Abstract
Methylene blue (MB) is commonly a persistent and perilous pollutant in industrial wastewater effluent. While MB has a massive negative effect on human and ecology safety. The neutralization of such harmful material would save a huge clean water amount. This work proposes the natural and abundant black sand as a catalyst for MB photodegradation. The sand sample was calcined at three temperatures of 300°, 400°, and 600 °C. The properties of samples were analyzed with different micro- as well as spectroscopic techniques. The experimental data revealed the sample's main composition of SiO2, Fe2O3, and Al2O3. XRD and XPS results proved the formation of mixed oxides composites due to calcination, especially at 400 °C. The UV–vis analysis conducted showed that samples can absorb light in the visible range. The photodegradation reached a maximum of 975.3%, 82.7%, and 75.7% for BS400, BS600, and BS300 respectively; by illumination for 2.5 h in the visible range. The observed distinguishable catalytic performance was discussed via the spectroscopic analysis and oxygen availability in the catalyst lattice. When AO, t-BuOH, and BQ are used as scavengers instead of just BQ during the photodegradation of MB dye over BS400 catalyst, the photodegradation rate drops significantly. The degradation % of MB dye diminished to 60%, and 70% by using AO, t-BuOH, and BQ scavengers; respectively. This proved that rather than hydroxyl radicals, superoxide radicals or positive hole production is the main determining step in MB photo-degradation over BS400 catalyst.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Garment dyeing is one of the essential industries in human life. However, the dramatic dyes effluent in freshwater causes serious environmental impact and health crises [1]. Around 15% of the produced dyes are wasted in the environment during the dyeing process [2]. Azo dyes, especially methylene blue (MB), has extensively applied in the textile industry [3]. This dye can be reduced to persistent carcinogenic aromatic amines in the ecosystem [4]. Additionally, the distribution of this dye into aquatic systems causes an obvious alteration in water's physical properties and ecological equilibrium [5]. Conventional technologies have failed to neutralize methylene blue pollutant in aqueous media due to its complex structure, high water solubility, and stability against temperature and chemicals. Recently, photocatalytic MB neutralization has been broadly explored as an effective methodology [6]. The advanced oxidation process (AOP) technique is economical, environmentally, efficient, and required simple equipment. AOP is an attractive process that has been intensively considered recently to decline or mineralize the dyes. This is because it is an environmentally friendly process applying the solar cell in the presence of a semiconductor for dyes removal achievement [7, 8]. Also, it is an economic process with minimal requirements to provide cleaner water that meets global legalizations [9, 10].
Especially, TiO2 has effectively been applied as a photocatalyst for MB elimination from wastewater [11]. However, titania can effectively absorb light in the UV region only accounts for 2–4% of the received sun radiation which hinders its practical application. On the contrary, Fe2O3 can highly neutralize the MB in a visible light range which represents ~ 45% of the light spectrum [11, 12]. Fe2O3 is an abundant, cheap semiconductor that has a narrow band gap (2–2.2 eV) [13] and is stable in aqueous solutions with pH > 3 [14]. However, pure Fe2O3 is generally characterized by weak conductivity accompanied by a fast e−/h+ recombination rate which hinders its industrial utilization as a photocatalyst [15]. So, coupling the Fe2O3 with a wide band gap semiconductor to influence its catalytic performance is encouraged [16].
Among several semiconductors, Fe2O3-Al2O3 coupling combines both virtues of unique alumina properties of considerable surface area (~ 100 m2/g), mechanical and thermal stability (up to 700 °C) along with Fe2O3 photoactivity in the visible range. Moreover, the doping of Fe2O3 influences the anionic dye adsorption in a wide pH range (4–9) over alumina [17]. Furthermore, doping of Fe3+ ions into Al2O3 framework enhances their structure through the produced synergetic effect due to the structure stress and hence more Lewis centers formation [18]. The incorporated Fe3+ to alumina catalyst has exhibited higher Congo red dye adsorption over the neat F2O3 and Al2O3 [19]. Also, the Fe2O3-Al2O3 nanocomposite had improved the photo desulfurization efficiency of dibenzothiophene by three folds than the pure F2O3 and Al2O3 [20]. Silica is another effective support for Fe2O3 as catalyst owing to its reputational surface area (80–160 m2/g), porous structure and stability. A Fe3+ incorporated silica exhibited a 98.5% removal of methyl red dye after 120 min [21]. Also, a premium NO conversion over Fe2O3-SiO2 nanocomposite accompanied with high selectivity of 72% to NO oxidation was achieved [22]. A Fe2O3/SiO2 photocatalyst with only 20 wt.% Fe2O3 content has considerable photocatalytic activity of 88% towards methylene blue and Congo red dyes [23].
Although the Fe2O3-Al2O3 and Fe2O3-SiO2 are highly efficient catalysts, their preparation procedures involve the application of high calcination temperature, solvents, and/or some hazardous materials. So, applying naturally occurring material that contains the Fe2O3-Al2O3 or SiO2 mixture is mostly favorable. This has manifested in the abundant black sand. It is a cheap, non-toxic material with high chemical stability. Nemours research has demonstrated the presence of silica, iron titanate, iron oxide, titania, and other oxides in small portions [24]. This valuable and particular collection of metal oxides and metals provides a useful synergetic electron transfer that delays the e−/h+ recombination and facilitates photo-activity [25]. Also, the presented Fe2TiO5 (ilmenite) has a 2.2 eV band gap which absorbs the visible range of solar spectrum as well as hematite. Where inorganics such as ilmenite and sphalerite were utilized as catalysts for chlorinated organics photo-degradation [26]. Also, the black sand as sediments was applied as a photocatalyst with high efficiency to produce H2 through the water splitting procedure [27]. Nationally, the black sand is presented along ~ 600 km on the northern coast of Egypt which makes it available for environmental and industrial applications.
In this work, the catalytic performance of black sand for the MB photo-neutralization under illumination in the visible range was investigated. The radical scavenging was investigated to understand the role of hydroxyl, superoxide radicals, and hole recombination in MB degradation. The process is simple, sustainable and cheap sand photocatalyst utility and the elimination would occur under visible irradiation, actually; it is just like a “waste by waste self-assembly process”.
2 Experimental
2.1 Materials
Catalyst samples were obtained from the sandy Beach of Abu Khashaba located on the Egyptian north coast. The methylene blue dye was delivered from Sigma-Aldrich Company as a dye for investigations.
2.2 Catalysts preparation
Three portions of the obtained black sand were calcined at three temperatures of 300°, 400°, and 600 °C for 4 h. Samples had denoted as BS300, BS400, and BS600 with respect to their calcination temperature.
2.3 Catalysts characterization
The catalyst’s crystallography and formed phases during calcination were determined by XRD analysis. The X’PertPRO PANalytical apparatus was used for investigation. It is equipped with a filter of Ni that has Cu Kα radiation with λ of 15418 nm at 0.02 s−1 rate of scanning. Raman's investigation of the photocatalysts was executed at room temperature utilizing the SENTERRA microscope provided by Burker. The apparatus is furnished with an Nd: YAG laser diode of λ = 532 nm. The spectrum has measured in the range of 10 to 2000 cm−1. The catalyst’s optical properties were determined by the UV–vis DRS technique. The spectra have measured using JASCO (Japan) model V-600, UV-spectrophotometer. The obtained data were used to calculate the band gap through the Tauc curve. The catalyst’s e−/h+ recombination proportionality was determined through the PL analysis. The measurements were operated at ambient temperature using (JASCO FP-6500, Japan) Spectrofluorometer. The composition and oxidation state of the catalyst’s surface was determined by X-ray photoelectron spectroscopy (XPS). The collected data were conducted through-ALPHA, thermo-scientific equipment with Al Kα surface analysis. A 400 μm spot size was applied at 10–9 mbar .
2.4 Catalytic performance
The experiments were carried out in a 250 ml vessel made of glass with a water-cooling system. A 0.05 g MB was desoluted in 1000 ml distilled water to prepare a 50 ppm of dye solution. Then catalyst with 0.5 g was introduced to the 100 ml of dye solution. The suspension with catalyst/dye ratio was so 5 g/l is formed. The experiment was conducted under natural pH of MB dye of 8. The suspension was then aerated using an air pump. The vessel was then loaded into a wooden box in an dark area for 60 min to attain equilibrium. Lately, the suspension was illuminated by a tungsten lamp that has > 400 nm wavelength under vigorous stirring for 150 min. The lamp has a power of 100 W. Throughout the experiment, a 5 ml Aliquot was collected at a constant time interval to be analyzed. The sample was filtered and investigated by a JANEWAY-6505 UV–visible photometer using λmax = 663 nm. The dye degradation % is calculated according to Eq. 1:
The Co is the dye starting concentration; Ct is the concentration at time t. The kinetics of photo-catalytic dye degradation was estimated according to the first-order equation illustrated in Eq. 2:
3 Results and discussion
3.1 Catalyst compositional characteristics
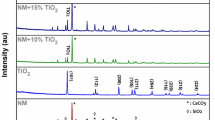
The phases and crystallography of the prepared catalysts were determined by the XRD. The data were represented in Fig. 1a. The original black sand (BS) spectra showed well-defined sharp peaks at 20.6°, and 26.2° accompanied by high-intensity sharp peaks at 38.9°. Those peaks were attributed to the (100), (101), and (102) crystalline quartz silica [28]. A peak with small intensity at 41.5° assigned to the Fe2O3 was recorded. Also, a small peak at 45.5° ascribed to Al2O3 was observed. The BS300 sample possessed peaks at 20.9° and 22.1°, 28.05°, and 38.4° for the silica [29]. The other two peaks at 35.6° and 41.6° due to the (110) and (113) miller indices of Fe2O3 were recognized. The peak at 26.8° and 51.1° are ascribed to alumina. The BS400 exhibited sharp peaks related to quartz as in the previous two samples but with lower intensity. The peaks at 35.6° and 41.6° were due to the Fe2O3 [30]. The peaks assigned to the Fe2O3 were so tiny in BS300 and BS600 while recognized in BS400. The absence or low intensity of F2O3 characteristic peaks can be due to the low Fe2O3 concentration and/or because of the nanocomposite formation of Fe2O3 with either Al2O3 or SiO2.
A tiny peek at 67.6° appeared due to alumina. The spectra of BS600 showed the same peaks as those presented in the previous samples assigned to the quartz, alumina and Fe2O3.
Figure 1b represented the Raman analyses of samples calcined at varied temperatures. The intense peak at ~ 209 and 464 cm−1 ascribed to quartz was detected in all samples. This high-intensity peak revealed the sample's silica crystallinity and phase domination. A small peak at ~ 288 cm−1 assigned to the bond Fe–O in Fe2O3 was indicated in the BS600 only.
3.2 Catalysts’ optical properties
The catalysts light sensitivity features were determined through several techniques. Where the photocatalyst's light absorption profiles were determined through the UV–vis technique, results were represented in Fig. 2a. All samples exhibited absorption edges at a wavelength higher than the 400 nm region which characterizes the visible light range. The absorbance in the visible region may be attributed to the presented Fe2O3 and alumina. All samples have possessed an intense peak at ~ 250 nm which is normally dedicated to the Fe-doped materials charge delocalization. While another broad peak in the range of 550–800 nm describes the Fe3+-loaded material transitions [31].
From Fig. 2a, the BS300, BS400, and BS600 samples can absorb visible light at 450, 425 and 410 nm respectively. Meanwhile, the BS400 spectra have exhibited a higher absorbance % than the other two samples. This can be explained by the beginning of Fe2O3 phase formation at 400 °C. The band gap for the synthesized photocatalysts was determined from the Tauc curve. The curve was derived from the Kubelka–Munk function and data were illustrated in Fig. 2b. The BS300 sample show showing intensity while the BS400 and BS600 have a relatively lower intensity. The band gap values were 1.9, 1.75, and 1.55 eV for BS600, BS300, and BS400 samples respectively. The catalysts e−/h+ recombination proportion was determined by the PL analysis using λ = 325 nm. The outcoming data were displayed in Fig. 2c. All samples possessed a narrow peak in the range of 495–505 nm. This observation indicated the presence of induced oxygen lattice deficiency [32]. BS300 showed an emission peak at 498 nm while the BS400 and BS600 possessed peaks at 500 nm.
3.3 XPS analysis
The oxidation state and surface chemical configuration of the samples calcined at three calcination temperatures were examined by XPS analysis. The results were collected and illustrated in Table 1 and Fig. 3. As indicated in Table 1. The samples mainly consist of Al2O3, SiO2 and Fe2O3 as mixed oxides. The table shows that the percentage of oxygen in BS400 is less than the other two samples. While, the surface atomic Si% was increased with increasing the calcination temperature. Those results reflect that sample was composed of Fe-Al composite that impregnated or finely surface dispersed in silica. Additionally, all those observations together confirm the existence of oxygen deficiency in BS400 which would influence its photoactivity. In addition, the indicated results proved further the XRD results of Fe-Al composite formation. Meanwhile, most of elements percent is much higher in the case of BS600. This can be explained via the segregation of Fe2O3 and alumina onto the silica surface as raising the calcination temperature up to 600 °C. Reportedly, Fe atoms tends to segregate from the composites structure to form a more thermodynamically stable Fe2O3 by calcination at temperatures ≥ 600 °C and/or higher Fe2O3 content than 30% [20, 33,34,35].
Figure 3 generally indicated that as the sample calcination temperature increased as the identified peaks for all elements were shifted to higher binding energy and become more broadening. This result can be due to the higher metal oxide interaction [36]. Meanwhile, the peaks shift is considerably higher in case of BS400 than the BS600 sample. In addition, the recognized peaks have a distinguishable higher intensity as the sample calcination temperature was increased. This observation indicated a higher surface metals content due to the calcination temperature.
The Al 2p spectra have shown a broad beak in range of 72.5–76 eV. The peak deconvolution possesses two peaks at a binding energy of 74.5 and 75.8 eV related to Al3+ in Al2O3 and Al(OH)3 respectively [37]. While, the peak due to the Al(OH) is more highly recognized in the BS600 spectrum than the BS400. This confirms the influenced surface structure of the second sample. The Si 2p fig. displayed a wide peak at around 102.5 eV. The peak can be separated into 3 main peaks at 102.4, 102.7 and 103.0 eV due to the Si4+ in Si–Si-O, Si 2p3/2 orbital spins as well as Si 2p1/2 in SiO2 respectively [38]. Clearly, the recorded peak values have a red shift than common values in literature. The observation can be due to the SiO2 interaction with other oxides as a solid solution [20]. The peak at 102.4 eV is absent in the BS400 sample spectrum which indicates that all Si atoms are located as SiO2 while no Si–Si interaction was found in this sample. This observation also indicated the higher acidity of the BS400 sample.
The Fe 2p core level spectra showed two extreme intense peaks at 712.9 and 726.1 eV due to Fe 2p3/2 and Fe 2p1/2 orbital-doublet spin respectively. Other two relatively tiny peaks at 719.2 and 733.3 were assigned to the Fe shake-up satellite. Those results imply the existence of Fe3+ ions in all samples [39]. The O 1 s core level spectra of formed samples have exhibited a broad peak that deconvoluted to 3 main peaks at 530.4, 532.2 and 533.6 eV. The peaks describe the lattice oxygen, M–O interaction, and surface adsorbed O species respectively. As indicated, the BS400 and BS600 samples exhibited lower intensity for the lattice oxygen peak which indicate the oxygen deficiency in those samples. The BS400 showed an additional peak at 434.1 eV which can assigned to the adsorbed H2O molecules [40].
3.4 Photocatalytic activity
As shown in the previous characterization techniques the photocatalyst composite formed by calcination of black sand at three different temperature is Fe2O3/Al2O3/SiO2 with various metal ratios.
The catalytic performance of the black sand was tested towered 50 ppm of MB degradation under visible light for 150 min of irradiation time. The photodegradation of 100 ml of MB dye using 0.5 gm of different calcined black sand BS300, BS400, and BS600 are shown in Fig. 4. Initially; the experimental was operated in dark to study the effect of dye adsorption on the catalyst surface of composite, whereas the composite showed best adsorption capacity was most photoactivity, when exposed to light, promote chemical reactions that break down the pollutants into harmless products [41, 42]. Where the adsorption ability of MB dye pollutant over black sand series can rank as BS400 > BS600 > BS300. As represented in Fig. 4 the photodegradation reached the maximum of 95.3%, 82.7%, and 75.7% for BS400, BS600, and BS300 respectively; after 150 min under visible light. According to Eq. 2; the apparent first order of the generated photocatalysts under visible illumination is 0.0154, 0.0121, and 0 0.00052 min−1 for BS400, BS600, and BS300 respectively.
The super photoactivity of the BS400 sample correlated to its physical properties like highest adsorption capacity towards MB dye due to the formation of crystalline silica (SiO2) which inspire the acidity of the BS400 sample; as shown in XPS analysis. The acidity of the catalyst can promote the adsorption and the reactants activation over the catalyst surface through the generation of reactive intermediates or can facilitate proton transfer steps, leading to increased photoactivity [43, 44]. The peaks assigned to the Fe2O3 were so tiny in BS300 and BS600 while recognized in BS400; as confirmed from XRD and XPS analysis. Also, XPS confirmed that the surface metal content and interaction are highest observed in the BS400 sample. Also; the low band gap value of 1.55 Ev for BS400 comparison to 1.9, 1.75 for BS600, and BS300, respectively increase its response under visible light [45]. XPS analysis confirmed the presence of adsorbed H2O molecules; adsorbed water can enhance the photoactivity of the catalyst by facilitating the charge transfer and reducing e−/h+ recombination [46, 47]. Water can act as electron traps and inhibit charge transfer processes [48].
In order to discover the role of hydroxyl radicals (•OH), hole formation, and over oxide radicals (O2•−), and in MB degradation, scavengers such as tertiary-butanol (t-BuOH), 1,4-benzoquinone (BQ), and ammonium oxalate (AO), were used respectively [46, 49]. The scavenger has declined the degradation efficiency, confirming that the radical was exclusively in charge for dye degradation. Figure 5 shows the extremely efficient MB photo-neutralization using BS400 sample in the absence and presence of scavengers. The photodegradation rate decreases dramatically when using AO, and t-BuOH as scavenger then and BQ; as shown in Fig. 5. This proved the positive hole formation responsibility as main reactive species in the MB degradation over BS400 catalysts rather than hydroxyl radical and superoxide radicals.
In general; the photodegradation mechanism under visible light in the presence of Fe2O3/Al2O3/SiO2 composite proceeds in the continued steps; as shown in Scheme 1. The absorption of light energy by Fe2O3 particles can result in its electron's excitation to upper energy levels. This can generate electrons and holes (e−/h+) in conduction and valence bands respectively. The existing surface Fe3+ ions act as electron acceptor or maybe as hole donor to versatile delocalizes the charge carrier and hence delay the e−/h+ combination. This occurred as a result of electrons trapping at energy levels near the conduction or valence bands, respectively [43, 47, 49]. The presence of e−/h+ radicals, which are highly reactive species that can initiate chain reactions that break down the MB dye to form less stable and more prone to degradation compounds. The photo-induced electrons (e−Cb) can react with the catalyst surface adsorbed O2 acceptors or be reduced via dissolute into water, it to superoxide radical anion O2−. The photogenerated holes (h+Vb) can react with OH− into H2O to form the hydroxyl radicals (OH•), and peroxide radicals or HO2• radicals. The created extreme oxidant species are reported for efficient MB photodecomposition over BS400 catalyst; as confirmed by scavenger experiment.
To further ensure the catalyst efficiency the catalyst stability and reusability were investigated. The collected data were interpreted and represented in Fig. 6. Figure 6a depicted the slight decline in degradation% as, 14.6%, 5.3%, and 9.6% for BS300, BS400, and BS 600 respectively. As indicated from fig., the catalysts were relatively active up to 8 h of working time. While, the BS400 exhibited the higher activity and smallest decrease in degradation% along the investigated catalysts.
Figure 6b. implies the ability of the applied catalysts in this study to survive along 6 cycles of MB photodegradation. All catalysts have showed considerable activity toward the degradation process with highly acceptable percentages. Among the investigated catalysts, the BS400 had the lowest degradation% of about 6%. All those observations indicated the high catalytic performance of the applied catalysts and their considerable reusability and sustainability.
For more convenience the efficiency of the utilized catalyst BS400 was correlated to some other catalysts stated in literature for MB photodegradation. The comparisons were illustrated in Table 2. For clearer and fair data analysis, the photodegradation conditions were also represented in the table. The BS400 can achieve an extreme MB degradation by applying the visible light. Despite BS400 being a naturally occurring material with bulk size, it exhibited a close degradation present to the catalysts in nanoscale which prepared with complicated or multi-step procedures. In addition, the comparable activity of BS400 was attained although the application of high concentration of 8 ppm. The BS400 also exhibited a higher MB removal by ~ 2% over the 6% Pt-CeO2 catalyst which was prepared by hydrothermal methods as in [50]. This indicated that applying of black sand with calcination at only 400° C can produce a comparable result to nanoscale noble metal-based catalysts. Table 2 also indicated that the BS400 can achieve a similar MB degradation to that measured for 20%SnO-AC at the same conditions while applying a smaller operation time [51]. The comparison is to be said in favor of the BS400 catalyst in a penalty of point of view. Where it is natural and abundant material with high activity toward MB degradation.
4 Conclusion
The Abu Khashaba Beach Area on the north coast of Egypt is where the black sand was gathered. The obtained black sand was divided into three sections and calcined for four hours at temperatures of 300, 400, and 600 °C. According to XRD analyses and Raman spectra of three calcined samples, the original black sand (BS) spectra had well-defined sharp peaks due to the crystalline quartz silica. In the XRD examination, there were additional two faint peaks that belonged to Fe2O3 and Al2O3. The produced photocatalysts showed absorption edges at wavelengths above the 400 nm range, which was attributed to the Fe2O3 and Al2O3 that were present. All samples had a narrow peak in the 495–505 nm range in the PL analysis. This finding demonstrated the existence of a lattice defect brought on by an oxygen shortage. The band gap values for the BS600, BS300, and BS400 samples were 1.9, 1.75, and 1.55 eV, respectively. The samples' predominant oxide composition was confirmed by XPS analysis to be a mixture of Al2O3, SiO2, and Fe2O3. In addition, as the sample calcination temperature was raised, the recognised peaks showed a discernible higher intensity. This finding suggested that the increased surface metal concentration was caused by the higher calcination temperature.
The photo-reactivity of the black sand was investigated towered 50 ppm MB elimination under visible ranged luminance. The degradation had reached maxima of 95.3%, 82.7%, and 75.7% after 150 min visible light irradiation for BS400, BS600, and BS300 respectively.
The super performance of the BS400 sample was associated with its physical characteristics, including its ability to adsorb MB dye and the acidity of the catalyst. Additionally, XPS indicated that the BS400 sample had the highest surface metal concentration and interaction. Lowest band gap value, which improves responsiveness to visible light. Although water can operate as an electron trap and obstruct charge transfer activities, XPS examination showed the existence of adsorbed H2O molecules, which is realistically advantageous for photocatalytic reactions that include water splitting or the oxidation of organic compounds. The primary reactive species in the photocatalytic elimination of MB over BS400 catalyst is the creation of positive holes.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.
References
Rauf MA, Ashraf SS. Fundamental principles and application of heterogeneous photocatalytic degradation of dyes in solution. Chem Eng J. 2009;151:10–8.
Houas A, Lachheb H, Ksibi M, Elaloui E, Guillard C, Herrmann J-M. Photocatalytic degradation pathway of methylene blue in water. Appl Catal B. 2001;31:145–57.
Oladoye PO, Ajiboye TO, Omotola EO, Oyewola OJ. Methylene blue dye: toxicity and potential elimination technology from wastewater. Results Eng. 2022;16: 100678.
Huang J, Liu Y, Jin Q, Wang X, Yang J. Adsorption studies of a water soluble dye, Reactive Red MF-3B, using sonication-surfactant-modified attapulgite clay. J Hazard Mater. 2007;143:541–8.
Saadoon SJ, Jarosova M, Machek P, Kadhim MM, Ali MH, Khalaji AD. Methylene blue photodegradation using as-synthesized CeO2 nanoparticles. J Chin Chem Soc. 2022;69:280–8.
Pouretedal HR, Kadkhodaie A. Synthetic CeO2 nanoparticle catalysis of methylene blue photodegradation: kinetics and mechanism. Chin J Catal. 2010;31:1328–34.
Oviedo LR, Muraro PCL, Pavoski G, Espinosa DCR, Ruiz YPM, Galembeck A, Rhoden CRB, da Silva WL. Synthesis and characterization of nanozeolite from (agro) industrial waste for application in heterogeneous photocatalysis. Environ Sci Pollut Res. 2022;29:3794–807.
Da Silva WL, Lansarin MA, dos Santos JH, Da Rocha ZN, Pepe IM. Electrochemical and catalytic studies of a supported photocatalyst produced from petrochemical residue in the photocatalytic degradation of dexamethasone and guaifenesin drugs. Water Air Soil Pollut. 2016;227:1–9.
da Silva WL, BrC L, Ziulkoski AL, van Leeuwen PWNM, dos Santos JoHZ, Schrekker HS. Petrochemical residue-derived silica-supported titania-magnesium catalysts for the photocatalytic degradation of imidazolium ionic liquids in water. Separat Purific Technol. 2019;218:191–9.
Silva WL, Lansarin MA, Moro CC. Synthesis, characterization and photocatalytic activity of nanostructured TiO2 catalysts doped with metals. Quim Nova. 2013;36:382–6.
Chen W, Xiao H, Xu H, Ding T, Gu Y. Photodegradation of methylene blue by TiO2-Fe3O4-bentonite magnetic nanocomposite. Int J Photoenergy. 2015. https://doi.org/10.1155/2015/591428.
Zhang R, Du B, Li Q, Cao Z, Feng G, Wang X. α-Fe2O3 nanoclusters confined into UiO-66 for efficient visible-light photodegradation performance. Appl Surf Sci. 2019;466:956–63.
Dai X, Lu G, Hu Y, Xie X, Wang X, Sun J. Reversible redox behavior of Fe2O3/TiO2 composites in the gaseous photodegradation process. Ceram Int. 2019;45:13187–92.
Noruozi A, Nezamzadeh-Ejhieh A. Preparation, characterization, and investigation of the catalytic property of α-Fe2O3-ZnO nanoparticles in the photodegradation and mineralization of methylene blue. Chem Phys Lett. 2020;752: 137587.
Mishra M, Chun D-M. α-Fe2O3 as a photocatalytic material: a review. Appl Catal A. 2015;498:126–41.
Akhavan O. Thickness dependent activity of nanostructured TiO2/α-Fe2O3 photocatalyst thin films. Appl Surf Sci. 2010;257:1724–8.
Jiang Y, Mao Q, Ma T, Liu X, Li Y, Ren S, Sun J. Facile preparation of Fe2O3 Al2O3 composite with excellent adsorption properties towards Congo red. Ceram Int. 2021;47:13884–94.
Gulshan F, Kameshima Y, Nakajima A, Okada K. Preparation of alumina–iron oxide compounds by gel evaporation method and its simultaneous uptake properties for Ni2+, NH4+ and H2PO4−. J Hazard Mater. 2009;169:697–702.
Mahapatra A, Mishra BG, Hota G. Adsorptive removal of Congo red dye from wastewater by mixed iron oxide–alumina nanocomposites. Ceram Int. 2013;39:5443–51.
Ali AO, El Naggar AM, Morshedy AS, Aboutaleb WA, Metwally NH. Synthesis and characterization of iron-alumina composites as novel efficient photocatalysts for removal of DBT. Chemosphere. 2022;307: 136011.
Saghi M, Shokri A, Arastehnodeh A, Khazaeinejad M, Nozari A. The photo degradation of methyl red in aqueous solutions by α-Fe2O3/SiO2 nano photocatalyst. J Nanoanal. 2018;5:163–70.
Balbuena J, Cruz-Yusta M, Pastor A, Sánchez L. α-Fe2O3/SiO2 composites for the enhanced photocatalytic NO oxidation. J Alloys Comp. 2018;735:1553–61.
Mandal S, Adhikari S, Pu S, Wang X, Kim D-H, Patel RK. Interactive Fe2O3/porous SiO2 nanospheres for photocatalytic degradation of organic pollutants: Kinetic and mechanistic approach. Chemosphere. 2019;234:596–607.
Mahmoud HH, Abdel-Lateef AM, Attiah AM. Distribution of some elements in the Egyptian black sands from Abu Khashaba beach area. JASMI. 2013. https://doi.org/10.4236/jasmi.2013.31007.
Moniz SJA, Shevlin SA, An X, Guo ZX, Tang J. Fe2O3-TiO2 nanocomposites for enhanced charge separation and photocatalytic activity. Chem A European J. 2014;20:15571–9.
Ping TAN, Hu H-P, Zhang L. Effects of mechanical activation and oxidation-reduction on hydrochloric acid leaching of Panxi ilmenite concentration. Trans Nonferrous Metals Soc China. 2011;21:1414–21.
López-Vásquez A, López-Suárez FE. Black Sand-based photocatalyst for hydrogen production from EDTA solutions under UV-Vis irradiation. Topics Catal. 2020;63:1325–35.
Zhang C, Xu Z, Hu Y, He J, Tian M, Zhou J, Zhou Q, Chen S, Chen D, Chen P. Novel insights into the hydroxylation behaviors of α-quartz (101) surface and its effects on the adsorption of sodium oleate. Minerals. 2019;9:450.
Chisholm JIM. Comparison of quartz standards for X-ray diffraction analysis: HSE A9950 (Sikron F600) and NIST SRM 1878. Ann Occup Hyg. 2005;49:351–8.
Yazirin C, Puspitasari P, Sasongko MIN, Tsamroh DI, Risdanareni P. Phase identification and morphology study of hematite (Fe2O3) with sintering time varitions. AIP Conference Proceedings vol. 1887: AIP Publishing LLC; 2017. p. 020038.
Adhlakha N, Yadav KL. Structural, dielectric, magnetic, and optical properties of Ni 0.75 Zn 0.25 Fe 2 O 4-BiFeO 3 composites. J Mat Sci. 2014;49:4423–38.
Corma A, Atienzar P, Garcia H, Chane-Ching J-Y. Hierarchically mesostructured doped CeO2 with potential for solar-cell use. Nat Mater. 2004;3:394–7.
Gobara HM, Aboutaleb WA, Hashem KM, Hassan SA, Henein SA. A novel route for synthesis of α-Fe 2 O 3–CeO 2 nanocomposites for ethanol conversion. J Mater Sci. 2017;52:550–68.
Liu R, Gao N, Zhen F, Zhang Y, Mei L, Zeng X. Doping effect of Al2O3 and CeO2 on Fe2O3 support for gold catalyst in CO oxidation at low-temperature. Chem Eng J. 2013;225:245–53.
Li K, Wang H, Wei Y, Yan D. Syngas production from methane and air via a redox process using Ce–Fe mixed oxides as oxygen carriers. Appl Catal B. 2010;97:361–72.
Abdalla ZEA, Li B, Tufail A. Direct synthesis of mesoporous (C19H42N) 4H3 (PW11O39)/SiO2 and its catalytic performance in oxidative desulfurization. Colloids Surf, A. 2009;341:86–92.
El-Deeb ZM, Aboutaleb WA, Dhmees AS, El Naggar AMA, Emara K, Elgendy AT, Ahmed AI. Bio-fuels production through waste tires pyrolytic oil upgrading over Ni-W/zeolite composites derived from blast furnace slag. Int J Energy Res. 2022;46:17376–90.
Ma H-P, Yang J-H, Yang J-G, Zhu L-Y, Huang W, Yuan G-J, Feng J-J, Jen T-C, Lu H-L. Systematic study of the SiOx film with different stoichiometry by plasma-enhanced atomic layer deposition and its application in SiOx/SiO2 super-lattice. Nanomaterials. 2019;9:55.
Yamashita T, Hayes P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl Surf Sci. 2008;254:2441–9.
Shu-Ping W, Xiu-Cheng Z, Xiao-Ying W, Wang S-R, Shou-Min Z, Li-Hua Y, Huang W-P, Shi-Hua W. Comparison of CuO/Ce 0.8 Zr 0.2 O 2 and CuO/CeO 2 catalysts for low-temperature CO oxidation. Catal Lett. 2005;105:163–8.
El-Salamony RA, Morsi RE. Stable gallium oxide@ silica/polyvinyl pyrrolidone hybrid nanofluids: preparation, characterization, and photo-activity toward removal of malachite green dye. J Mol Liq. 2018;271:589–98.
El-Salamony RA, Gobara HM, Younis SA, Moustafa YM. Zn+ 2-doped x-Ti–SiO 2 tricomposites for enhancement the photo-catalytic degradation of phenol under UV irradiation. J Sol-Gel Sci Technol. 2017;83:422–35.
Aboutaleb WA, El-Salamony RA. Effect of Fe2O3-CeO2 nanocomposite synthesis method on the Congo red dye photodegradation under visible light irradiation. Mater Chem Phys. 2019;236: 121724.
Younis SA, Amdeha E, El-Salamony RA. Enhanced removal of p-nitrophenol by êžµ-Ga2O3-TiO2 photocatalyst immobilized onto rice straw-based SiO2 via factorial optimization of the synergy between adsorption and photocatalysis. J Environ Chem Eng. 2021;9: 104619.
El-Salamony RA, Amdeha E, Ghoneim SA, Badawy NA, Salem KM, Al-Sabagh AM. Titania modified activated carbon prepared from sugarcane bagasse: adsorption and photocatalytic degradation of methylene blue under visible light irradiation. Environ Technol. 2017;38:3122–36.
Amdeha E, El-Salamony RA, Al-Sabagh AM. Enhancing the photocatalytic activity of Ga2O3–TiO2 nanocomposites using sonication amplitudes for the degradation of Rhodamine B dye. Appl Organomet Chem. 2020;34:e5336.
El-Salamony RA, Aboutaleb WA, Dhmees AS. Photodegradation of amido black 10b dye under visible light using ni and zn ferrite catalysts prepared by a simple modified Sol–Gel method. Arabian J Sci Eng. 2023. https://doi.org/10.1007/s13369-023-07676-1.
Srimuk P, Su X, Yoon J, Aurbach D, Presser V. Charge-transfer materials for electrochemical water desalination, ion separation and the recovery of elements. Nat Rev Mater. 2020;5:517–38.
El-Salamony RA, Aboutaleb WA. Preparation, characterization of green synthesis ceo2 nanoparticles and their photocatalytic activity towards malachite green dye. ChemistrySelect. 2022;7:e202103722.
Shajahan S, Arumugam P, Rajendran R, Munusamy AP. Optimization and detailed stability study on Pb doped ceria nanocubes for enhanced photodegradation of several anionic and cationic organic pollutants. Arab J Chem. 2020;13:1309–22.
El-Salamony RA, Amdeha E, Badawy NA, Ghoneim SA, Al-Sabagh AM. Visible light sensitive activated carbon-metal oxide (TiO2, WO3, NiO, and SnO) nano-catalysts for photo-degradation of methylene blue: a comparative study. Toxicol Environ Chem. 2018;100:143–56.
Asl EA, Haghighi M, Talati A. Sono-solvothermal fabrication of flowerlike Bi7O9I3-MgAl2O4 pn nano-heterostructure photocatalyst with enhanced solar-light-driven degradation of methylene blue. Sol Energy. 2019;184:426–39.
Fiaz M, Sohail M, Nafady A, Will G, Wahab MA. A facile two-step hydrothermal preparation of 2D/2D heterostructure of Bi2WO6/WS2 for the efficient photodegradation of methylene blue under sunlight. Environ Res. 2023;234: 116550.
Waseem S, Sittar T, Kayani ZN, Gillani SSA, Rafique M, Nawaz MA, Shaheen SM, Assiri MA. Plant mediated green synthesis of zinc oxide nanoparticles using Citrus jambhiri lushi leaves extract for photodegradation of methylene blue dye. Physica B. 2023;663: 415005.
Younis A, Chu D, Kaneti YV, Li S. Tuning the surface oxygen concentration of 111 surrounded ceria nanocrystals for enhanced photocatalytic activities. Nanoscale. 2016;8:378–87.
Alberoni C, Barroso-Martín I, Infantes-Molina A, Rodríguez-Castellón E, Talon A, Zhao H, You S, Vomiero A, Moretti E. Ceria doping boosts methylene blue photodegradation in titania nanostructures. Mat Chem Front. 2021;5:4138–52.
Ulfa M, Pertiwi YE, Saraswati TE, Bahruji H, Holilah H. Synthesis of iron triad metals-modified graphitic mesoporous carbon for methylene blue photodegradation. South African J Chem Eng. 2023. https://doi.org/10.1016/j.sajce.2023.05.008.
Acknowledgements
This work was supported by the labs of the Egyptian Petroleum Research Institute (EPRI).
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. This work was supported by the labs of the Egyptian Petroleum Research Institute (EPRI).
Author information
Authors and Affiliations
Contributions
Dr. R, Dr. I and Dr. A collect the black sand sample, characterized them and prepared the Figs. 1–3. Dr. Wael prepared samples and write the characterisation section. Prof. Dr. Radwa did the photo experiments, prepared the Figs. 4, 5, scheme 1, and write the photoactivity section.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aboutaleb, W.A., Nassar, I.M., Mahmoud, R.M. et al. Black sand as a cost-effective catalyst for methylene blue photo-remediation under visible light. Discov Sustain 4, 39 (2023). https://doi.org/10.1007/s43621-023-00157-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43621-023-00157-3