f) shows the Total solids comparison between wastewater and treated water. Algae decreases the total solids present in wastewater by the adsorption process. Due to the presence of heavy metals and impurities like microbes, total solids lead to higher in sample i.e. 180 mg/l in industrial effluent and 60 mg/l in river water. After the integration of Chlorella Minutissima, the value recorded for total solids is 10 mg/l in industrial effluent and river water whereas after the inclusion of Chlorella Singularis the value estimated for total solids is 20 mg/l.
Figure 5 illustrates the quantitative analysis of chemical parameters between two types of water i.e. industrial effluent and river water showing the fluctuation in pre-test and post-test after the inclusion of algae. The variation in the WQP illustrated in the figure is due to various impurities present in the sample which is tackled by the inclusion of various algae like chlorella Minutissima & chlorella Singularis.
Graph (a) shows the hardness comparison between wastewater and treated water recorded in the pre-test and post-test. Hardness is reduced in the treated water sample due to algae inclusion. Due to the presence of heavy metals and impurities, hardness recorded in industrial effluent is 65 mg/l whereas in river water it is recorded as 62 mg/l. After the inclusion of Chlorella Minutissima, the value of hardness recorded in industrial effluent is 28 mg/l whereas 24.6 in river water. However, after the integration of Chlorella Singularis, the value recorded for hardness in industrial effluent is 21 mg/l and 12.6 mg/l in river water. Graph (b) shows a chloride comparison between wastewater and treated water. Chlorides increase in the treated water sample due to the oleaginous property of algae and algae impart an increase in the chloride content of water.
Graph (c) deploys the alkalinity comparison between wastewater and treated water recorded in pre-test and post-test. Alkalinity alters in both industrial effluent and river water due to their characteristic properties. Algae lead the water from acidic to alkaline as demonstrated in the graph (c). Graph (d) represents the DO comparison between wastewater and treated water. Due to algae, the nutrients and microbes are removed from the wastewater therefore there is a rise in dissolved oxygen present in water. Due to the presence of microbes, the DO in water reaches to lower level i.e. estimated in the pre-test as 3.2 mg/l in industrial effluent and 5.6 mg/l in river water. After the integration of algae, the removal of microbes is seen which leads to a rise in DO of the sample collected as shown in the graph (d).
Graph (e) deploys the BOD comparison between wastewater and treated water recorded in the pre-test and post-test. BOD is calculated by the prescribed formula presented by CPCB. BOD decreases due to an increase in DO and the removal of nutrients present in a water sample. Removal of nutrients and microbes leads to a decrease in BOD as BOD is required for the decomposition of microbes and nutrients.
Figure 6 illustrates the heavy metal contamination in the sample collected for treatment using Chlorella Minutissima & Chlorella Singularis. Graph (a) shows the variation in heavy metal constituents i.e. Cd, Cu, Zn, Fe, Pb, Na+, and K+ in both industrial effluent and river water demonstrated in Table 4. The quantity of heavy metal present in wastewater in the pre-test is estimated by FAAS equipment and shown in the variation in graph (a). Graph (b) deploys the variation in heavy metal in different water samples after the post-test. The value of some constituents leads to 0 as shown in Table 4 and demonstrated graphically in graph (b).
4.4 Optimized impurities removal and heavy metal analysis
Figure 7 illustrates the quantitative analysis of physical and chemical parameters and heavy metal concentration between two types of water i.e. industrial effluent and river water showing the fluctuation in pre-test and post-test after the inclusion of algae. The variation in the WQP is illustrated in the figure shown with the optimization of parameters using the ANOVA Model. The graph illustrates variation between groups, variation within groups, ANOVA F-statistics, P-value, F-crit value, SS, df, MS.
5 Summary of the proposed work
Microalgal bioremediation is considered a very innovative method that is used for removing impurities and heavy metals from wastewater. In the current evaluation, two samples were taken, one from industry and another from impure river. First, the sample is taken for the pre-treatment for quality check of water quality parameters then after the pre-test the algal growth in prepared Bold Bascal’s Media has been recorded, then the algae is inculcated in the wastewater. After 15 days of evaluation, the sample is filtered and passed through post-treatment which gives subsequent results highlighted in the current study. Additionally, the ANOVA model—which stands out in the experimental evaluation—is used to optimize the result and P-value, F-crit values have been highlighted and the outcome is shown in graphical form.
5.1 Challenges and future work
The challenges faced in implementing the methodology are illustrated below:
-
After studying various studies and research got to know that the researcher doesn’t go beyond its scales and bringing up this method over industrial scale which will help to expose microalgae to harsher environmental and seasonal variable conditions and concentration of pollutants as well which is responsible for reducing treatment efficiency and yield biomass. As an outcome, there must be adoption of microbial bioremediation to adapt to a greater challenging environmental condition which is used to maintain the ability to degrade pollutants which must be investigated shortly.
-
After the bioremediation process the separation of microbial biomass from the treated wastewater is a very challenging task. In the current study, the wastewater considered is taken up in a small quantity by which the separation becomes easier using the filtration method. But in terms of the near future, the process can be used over an industrial scale, and then the separation becomes challenging which can be dealt with by attaching microalgae cultivation to a media that makes the process more accessible and easier.
-
There have been a few studies completed, but more needs to be done to determine the viability of using microalgae to clean wastewater and how it compares to other chemical approaches. On the other hand, the technique design and operation guidelines for microalgae bioremediation of wastewater encourage researchers to put forth a lot of effort to offer fundamental advice and recommendations that enhance the resilience and flexibility of microalgae strains to remove contaminants from wastewater.
-
In the experiments mentioned above, researchers utilize microalgae bioremediation to remove usual contaminants like fertilizers and heavy metals. As a result, several studies must be conducted to further remove heavy metals, colors, and contaminants including personal care items and antibiotics. Much research has been done on the removal of nutrients and heavy metals from wastewater using microalgae bioremediation. Furthermore, there must be a complete grasp of biological treatments utilizing microalgae. More studies must be done in this area.
-
Due to the dark color and dangerous contaminants in wastewater, microbial growth is the major drawback of employing large-scale wastewater treatment facilities. Controlling the effects and pre-treating the sample before adding microalgae are suggested solutions to this problem. The sophisticated operation and monitoring of the entire process, which necessitates the development of new technologies for online monitoring and remote control, presents another obstacle to the operation of large-scale WWTPs, particularly the microalgae-based wastewater treatment process.
6 Conclusion
Sustainable management of water and natural resources with consideration the quality becomes a great demand in today’s society. Various methods are there for the treatment of wastewater and the removal of hazardous impurities present in water. One of the hazardous impurities of water is heavy metal contamination which leads to the destruction of livelihood. The current work highlights the previous studies deployed on the techniques used for the removal of heavy metals and shown in the extensive literature. For the removal of heavy metals from the water the study presented various methods and also provided an efficient technique i.e., Bioremediation. The potential of Chlorella minutissima and Chlorella singularis microalgae for wastewater bioremediation has been carefully assessed in this work.
The present study focuses on the removal of heavy metals from wastewater and provides a framework for the implementation process to be conducted. The quality check of water is done by analysis of WQP and results are represented in the form of graphs. The findings show that both species of algae are remarkably adept in eliminating organic pollutants and nutrients from the wastewater matrix, among other contaminants. The observation collected by the analysis of WQP is that Alkalinity leads to the permissible value of 20–40 mg/l, and pH is estimated in a range of 6.5 to 8. DO recorded after the analysis of WQP comes in a range of 6.5 to 8 mg/l and BOD decreases due to the removal of heavy metals and comes in a range of 1–2 mg/l. The technique presented in the current study is marked as the efficient technique used for heavy metal removal. The inclusion of the ANOVA model provides the optimization of the result in which the P-value and F-crit value have been highlighted. The highest P-value is 0.97106, which is of Heavy metal concentration check after post-testing and the highest value of F-crit is 3.865969, which is of Heavy metal concentration check at the time of pre-testing. Further, the challenges faced in implementing the methodology have been elaborated and future recommendations have been provided. Overall, this study offers strong evidence that Chlorella singularis and minutissima are effective in wastewater bioremediation. Their incorporation into wastewater treatment plans has a great deal of potential to reduce pollution in the environment and advance environmentally friendly water management techniques.
The novelty of the article is as follows: The article discusses the methods for heavy metal removal and further the use of algae for the bioremediation of wastewater is detailed in the present article which was lacking in previous studies. The article presents the bioremediation technique for removal of wastewater and experimental results have been illustrated in the current study. Also, a framework for implementation methodology has been generated and broadly explained further in the study. Further, the inclusion of the ANOVA model shown in the outcome result has been assembled and shown in graphical form.
Data availability
Data sharing does not apply to this article as no datasets were generated or analyzed during the current study.
References
Pandey S, Twala B, Singh R, Gehlot A, Singh A, Montero EC, Priyadarshi N. Wastewater treatment with technical intervention inclination towards smart cities. Sustainability. 2022;14(18):11563.
Alcamo J. Water quality and its interlinkages with the Sustainable Development Goals. Curr Opin Environ Sustain. 2019;36:126–40.
Ait-Kadi M. Water for development and development for water: realizing the sustainable development goals (SDGs) vision. Aquatic Procedia. 2016;6:106–10.
Ahmed F, Hasan S, Rana MS, Sharmin N. A conceptual framework for zero waste management in Bangladesh. Int J Environ Sci Technol. 2023;20(2):1887–904.
Jayaswal K, Sahu V, Gurjar BR Water pollution, human health and remediation. Water remediation, 2018, 11–27.
Qiao XJ, Liao KH, Randrup TB. Sustainable stormwater management: a qualitative case study of the Sponge Cities initiative in China. Sustain Cities Soc. 2020;53: 101963.
Elbehiry F, Alshaal T, Elhawat N, Elbasiouny H. Environmental-friendly and cost-effective agricultural wastes for heavy metals and toxicants removal from wastewater. 2021.
Ahmed SF, Mofijur M, Parisa TA, Islam N, Kusumo F, Inayat A, Le VG, Badruddin IA, Yunus Khan TM, Ong HC. Progress and challenges of contaminate removal from wastewater using microalgae biomass. Chemosphere. 2022;286: 131656.
Wang Y, Ho SH, Cheng CL, Guo WQ, Nagarajan D, Ren NQ, Lee D-J, Chang JS. Perspectives on the feasibility of using microalgae for industrial wastewater treatment. Bioresour Technol. 2016;222:485–97.
Hena S, Gutierrez L, Croué JP. Removal of pharmaceutical and personal care products (PPCPs) from wastewater using microalgae: a review. J Hazard Mater. 2021;403: 124041.
Jia H, Yuan Q. Removal of nitrogen from wastewater using microalgae and microalgae–bacteria consortia. Cogent Environ Sci. 2016;2(1):1275089.
Lokuliyana S, Jayakody A, Bandara, NB, Deshapriya JWP, Kavinda PC, Wijewickrema TL. A survey: IoT enable a framework for water quality measurement and distribution. In 2018 13th International Conference on Computer Science & Education (ICCSE) (pp. 1–6). IEEE. (2018)
Sekomo CB, Rousseau DP, Saleh SA, Lens PN. Heavy metal removal in duckweed and algae ponds as a polishing step for textile wastewater treatment. Ecol Eng. 2012;44:102–10.
Richards RG, Mullins BJ. Using microalgae for combined lipid production and heavy metal removal from leachate. Ecol Model. 2013;249:59–67.
Malla FA, Khan SA, Sharma GK, Gupta N, Abraham GJEE. Phycoremediation potential of Chlorella minutissima on primary and tertiary treated wastewater for nutrient removal and biodiesel production. Ecol Eng. 2015;75:343–9.
Choi HJ, Lee SM. Heavy metal removal from acid mine drainage by calcined eggshell and microalgae hybrid system. Environ Sci Pollut Res. 2015;22:13404–11.
Rajasulochana P, Preethy V. Comparison on the efficiency of various techniques in the treatment of and sewage water–a comprehensive review. Resour Effic Technol. 2016;2(4):175–84.
Salama ES, Kurade MB, Abou-Shanab RA, El-Dalatony MM, Yang IS, Min B, Jeon BH. Recent progress in microalgal biomass production coupled with wastewater treatment for biofuel generation. Renew Sustain Energy Rev. 2017;79:1189–211.
Cuellar-Bermudez SP, Aleman-Nava GS, Chandra R, Garcia-Perez JS, Contreras-Angulo JR, Markou G, Muylaert K, Rittmann BE, Parra-Saldivar R. Nutrients utilization and contaminants removal. A review of two approaches of algae and cyanobacteria in wastewater. Algal Res. 2017;24:438–49.
Jaafari J, Yaghmaeian K. Optimization of heavy metal biosorption onto freshwater algae (Chlorella colonies) using response surface methodology (RSM). Chemosphere. 2019;217:447–55.
Tytła M. Assessment of heavy metal pollution and potential ecological risk in sewage sludge from municipal wastewater treatment plant located in the most industrialized region in Poland—case study. Int J Environ Res Public Health. 2019;16(13):2430.
Cheng SY, Show PL, Lau BF, Chang JS, Ling TC. New prospects for modified algae in heavy metal adsorption. Trends Biotechnol. 2019;37(11):1255–68.
Arora N, Jaiswal KK, Kumar V, Vlaskin MS, Nanda M, Pruthi V, Chauhan PK. Small-scale physio-mitigation of raw urban wastewater integrated with biodiesel production and its utilization for aquaculture. Biores Technol. 2020;297: 122489.
Al Ketife AM, Al Momani F, Judd S. A bioassimilation and bioaccumulation model for the removal of heavy metals from wastewater using algae: new strategy. Process Saf Environ Prot. 2020;144:52–64.
Elgarahy AM, Elwakeel KZ, Mohammad SH, Elshoubaky GA. A critical review of biosorption of dyes, heavy metals and metalloids from wastewater as an efficient and green process. Cleaner Eng Technol. 2021;4: 100209.
Saravanan A, Kumar PS, Varjani S, Jeevanantham S, Yaashikaa PR, Thamarai P, Abirami B, George CS. A review on the algal-bacterial symbiotic system for effective treatment of wastewater. Chemosphere. 2021;271: 129540.
Arokiasamy P, Abdullah MMAB, Abd Rahim SZ, Sadique M, Ming LY, Salleh MAAM, Zainol MRRMA, Ghazali CR. Diverse material based geopolymer towards heavy metals removal: a review. J Mater Res Technol. 2022;22:126–56.
Chugh M, Kumar L, Shah MP, Bharadvaja N. Algal bioremediation of heavy metals: an insight into removal mechanisms, recovery of by-products, challenges, and future opportunities. Energy Nexus. 2022, 100129.
Greeshma K, Kim HS, Ramanan R. The emerging potential of natural and synthetic algae-based microbiomes for heavy metal removal and recovery from wastewaters. Environ Res. 2022;215: 114238.
Sharma P, Bano A, Singh SP, Dubey NK, Chandra R, Iqbal HM. Recent advancements in microbial-assisted remediation strategies for toxic contaminants. Cleaner Chem Eng. 2022;2: 100020.
Rajendran S, Priya TAK, Khoo KS, Hoang TK, Ng HS, Munawaroh HSH, Karaman C, Orooji Y, Show PL. A critical review of various remediation approaches for heavy metal contaminants removal from contaminated soils. Chemosphere. 2022;287: 132369.
Freitas TKFS, Almeida CA, Manholer DD, Geraldine HCL, de Souza MTF, Garcia JC. Review of utilization plant-based coagulants as alternatives to textile wastewater treatment. Detox fashion: wastewater treatment, 2018, 27–79
Dahasahastra AV, Balasundaram K, Latkar MV. Turbidity removal from synthetic turbid water using coagulant recovered from water treatment sludge: a potential method to recycle and conserve aluminum. Hydrometallurgy. 2022;213: 105939.
Chen Q, Yao Y, Li X, Lu J, Zhou J, Huang Z. Comparison of heavy metal removals from aqueous solutions by chemical precipitation and characteristics of precipitates. J Water Process Eng. 2018;26:289–300.
Pohl A. Removal of heavy metal ions from water and wastewaters by sulfur-containing precipitation agents. Water Air Soil Pollut. 2020;231(10):503.
Swanckaert B, Geltmeyer J, Rabaey K, De Buysser K, Bonin L, De Clerck K. A review on ion-exchange nanofiber membranes: properties, structure and application in electrochemical (waste) water treatment. Sep Purif Technol. 2022;287: 120529.
Pachouri V, Singh R, Gehlot A, Pandey S, Akram SV, Abbas M. Empowering sustainability in the built environment: a technological Lens on industry 40 Enablers. Technol Soc. 2023;76: 102427.
Rashid R, Shafiq I, Akhter P, Iqbal MJ, Hussain M. A state-of-the-art review on wastewater treatment techniques: the effectiveness of adsorption method. Environ Sci Pollut Res. 2021;28:9050–66.
Wang W, Guo Q, Yang Z, Jiang Y, Xu J. A state-of-the-art review on robotic milling of complex parts with high efficiency and precision. Robot Comput Integr Manuf. 2023;79: 102436.
Lewoyehu M. Comprehensive review on synthesis and application of activated carbon from agricultural residues for the remediation of venomous pollutants in wastewater. J Anal Appl Pyrol. 2021;159: 105279.
Ahmed SF, Mofijur M, Nuzhat S, Chowdhury AT, Rafa N, Uddin MA, Inayat A, Mahlia TMI, Ong HC, Chia WY, Show PL. Recent developments in physical, biological, chemical, and hybrid treatment techniques for removing emerging contaminants from wastewater. J Hazard Mater. 2021;416: 125912.
Rajoria S, Vashishtha M, Sangal VK. Treatment of electroplating industry wastewater: a review on the various techniques. Environ Sci Pollut Res. 2022;29(48):72196–246.
Ray P, Singh PS, Polisetti V. Synthetic polymeric membranes for the removal of toxic pollutants and other harmful contaminants from water. In Removal of toxic pollutants through microbiological and tertiary treatment, pp. 43–99. Elsevier; 2020.
Qin M, Deshmukh A, Epstein R, Patel SK, Owoseni OM, Walker WS, Elimelech M. Comparison of energy consumption in desalination by capacitive deionization and reverse osmosis. Desalination. 2019;455:100–14.
Goudarzi S, Azizi N, Eslami R, Zarrin H. Polymer-based nanoenhanced nanofiltration membranes. In Advancement in polymer-based membranes for water remediation, pp. 197–235. Elsevier; 2022.
Wickramaarachchi WKP, Minakshi M, Gao X, Dabare R, Wong KW. Hierarchical porous carbon from mango seed husk for electrochemical energy storage. Chem Eng J Adv. 2021;8: 100158.
Eslami A, Kashani MRK, Khodadadi A, Varank G, Kadier A, Ma PC, Madihi-Bidgoli S, Ghanbari F. Sono-peroxi-coagulation (SPC) as an effective treatment for pulp and paper wastewater: focus on pH effect, biodegradability, and toxicity. J Water Process Eng. 2021;44: 102330.
Kumar V, Shahi SK, Singh S. Bioremediation: an eco-sustainable approach for restoration of contaminated sites. Microbial bioprospecting for sustainable development, 2018, 115–136.
Azubuike CC, Chikere CB, Okpokwasili GC. Bioremediation techniques–classification based on site of application: principles, advantages, limitations, and prospects. World J Microbiol Biotechnol. 2016;32:1–18.
Kumar AK, Sharma S, Dixit G, Shah E, Patel A. Techno-economic analysis of microalgae production with simultaneous dairy effluent treatment using a pilot-scale High Volume V-shape Pond system. Renew Energy. 2020;145:1620–32.
Abdulbaki D, Al-Hindi M, Yassine A, Abou Najm M. An optimization model for the allocation of water resources. J Clean Prod. 2017;164:994–1006.
Meindersma GW, Guijt CM, De Haan AB. Desalination and water recycling by air gap membrane distillation. Desalination. 2006;187(1–3):291–301.
Singh P, Jain R, Srivastava N, Borthakur A, Pal DB, Singh R, Madhav S, Srivastava P, Tiwary D, Mishra PK. Current and emerging trends in bioremediation of petrochemical waste: a review. Crit Rev Environ Sci Technol. 2017;47(3):155–201.
Dhanarajan G, Sen R. Cost analysis of biosurfactant production from a scientist’s perspective. Biosurfactants. 2014;159:153.
Funding
The APC was funded by Tshwane University of Technology, South Africa.
Author information
Authors and Affiliations
Contributions
Conceptualization, VP and AC; methodology, RS.; data curation, AG.; writing—original draft preparation, VP; writing—review and editing, AG and NP; visualization, RS; funding acquisition, NP and BT.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pachouri, V., Chandramauli, A., Singh, R. et al. Removal of contaminants by chlorella species: an effort towards sustainable remediation. Discov Sustain 5, 19 (2024). https://doi.org/10.1007/s43621-024-00199-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43621-024-00199-1