Abstract
This paper aimed to investigate the selected physicochemical and biological properties of titanium dioxide thin films deposited by atomic layer deposition on 316LVM stainless steel dedicated for cardiovascular implants. The main challenge in surface modification of these implants is the complexity of the processes taking place in the circulatory system. The atomic layer deposition was carried out for a number of cycles 500 and temperature 200 °C for 316LVM stainless steel substrate. The surface topography and surface microstructure were examined. Mouse fibroblasts L929 and Human Dermal Fibroblasts (NHDF-Ad) were used for cytotoxicity assays. The following biocompatibility aspects were investigated in vitro: direct cytotoxicity, hemolysis, platelet activation and aggregation, and pro-inflammatory cytokine levels. The titanium dioxide thin films inherited the substrate topography. The surface microstructure was amorphous with the typical layer by layer growth. The film improved the in vitro cell response in terms of cell viability. The cells were also able to proliferate and adhere; however, differences in the cell morphology and the distribution of cell nuclei were observed. The host cell damage was not noted in terms of lactate dehydrogenase levels. The proposed surface modification reduced the hemolysis index and did not significantly affect platelet activation and aggregation. Acute cytotoxicity of the thin films is not predicted basing on the in vitro pro-inflammatory cytokine assay. The results of the biological tests may be basis for further biological assessment proving the full biocompatibility of the proposed surface modification dedicated for specific cardiovascular implants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
For blood contacting devices, various interactions of the biomaterials with blood and surrounding tissues should be considered. Circulating blood contacting devices (stents, heart valves, artificial hearts, and ventricular-assist devices) should be subject to thrombosis and hematology assays. Depending on the type of implant, interactions with various types of cells should be investigated. Platelet response (platelet activation and aggregation) is a helpful tool for determining non-thrombogenic properties of the implant surface. Hemolysis induced by circulating blood contacting device may be caused by mechanical and biomechanical factors and induce toxic effects or promote thrombosis [1].
The assessment of cytotoxicity is a basic assay for all tissue contacting medical devices and may include various aspects of the cellular response depending on the type of contact expected. Cytotoxicity can be determined by evaluation of cell morphology, cell damage, cell growth, or cellular activity. Stainless steel 316LVM is commonly used in biomedical applications as coronary and pulmonary stents. As artery needs mechanical support for its healing without risk of recoil, stainless steel is considered for such applications. However, compared to other metallic biomaterials (e.g., Ti-based alloys), corrosion resistance of biomedical-grade of stainless steel 316LVM is significantly low. Passive layer does not protect the steel sufficiently when subjected to extremely corrosive environments. Therefore, providing coatings for corrosion protection which does not change the mechanical properties of the material are subject to current research, as corrosion of a metallic material is one of the most important issues to be considered when it is designed for implants.
Taking into account the above requirements, low-temperature surface modification techniques with ability to cover geometrically complex surfaces (miniaturized implants) on all sides should be considered. The ALD technology, which is a variation of the CVD method, allows for the production of ultra-thin, homogeneous and repeatable coatings with a wide range of potential biological applications. In ALD, alternating pulses of precursor and reactant gases are separated by purging steps with an inert carrier gas (N2 or Ar) to achieve self-limiting surface reactions. The ALD reaction ends when all available sites on the reactive surface are occupied. This feature is referred to as self-limiting, which enables ALD to deposit uniform layers of high conformation and precisely controlled thickness on all types of substrates [2].
Numerous tests of coatings using the ALD method show an improvement in the corrosion resistance of the substrate depending on chemical composition (SiO2, Al2O3, SiO2/TiO2, ZnO, and ZrO2–Al2O3) of the layers and ALD process parameters [3,4,5,6,7]. Some single aspects of biological properties were also studied, indicating the influence of ALD process parameters on various aspects of biocompatibility [8,9,10,11,12,13,14,15,16,17,18].
The third-generation stents currently used in clinical practice are the result of many years of research aimed at minimizing the risk of restenosis and thrombosis. While the problem of restenosis has been effectively solved by the use of modern anti-proliferative drugs and matrices of biodegradable drug-releasing polymers, the problem of late thrombosis has not been fully solved. The use of dual antiplatelet therapy is still the standard after stenting, which in some cases increases the risk of bleeding. The optimal stent surface should prevent the adsorption of fibrinogen, prevent the adhesion, aggregation, and activation of platelets, inhibit the adhesion and proliferation of smooth muscle cells, and promote the adhesion and growth of endothelial cells. Various surface modification techniques based on deposition of titanium dioxide, has been widely used for biomedical applications, showing a positive influence on various aspects of biocompatibility. The presence of hydroxyl groups on the surface promotes osseointegration in bone implants. The same property can be also used in surface modification of stents, consisting of surface immobilization leading to generation of nitric oxide to promote reendothelialization [18]. The ongoing intensive research into the creation of biocompatible implant surfaces in contact with the circulating blood indicates the lack of satisfactory solutions in this field, mainly due to the complexity of the physiological processes occurring in the cardiovascular system. In this case, the biological testing program should cover many aspects of biocompatibility. The combination of biological properties with the appropriate physicochemical properties of implants is also an important factor influencing the selection of appropriate process parameters for implant surface modification.
The aim of this study is to investigate the biological properties of titanium dioxide thin films deposited by atomic layer deposition on 316 LVM stainless steel dedicated for circulating blood contacting medical devices. The precursors used in the ALD process (TiCl4/H2O) are thermally stable up to high temperatures, but the corrosive byproduct HCl may be harmful for the deposition instruments; therefore, deposition temperature 200 °C was proposed for the ALD process. The most important aspects of the biocompatibility were studied, including: direct cytotoxicity, platelet activation and aggregation, hemolysis, and prediction of acute toxicity.
2 Materials and methods
2.1 Materials
The test specimens are described in Table 1. 5 pcs. of each specimen were used for testing. Average values are presented in the test results. The test sample of 316LVM was subject to preliminary surface finishing typical for stent fabrication and deposition of TiO2 by the means of ALD.
The test material was 316LVM stainless steel in the annealed state in the form of discs with diameter d = 14 mm and thickness g = 3 mm. Modification of the surface of the analyzed biomaterial was carried out using such procedures as: electrochemical polishing, passivation, application of a TiO2 coating using the low-temperature Atomic Layer Deposition (ALD) method, as well as medical sterilization. To ensure the required physical and chemical properties of the 316LVM steel surface, conditions for its surface treatment have been developed. The first surface treatment was electrochemical polishing, which was carried out in a bath based on a mixture of orthophosphoric acid and sulfuric acid (VI). This process was carried out until the surface roughness of Ra < 0.16 µm, recommended for blood contacting implants, was obtained. Then, the samples were chemically passivated in 40% HNO3 acid. These are the basic stages of shaping the surface of metal biomaterials used for implants with miniaturized geometric features. Then, a layer of TiO2 was deposited on the prepared samples using the ALD method. TiCl4 (Titanium (IV) chloride) + H2O was used as a precursor. The parameters of the deposition are described in Table 2.
The last stage of the proposed surface treatment included medical sterilization, which was carried out using the steam method in an autoclave at temperature T = 135 °C and pressure p = 2.1 bar.
2.2 Microstructure
Characterization of the microstructure of the surface layer was performed using transmission electron microscopy (TEM). The microstructure was characterized by the TEM technique in the so-called bright-field observation BF TEM, scanning transmission electron microscopy (STEM), and high-resolution TEM (HRTEM). Qualitative chemical analysis was performed by X-ray spectroscopy with energy dispersion (EDS). Comprehensive TEM analysis was performed using TECNAI G2 F20 (200 kV) FEG (FEI) and THEMIS (200 kV) FEG (Thermo Fisher Company) microscopes. Thin films for TEM observation were prepared by the focused gallium ion beam (FIB) technique on an SCIOS II DualBeam (ThermoFisher Company) equipped with an EasyLift in situ micromanipulator.
2.3 Surface topography
Surface topography testing was performed using an optical profilometer 3D Surface Metrology Microscope Leica DCM8. Surface scans were processed in Leica Map software, resulting in average Sa values and 3D surface visualizations.
2.4 Cell viability assay
The cell viability assay was performed by the direct method according to the PN-EN ISO 10993-5:2009 standard [19] on fibroblasts (L929 ATCC). The supplemented PromoCell Fibroblast Growth Medium 2 enriched with antibiotics (SIGMA ALDRICH Antibiotic Antimicotic Solution) was used as a medium. Cell viability was assessed after a 24-h incubation using fluorescence microscopy. FDA (fluorescein diacetate) and PI (propidium iodide) were used in cell staining. The research was carried out using a Carl Zeiss Exciter 5 scanning laser confocal microscope. Image analysis and cell counting were performed using ZEN 2008 software and AxioVision 4 Module AutoMeasure.
The cytotoxicity studies were complemented by the direct method of testing the level of lactate dehydrogenase (LDH), a substance released from damaged cells. Human fibroblast cells (L929 ATCC) were used for the study, and Fibroblast Growth Medium 2 (PromoCell) enriched with supplements and antibiotics was used as the culture medium. After incubation, the medium was taken from the above samples and centrifuged, and then, the supernatant was taken from the centrifuged solution for the analysis of LDH level using the colorimetric Roche Cytotoxicity Assay Kit.
2.5 Studies of cell growth, proliferation, and adhesion
The proliferation study was performed on Human Dermal Fibroblasts in the adult skin version (NHDF-Ad). The study qualitatively assessed cell morphology, growth, adhesion, and direction of elongation processes based on the appearance of the cytoskeleton, as well as the appearance and distribution of cell nuclei and their ability to proliferate. The culture was carried out in a dedicated supplemented medium in an incubator at 37 °C, 99% humidity and 5% CO2. Cells were fixed with formaldehyde, permeabilized with TritonX-100 and blocked in 0.1% bovine serum albumin (BSA) solution in Tris-buffered saline (TBS). To assess cell proliferation, Invitrogen™ DAPI D1306 by Thermo Fisher was used to stain cell nuclei, allowing for nuclear and chromosomal counterstaining. DAPI emits blue fluorescence when bound to AT regions of DNA. Alexa Fluor 488 from Thermo Fisher (a rabbit IgG secondary antibody) with green fluorescence was used for F-actin (fibrillar actin) staining. Actin is an element of the cytoskeleton involved in cell division, maintenance, and changes in the shape and movement of the cell. Fluorescently labeled actin is an important tool for studying the structural dynamics of the cytoskeleton in the living and fixed cells.
2.6 Evaluation of the effect of biomaterial on red blood cells
The aim of the in vitro studies was to evaluate the effect of extracts contacted with materials on preserved whole blood components. The tests were carried out in accordance with the PN-EN ISO 10993-4:2018-02 [20] and ASTM F 756–00 [21] standards.
The tests were carried out on human whole blood (KPK) obtained from the Regional Center for Blood Donation and Hemotherapy in Katowice. The blood was anticoagulated with CPDA preservative fluid. The research included three study groups:
-
Blank test: CCP freestanding at room temperature, not subjected to the conditions of the experiment,
-
Negative control: CCP without contact with the test material, subjected to experimental conditions (slow mixing).
-
Study group: materials contacted with CCP in experimental conditions.
The KPK was subjected to a 3-h contact with the tested materials and extracts at a temperature of 37 °C, in a slow-mixing system. The impact of the tested materials on red blood cell parameters was determined by assessing the degree of hemolysis, determined by calculating the hemolysis index of the tested materials.
2.7 Studies of thrombogenic properties
Methods developed by Sanak et al. [22] were used for the study. P-selectin activating markers and the fibrinogen antigen-binding site in the GP IIb–IIIa assembly (PAC-1) were used as indicators of platelet activation and CD 61 marker was used for aggregation assessment. Positive and negative controls were prepared to analyze the amount of aggregates. Negative control was obtained from human blood drawn on sodium citrate. A positive control was prepared by mixing blood with adenosine di-phosphate (ADP).
3 Results
3.1 Microstructure
The results of the conducted microstructure tests are presented in Figs. 1, 2, 3, 4. Studies using diffraction contrast showed on the cross-section the growth of atomic layer by atomic layer, correct for the ALD technique, at a distance of about 5 nm from the substrate (Fig. 4). Further on, the structure was amorphous. In addition, undesirable blisters were also noticed, which may indicate a poorer quality of adhesion of the coating to the substrate (Fig. 1). The amorphous nature of the coating was also confirmed by scanning transmission electron microscopy (STEM) and high-resolution HRTEM images (Fig. 3 and 4). The results of STEM + EDS confirmed the presence of Ti atoms in the coating, which was also homogenously distributed along the thin films (Fig. 2). The absence of substrate originated atoms in the coating was confirmed.
3.2 Surface topography
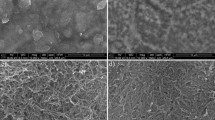
Example surface topographies of the substrate and TiO2-coated specimens are presented in Figs. 5 and 6. Sa (arithmetic mean height) and Ra (arithmetic average value of filtered roughness) values are presented in Table 3. Similar geometrical features of the surface are clearly visible, which indicates the tendency of the layer to inherit the substrate surface topography.
3.3 Cell viability assay
The results of the conducted cell survival studies are shown in Figs. 7, 8, 9, 10, 11. Analyzing the confocal microscopy photo, weak growth on the 316LVM steel substrate was observed. The cells had a characteristic round shape, which indicates the poor viability of cells with active mitochondria and a large number of necrotic cells. In turn, surface modification by applying a TiO2 coating improved the biological properties. A decrease in cytotoxicity was observed. The cells on the surface of the coating have an elongated shape and a reduced number of necrotic cells was found. Fibroblast cells belong to the group of adherent cells. In the case of the correct, from the point of view of substrate biology, membrane polarization occurs and the actin cytoskeleton is activated. The cells release the so-called phyllo- and lamellipodia, which is a positive phenomenon. Based on the studies, survival rates of 95% and 100% were found for the 316LVM and 316LVM with TiO2 samples, respectively. According to ISO 10993, reduction of cell viability by more than 30% is considered a cytotoxic effect, but this was not observed in the analyzed samples.
Figure 12 shows a graph of LDH lactate dehydrogenase levels. The lower the value for the tested sample, the smaller the amount of secreted isoenzyme, which means less cytotoxicity. For the test materials, all values are in the same range as the LDH value for the control material, which was polystyrene (PS) in this study. The positive control was presented as a critical value of 0.6, which indicates a 50% probability of cell membrane damage.
3.4 Studies of cell growth, proliferation, and adhesion
The results of the performed cell proliferation studies are shown in Figs. 13 and 14. Analyzing the confocal microscopy images, both the 316LVM reference sample and the TiO2 coating show a typical normal appearance of fibroblasts with a spindle elongated shape, focal adhesion (bright elongated spots), and varied direction of elongation. In both cases, numerous round or oval cell nuclei can be seen, some in the dividing phase (close to each other or partially overlapping blue spots). In the case of the TiO2 coating, a greater diversity of the cytoskeleton and a different density and length of actin filaments can be observed. Places are also visible with increased density of cell nuclei. In case of uncoated samples, interruptions of the actin fibers continuity in the form of dark lines were observed. On both the 316LVM reference sample and the TiO2 coating, round dark spots are observed.
3.5 Thrombogenicity studies
The results of the conducted studies are presented in Figs. 15, 16, 17. In the case of the P-selectin activation analysis, the TiO2 coating had little effect on the activation processes, without affecting the platelets that remained in the blood. In turn, the second analyzed receptor, PAC-1, was similarly activated. The TiO2 coating also had no significant effect on platelet aggregation.
3.6 Evaluation of the effect of biomaterial on red blood cells
The results of the Hemolytic Index (IH) of the tested materials are presented in Table 4. The calculations were performed in accordance with the guidelines of the ASTM F 756–00 standard. This standard defines three ranges of degree of hemolysis:
-
IH 0–2%—degree of hemolysis: non-hemolytic.
-
IH 2–5%—degree of hemolysis: slightly hemolyzing.
-
IH > 5%—degree of hemolysis: hemolytic.
On the basis of the conducted tests, various values of the Hemolytic Index were found. There was a decrease in the hemolysis index for the samples with the TiO2 coating compared to the samples in the initial state. The obtained IH values for the tested groups were below 2%, which means that the tested materials can be classified as non-hemolytic.
3.7 Determination of pro-inflammatory cytokines
The levels of all pro-inflammatory cytokines tested for the TiO2-coated sample were mostly lower than in the case of the negative control (Figs. 18 and 19). In the case of the reference sample 316LVM, a differential effect of the material on different levels of cytokines was observed. Elevated levels of cytokines in in vitro studies are predictive of acute toxicity. Deposition of the TiO2 coating caused a decrease in the levels of all tested pro-inflammatory cytokines compared to the starting material, which is a positive phenomenon.
4 Discussion
The fabrication of intracoronary stents requires high precision and adequate physicochemical properties. Thus, the surface quality of stents is one of the most important factors affecting their biocompatibility. Therefore it is important to optimize surface treatment technologies to obtain smooth stent surface. 316LVM stainless steel is commonly used for stent fabrication, as it offers high radial strength and minimum risk of recoil caused by radial forces. The proposed surface treatment for 316LVM resulted in smooth surface with nano-roughness desirable for cardiovascular implants. This surface property could be inherited by ALD of TiO2 deposited in 200 °C. Both surfaces did not influence platelet activation and aggregation significantly, showing similar acceptable activation levels. Therefore, it may be concluded that surface with nano-roughness does not affect thrombus formation.
It has been reported in the literature [23,24,25] that titanium dioxide deposited by means of ALD, using titanium dioxide and water steam as precursors, shows various crystal structures, depending on deposition parameters (literature). Anatase crystallites may appear in intermediate deposition temperatures (below 200 °C); however, no crystalline structures were noted in the examined layers. The deposition temperature 200 °C allows to obtain homogenic TiO2 layers without atoms originating from substrate. Both coated and uncoated specimens showed acceptable cytotoxicity levels; however, the cell viability was enhanced by the TiO2 coating. The cell proliferation assay showed differences in cell morphology and nuclei distribution between coated and uncoated specimens; however, in both cases, the cells were able to proliferate and adhere. Proliferation can readily be interpreted as viability.
The morphological differentiation of cells was increased by the TiO2 coating. As the examined layers were chemically homogenous and did not differ from substrate in terms of in topography, the factor influencing the cell morphology and nuclei distribution remains unidentified. Dark discontinuities observed in the actin fibers may result either from surface artifacts or mechanical damage of the fibers. It may indicate that the perfect implant surface quality influences conformal cell growth. A decrease in the hemolysis index was found for the samples with the TiO2 coating comparing to the samples in the initial state. Multiple factors can induce hemolysis, such as shear stress, interaction of red blood cells with leachables, chemicals, electrical forces, and metal ions. It may be predicted that the possible reason of the improvement of the red blood cells response is the improved corrosion resistance of the material.
The results of cytokine level tests showed that the TiO2 coating has a positive effect on inhibiting undesirable inflammatory reactions, while the 316LVM medium showed pro-inflammatory potential. It should be remembered that the inflammatory process and the coagulation process are closely related—tissue factor-induced inflammation initiates the production of thrombin, and thus, the thrombotic state, therefore, a material with anti-inflammatory properties may reduce the risk of inflammation-induced thrombosis. The in vitro pro-inflammatory cytokine assay is used as a predictor of acute cytotoxicity. The level of pro-inflammatory cytokines elevates if any undesirable effects on cells are detected.
5 Summary
TiO2 coating deposited by means of ALD improves biocompatibility of 316LVM substrate in terms of cell viability shows anti-inflammatory properties and decreases hemolytic index comparing to the substrate. Uncoated substrate shows acceptable biocompatibility in most aspects; however, some pro-inflammatory properties need to be further investigated to confirm the in vitro-based predictions for acute toxicity. Relationship with the possibility of release of toxic ions (Cr, Ni) should be further investigated, as the relationship between toxic ion release and late stent thrombosis and restenosis has not been fully recognized. The enhanced biocompatibility of the proposed surface modification is a basis for further studies aiming to prove its suitability for defined cardiovascular implants. With regard to stents, it will be necessary to evaluate the effect of modifications on the proliferation of endothelial cells and smooth muscle cells. In this case, it should be remembered that the desired effect will be the rapid restoration of damaged endothelial cells, which will prevent the formation of blood clots and excessive proliferation of smooth muscle cells, which could lead to re-narrowing of the vessel lumen. Due to differences in stent structures and the recent development of some of these implants, standardized in vitro testing and clinical outcomes are not always available. It should be remembered that coatings used for stents should be resistant to damage caused by implantation techniques, cyclic loading, dislocation, and tracking methods used.
Considering the ability of the ALD thin film to inherit the surface topography, it may be used for coating of stents with nanopatterned drug-dosing structures and surface immobilization to create bioactive surfaces.
Data availability
Results of experiments presented in this study are available from the corresponding author Marcin Basiaga on request.
References
Polish Normalistic Committee. PN-EN ISO 10993-4:2018-02 Biological evaluation of medical devices Part 4: selection of tests for interactions with blood. Warsaw; 2018.
Hu L, Qi W, Li Y. Coating strategies for atomic layer deposition. Nanotechnol Rev. 2017;6:527–47.
Matero R. Atomic layer deposition of oxide films—growth, characterisation and reaction mechanism studies. Helsinki: University of Helsinki-Finland; 2004.
Marin E, Lanzutti A, Guzman L, Fedrizzi L. Corrosion protection of AISI 316 stainless steel by ALD alumina/titania nanometric coatings. J Coat Technol Res. 2011;8:655.
Szewczenko J, Jaglarz J, Basiaga M, Kurzyk J, Paszenda Z. Optical methods applied in thickness and topography testing of passive layers on implantable titanium alloys. Opt Appl. 2013;43(1):173–80.
Walke W, Paszenda Z, Pustelny T, Opilski Z, Drewniak S, Kos̈cielniak-Ziemniak M. Evaluation of physicochemical properties of SiO2-coated stainless steel after sterilization. Mater Sci Eng. 2016;63:155–63.
Szewczenk J, Jaglarz J, Basiaga M, Kurzyk J, Skoczek E. Paszenda Z Topography and thickness of passive layers on anodically oxidized Ti6Al4V alloys. Przeglad Elektrotechniczny. 2012;88:228–31.
Dobrzański L, Dobrzańska-Danikiewicz A, Szindler M, Achtelik-Franczak A. Atomic layer deposition of TiO2 onto porous biomaterials. Arch Mater Sci Eng. 2015;75(1):5–11.
Liu L, Bhatia R. Webster T, „Atomic layer deposition of nano-TiO2 thin films with enhanced biocompatibility”. Int J Nanomed. 2017;12:234–9.
Chung H, Sung O, Yongjoo P, Jin-Sang K, Tae Joo P, Seong K. Atomic-layer deposition of TiO2 thin films with a thermally stable (CpMe5)Ti(OMe)3 precursor. Appl Surf Sci. 2021;550:149381.
Szindler M, Szindler M, Boryło P, Jung T. Structure and optical properties of TiO2 thin films deposited by ALD method. Open Phys. 2017;15:1067–71.
Huang JJ, Lee YT. Self-cleaning and antireflection properties of titanium oxide film by liquid phase deposition. Surf Coat Tech. 2013;231:257–60.
Donnell S, Jose F, Shiel K, Snelgrove M, McFeely C, McGill E, O’Connor R. Thermal and plasma enhanced atomic layer deposition of ultrathin TiO2 on silicon from amide and alkoxide precursors: growth chemistry and photoelectrochemical performance. J Phys D Appl Phys. 2022;55:085105.
Zhuiykov S, Akbari MK, Hai Z, Xue C, Xu H, Hyde L. Wafer-scale fabrication of conformal atomic-layered TiO2 by atomic layer deposition using tetrakis (dimethylamino) titanium and H2O precursors. Mater Des. 2017;120:99–108.
Śmieszek A, Seweryn A, Marcinkowska K, Sikora M, Lawniczak-Jablonska K, Witkowski BS, Kuzmiuk P, Godlewski M, Marycz K. Titanium dioxide thin films obtained by atomic layer deposition promotes osteoblasts’ viability and differentiation potential while inhibiting osteoclast activity—potential application for osteoporotic bone regeneration. Materials. 2020;13:1–2.
Yang F, Chang R, Webster T. Atomic layer deposition coating of TiO2 nano-thin films on magnesium-zinc alloys to enhance cytocompatibility for bioresorbable vascular stents. Int J Nanomed. 2019;14:9955–70.
Jugessur AS, Textor A, Grierson C. Nanometer scale coating using atomic layer deposition technique to enhance performance of bio-medical devices. In: Proceedings of the 12th IEEE International Conference on Nano/Molecular Medicine and Engineering; 2018.
Weng Y, Song Q, Zhou Y. Immobilization of selenocystamine on TiO2 surfaces for in situ catalytic generation of nitric oxide and potential application in intravascular stents. Bionaterials. 2011;32(5):1253–63.
Polish Committee for Standardization, Health, Environment and Medicine Sector, PN-EN ISO 10993-5:2009 Biological evaluation of medical devices—part 5: In vitro cytotoxicity testing, Warsaw, 2009.
Astaneh S, Faverani L, Sukotjo C, Takoudis C. Atomic layer deposition on dental materials: processing conditions and surface functionalization to improve physical, chemical, and clinical properties—a review. Acta Biomater. 2021;121:103–11.
ASTM International. Standard practice for assessment of hemolytic properties of materials. West Conshohocken: ASTM International; 2010.
Sanak M, Jakieła B, Węgrzyn W. Assessment of hemocompatibility of materials. Bull Pol Acad Sci Tech Sci. 2010;58:2.
Aarik J, Aidla A, Uustare T, Sammelselg V. Morphology and structure of TiO2 thin films grown by atomic layer deposition. J Cryst Growth. 1995;148(3):268–75.
Cheng H, Hsu C, Chen Y. Substrate materials and deposition temperature dependent growth characteristics and photocatalytic properties of ALD TiO2 films. J Electrochem Soc. 2009;156:8.
Jõgi I, Pärs M, Martti J, Aidla J. Conformity and structure of titanium oxide films grown by atomic layer deposition on silicon substrates. Thin Solid Films. 2008;516(15):4855–62.
Funding
No funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors of the article declare that during the implementation of the research presented in the article, there was no conflict of interest.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Does not require.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dyner, A., Major, R., Major, Ł. et al. Biological properties of surface modified 316 LVM steel. Archiv.Civ.Mech.Eng 23, 237 (2023). https://doi.org/10.1007/s43452-023-00776-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43452-023-00776-7