Abstract
Introduction
There are currently no standardised guidelines on whether antibiotic prophylaxis is required for Kirschner wire (K-wire) fixation to minimise the risk of surgical site infection when used in patients undergoing clean orthopaedic surgery.
Purpose
To compare the outcomes of antibiotic prophylaxis versus no antibiotic in K-wire fixation when used in either in trauma or elective orthopaedics.
Methods
A systematic review and meta-analysis were performed as per the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) Guidelines and a search of electronic information was conducted to identify all randomised controlled trials (RCTs) and non-randomised studies comparing the outcomes of antibiotic prophylaxis group versus those without antibiotic in patients undergoing orthopaedic surgery in which K-wire fixation was used. Incidence of surgical site infection (SSI) was the primary outcome. Random effects modelling was used for the analysis.
Results
Four retrospective cohort studies and one RCT were identified with a total of 2316 patients. There was no significant difference between the prophylactic antibiotic and no antibiotic groups in terms of incidence of SSI (odds ratio [OR] = 0.72, P = 0.18).
Conclusions
There is no significant difference in administering peri-operative antibiotics for patients undergoing orthopaedic surgery using K-wire.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Kirschner (K) wires are commonly used in orthopaedic surgery for fixation of unstable fractures such as supracondylar humerus fractures in paediatric patients [1], distal radius fractures in paediatric and adult patients [2] and elective surgery of the foot. They can be inserted percutaneously with minimal damage to soft tissues such as tendons and neurovascular structures, followed by cutting and bending of the wires [3, 4]. The wires are then either left protruding outside the skin or buried. The limb is immobilised with either a splint or cast [5, 6].
There are associated complications related to the use of K-wire; one being pin-tract infections with an incidence rate ranging between 1.9 and 34% in hand and wrist fractures [5]. Superficial infections may respond to regular change in dressing or a course of oral antibiotics. In more severe cases pin removal may be required which can be problematic if the fracture has not united. More serious infections including osteomyelitis may require hospital admission for intravenous antibiotics or a further operation [2, 5].
While there are published guidelines relating to the use of peri-operative antibiotics for orthopaedic surgery, currently, there is no specific guidance for surgery using K-wire. The National Institute for Health and Care Excellence (NICE) guidelines recommends the use of antibiotic prophylaxis before operations that include clean wounds with prosthesis or implant insertion as well as contaminated and clean-contaminated surgical incisions [7]. Additionally, Scottish Intercollegiate Guidelines Network (SIGN) recommend antibiotic prophylaxis to be administered in orthopaedic surgery if the procedure involves an insertion of a prosthetic device [8]. The guidelines also recommend prophylactic antibiotics whilst carrying out an open reduction of a closed fracture. However, these guidelines do not specify whether this recommendation is applicable for percutaneously inserted K-wire for closed fractures.
Some authors have suggested the use of post-operative antibiotics; however, this is not routine practice in the UK [9]. The use of prophylactic antibiotics post-operatively has been explored in previous studies. A retrospective study including 618 patients has shown that post-operative antibiotics did not lower the incidence of surgical site infection (SSI): 1.7% with antibiotic use compared to 1.8% for no antibiotic group [9].
Interestingly, with regards to peri-operative antibiotic use some authors have argued there is a low overall SSI risk following K-wire fixation; therefore, peri-operative antibiotics are not indicated [10]. However, in other studies patients who were not given peri-operative prophylaxis had a higher rate of post-operative infection [11].
Overall, there is no consensus or clear guidance on the administration of antibiotics for K-wire. Reducing the incidence of SSI needs to be balanced with potential risks of the unnecessary use of antibiotics, such as antibiotic resistance and allergic reaction. Currently, there are no systematic reviews or meta-analyses that investigate the use of prophylactic antibiotics for orthopaedic surgery using K-wire. The aim of the study therefore is to investigate incidence of SSI using prophylactic antibiotics compared to no antibiotics in the context of percutaneous fixation of fractures with K-wire.
Methods
A systematic review and meta-analysis was conducted as per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [12].
Eligibility Criteria
All randomised control trials and observational studies investigating the use of a prophylactic antibiotics group with a comparator for patients with fractures of the upper (e.g. supracondylar fractures of the humerus, distal radius fracture) or lower extremity (e.g. toe fractures) requiring K-wire fixation were eligible for inclusion. The group without prophylactic antibiotic was the intervention group of interest and the group with prophylactic antibiotic was the comparator. No age, gender or morbidity status restrictions were used but for language, restrictions were set to English only. Cases reports and observational studies with no comparator were excluded.
Outcome Measures
Incidence of SSI is the primary outcome which was assessed between the two groups. No secondary outcomes were identified.
Literature Search Strategy
Two authors M.K. and A.A independently searched the following electronic databases: MEDLINE, EMBASE, EMCARE, CINAHL, and the Cochrane Central Register of Controlled Trials (CENTRAL). The last search was run on 10th January 2022. Thesaurus headings, search operators, and limits in each of the above databases were adapted accordingly. In addition, World Health Organization International Clinical Trials Registry (http://apps.who.int/trialsearch/), ClinicalTrials.gov (http://clinical-trials.gov/), and ISRCTN Register (http://www.isrctn.com/) were searched for details of ongoing and unpublished studies. No language restrictions were applied in our search strategies. The search terminologies included: “Kirschner wires”, “K-wires”, "Percutaneous wires", “Antibiotic Prophylaxis”, “Wound Infection” and “Surgical Site Infection”. The bibliographic lists of relevant articles were also reviewed.
Selection of Studies
The title and abstract of articles identified from the literature searches were assessed independently by two authors (A.A. and M.K.). The full texts of relevant reports were retrieved and those articles that met the eligibility criteria of our review were selected. Any discrepancies in study selection were resolved by discussion between the authors.
Data Extraction and Management
An electronic data extraction spreadsheet was created in line with Cochrane’s data collection form for intervention reviews. The spreadsheet was pilot tested in randomly selected articles and adjusted accordingly. Our data extraction spreadsheet included study-related data (first author, year of publication, country of origin of the corresponding author, journal in which the study was published, study design, study size, clinical condition of the study participants, type of intervention, and comparison), baseline demographics of the included populations (age and gender) and primary outcome data. The authors A.A. and M.K. cooperatively collected and recorded the results and any disagreements were solved via discussion.
Data Synthesis
Data synthesis was conducted using the Review Manager 5.3 software. The extracted data was entered into Review Manager by two independent authors (A.A. and M.K.). The analysis used was based on the random effect model. The results were reported in forest plots with 95% confidence intervals (CIs).
For dichotomous outcomes, the odds ratio (OR) was calculated between the two groups. The OR is the risk of an event in the group receiving prophylactic antibiotic compared with the group without antibiotic. An OR of > 1 for the SSI would favour the prophylactic antibiotic group, an OR of < 1 would favour the group without antibiotic and an OR of 1 would favour neither group.
Assessment of Heterogeneity
Heterogeneity among the studies was assessed using the Cochran Q test (χ2). Inconsistency was quantified by calculating I2 and interpreted using the following guide: 0–25% represents low heterogeneity, 25–75% moderate heterogeneity, and 75–100% high heterogeneity.
Methodological Quality and Risk of Bias Assessment
The Cochrane Collaboration’s Tool was used to assess the quality of the RCTs included in the study (Table 2). For non-randomised studies, the Newcastle–Ottawa scale [13] was used to assess its quality which offers a star system for analysis (Table 3). It offers a maximum score of nine stars across three domains including selection, comparability and exposure. It offers a maximum score of nine stars across three domains including selection, comparability and exposure. The overall rating of either good, fair or poor quality was based on the Agency for Healthcare Research and Quality (AHRQ) standards [13]. This was assessed by two authors (M.K and A.A) and a third author; S.A.S was used as an adjudicator if consensus was required.
Results
Literature Search Results
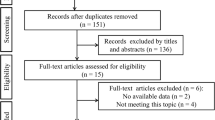
Our search strategy retrieved 181 studies and after a thorough screening of the retrieved articles, the authors identified five studies [5, 6, 14,15,16] in total which met the eligibility criteria (Fig. 1).
Description of Studies
Baseline characteristics of the included studies are summarised in Table 1. This includes the percentage SSI of the cohort for each of these studies.
Primary Outcome(s)
Incidence of SSI
The incidence of SSI was reported in four studies, enrolling a total of 2316 patients (Fig. 2). There was no significant difference between the prophylactic antibiotic and no antibiotic groups in terms of incidence of SSI (odds ratio (OR) = 0.72, confidence interval (CI) = 0.45–1.16, P = 0.16). A low level of heterogeneity was found amongst the studies (I2 = 0%, P = 0.42).
Methodological Quality and Risk of Bias Assessment
The Cochrane Collaboration’s Tool was used to assess the quality of the Mangwani et al. [16] showing an overall low risk of bias (Table 2). For non-randomised studies, the Newcastle–Ottawa Scale [13] was used to assess the quality of the four non-randomised studies (Table 3), which offers a star system for analysis. All four studies demonstrated a high selection and exposure but a low comparability score [5, 6, 13, 14]. Overall, all studies, except for van Leeuwen [5], were of good quality based on the AHRQ standards [13].
Discussion
This study demonstrated that there was no significant difference (OR 0.72, P = 0.18) in the incidence of reported SSI in patients undergoing K-wire fixation who receive prophylactic antibiotics when compared to patients who did not receive antibiotic prophylaxis. However, there is a slight trend that is in favour towards patients who received prophylactic antibiotics as seen in Fig. 1.
Cohort studies without comparators support this finding, in that the incidence of SSI reported across studies is similar whether antibiotics were used or not. For example, Schroeder et al. reported an SSI of 1.8% (11/618) in their cohort study in which all infected cases received pre-operative antibiotic [9]. Subramanian et al. reported on 100 patients (173 unburied K-wires) inserted without receiving prophylactic antibiotics with only 2% infection rate [17]. Studies concentrating on post-operative antibiotics found no significant link in the use of antibiotic therapy and the development of SSI. [9, 18].
Previous studies have elaborated on the importance of mechanism of action for antibiotic prophylaxis used in surgery [19]. The antibiotics used are bactericidal meaning they kill rather than prevent further growth of bacteria. However, concentrations of antibiotics seem to affect their properties, for example clindamycin being bacteriostatic at lower concentrations and bactericidal at higher concentrations [20]. Although in our study, peri-operative antibiotics are seen to be ineffective in reducing infection rate, other elective procedures such as hip surgery have much better success rates in the use of peri-operative antibiotics [21] which may be related to concentrations of antibiotics used as well as local antibiotics (in cement etc.).
Resistance to antimicrobials is becoming increasingly prevalent [2] which raises concerns when unnecessary antibiotics are used routinely. Routine administration of antibiotics has also been linked to other complications, for example, enterotoxaemia due to Clostridium difficile and the development of adverse reactions (22). Reduction of routine administration of antimicrobials may be cost efficient due to the overall reduction in healthcare costs [23]. It is therefore imperative to weigh the advantages and disadvantages of the prophylactic use of antibiotics.
A systematic approach was used in this review to provide a summary of the best available evidence and to assess the risk of bias of relevant retrospective studies [5, 6, 14,15,16]. With regard to the between-study heterogeneity, it was low (I2 = 0%). Based on the design and included population, the one RCT [16] and four non-randomised studies [5, 6, 13, 14] were standardised. All of these would make the conclusions of this study robust from the best available evidence.
Nevertheless, the reported outcomes of the current review should be studied in the context of inherent limitations. Only five studies were identified enrolling 2316 participants, which may not be sufficient for definite conclusions, exposing the findings to a potential type two error. Additionally, due to the limited number of studies included and the data available, it was not possible to perform a sensitivity analysis to separate the RCT study from the observational studies and which further weakens our analysis. The gap in sample sizes between the individual studies may have affected the analysis, such as van Leeuwen et al. [5] with a sample of 1213 participants compared to Heras et al. [14] with a sample of only 77 subjects. Additionally, Mangwani et al. [16] was the only RCT found to be included in the study which might limit the overall conclusions due to high selection bias of the retrospective studies. Furthermore, different studies had different approaches in K-wire fixation (either buried or exposed) which could have affected infection rates.
Noteworthy, a survey of Orthopaedic surgeons demonstrated a lack of consensus in routine practice [24]. Furthermore, no clear guidelines are available from NICE or SIGN [7, 8].
In conclusion, based on the current available evidence there is no significant difference in administering peri-operative antibiotics for patients undergoing fracture fixation with K-wire. Further high quality, prospective studies are required to improve the evidence base to influence guidelines.
Data Availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
References
Gopinath, P., Singh, S., & Ravoof, A. (2019). Study of percutaneous K wire fixation in supracondylar fracture of humerus in children. International Journal of Research in Orthopaedics, 5(3), 427.
Madhukar, K. T., Gopalkrishna, G., & Mohan, J. A. (2013). Percutaneous K-wire fixation of distal radius fractures Our results of leaving the wire outside. International Journal of Health & Allied Sciences, 2(1), 23.
Song, J., Yu, A. X., & Li, Z. H. (2015). Comparison of conservative and operative treatment for distal radius fracture: a meta-analysis of randomized controlled trials. International journal of clinical and experimental medicine., 8(10), 17023.
Iorio, C., Crostelli, M., Mazza, O., Rota, P., Polito, V., & Perugia, D. (2019). Conservative versus surgical treatment of Gartland type 2 supracondylar humeral fractures: what can help us choosing? Journal of orthopaedics, 16(1), 31–35.
Van Leeuwen, W. F., Van Hoorn, B. T., Chen, N., & Ring, D. (2016). Kirschner wire pin site infection in hand and wrist fractures: incidence rate and risk factors. Journal of Hand Surgery (European Volume), 41(9), 990–994.
Lobst, C. A., Spurdle, C., King, W. F., & Lopez, M. (2007). Percutaneous pinning of pediatric supracondylar humerus fractures with the semisterile technique: the Miami experience. Journal of Pediatric Orthopaedics, 27(1), 17–22.
National Institute for Health and Care Excellence (NICE). (2019): Surgical site infections: prevention and treatment. Retrieved from: https://www.nice.org.uk/guidance/ng125
Scottish Intercollegiate Guidelines Network (SIGN). (2008): Antibiotic prophylaxis in surgery: A national clinical guideline. http://www.just.edu.jo/ar/DIC/ClinicGuidlines/Antibiotic%20prophylaxis%20in%20surgery.pdf
Schroeder, N. O., Seeley, M. A., Hariharan, A., Farley, F. A., Caird, M. S., & Li, Y. (2017). Utility of postoperative antibiotics after percutaneous pinning of pediatric supracondylar humerus fractures. Journal of Pediatric Orthopaedics, 37(6), 363–367.
Sharma, H., Taylor, G. R., & Clarke, N. M. (2007). A review of K-wire related complications in the emergency management of paediatric upper extremity trauma. The Annals of The Royal College of Surgeons of England, 89(3), 252–258.
Lakshmanan, P., Dixit, V., Reed, M. R., & Sher, J. L. (2010). Infection rate of percutaneous Kirschner wire fixation for distal radius fractures. Journal of Orthopaedic Surgery, 18(1), 85.
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ, 372, n71. https://doi.org/10.1136/bmj.n71
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses (www.ohri.ca/programs/clinical_epidemiology/oxford.htm)
Heras, J., Durán, D., de la Cerda, J., Romanillos, O., Martínez-Miranda, J., & Rodríguez-Merchán, E. C. (2005). Supracondylar fractures of the humerus in children. Clinical Orthopaedics and Related Research, 432, 57–64.
Bashyal, R. K., Chu, J. Y., Schoenecker, P. L., Dobbs, M. B., Luhmann, S. J., & Gordon, J. E. (2009). Complications after pinning of supracondylar distal humerus fractures. Journal of Pediatric Orthopaedics, 29(7), 704–708.
Mangwani, J., Gulati, A., Benson, R., Cichero, M., & Williamson, D. M. (2017). Role of prophylactic antibiotics in lesser toe fusion surgery: a prospective randomised controlled trial. Foot and Ankle Surgery, 23(1), 50–52.
Subramanian, P., Kantharuban, S., Shilston, S., & Pearce, O. J. (2012). Complications of Kirschner-wire fixation in distal radius fractures. Techniques in hand & upper extremity surgery, 16(3), 120–123.
Hsu, L. P., Schwartz, E. G., Kalainov, D. M., Chen, F., & Makowiec, R. L. (2011). Complications of K-wire fixation in procedures involving the hand and wrist. The Journal of hand surgery, 36(4), 610–616.
Crader MF, Varacallo M (2017) Preoperative antibiotic prophylaxis
Yang, X., Xiao, X., Wang, L., Ao, Y., Song, Y., Wang, H., & Wang, H. (2018). Application of antimicrobial drugs in perioperative surgical incision. Annals of clinical microbiology and antimicrobials, 17(1), 2.
Bykowski, M. R., Sivak, W. N., Cray, J., Buterbaugh, G., Imbriglia, J. E., & Lee, W. A. (2011). Assessing the impact of antibiotic prophylaxis in outpatient elective hand surgery: a single-center, retrospective review of 8,850 cases. The Journal of hand surgery, 36(11), 1741–1747.
Harbarth, S., Samore, M. H., Lichtenberg, D., & Carmeli, Y. (2000). Prolonged antibiotic prophylaxis after cardiovascular surgery and its effect on surgical site infections and antimicrobial resistance. Circulation, 101(25), 2916–2921.
Formaini, N., Jacob, P., Willis, L., & Kean, J. R. (2012). Evaluating the use of preoperative antibiotics in pediatric orthopaedic surgery. Journal of Pediatric Orthopaedics, 32(7), 732–735.
Gulati, A., Dixit, A., & Williamson, D. M. (2011). The role of prophylactic antibiotics for percutaneous procedures in orthopaedic surgery. Surgical Science, 2(2), 5.
Acknowledgements
The authors acknowledge Mohammad Al-Ashqar for contributing towards the project conception and design.
Funding
This manuscript did not need any financial support or any method of funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author(s) declared that they have no competing interests.
Ethical Approval and Consent to Participate
Not applicable.
Human and Animal Rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
For this type of study, informed consent was not required.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abul, A., Karam, M., Al-Shammari, S. et al. Peri-operative Antibiotic Prophylaxis in K-Wire Fixation: A Systematic Review and Meta-analysis. JOIO 57, 1000–1007 (2023). https://doi.org/10.1007/s43465-023-00879-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43465-023-00879-6