Abstract
The paper shows a significant influence of the multi-stage wire drawing technology on deformability and phase transformations in the zinc coating. SEM tests proved that the coating after hot-dip galvanizing consists of a number of thin layers, ranging from 1 to 5 µm, and differing in thickness, chemical composition and properties. When pulled through the drawing die the zinc coating heats up (as a result of friction between the material and the tool) and its dynamic plastic deformation. It resulted in the fracture and partial crushing of the hard-intermetallic phases. It has been proven that as the wire passes through successive drawing dies, the coating is thinned and diffusion as well as phase remodelling of individual structural components occurs; in the place of phase ζ, the intermetallic phase δ1 develops, increasing its share in the diffusion layer. The crystals of intermetallic phases located on the border of the diffusion and outer layers break up and remain dispersed in the zinc. An analysis of the microhardness of the coating has proven that the level of the increase in the microhardness of the zinc coating is contingent on percentage of iron in particular layers of coating.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Hot galvanizing is a widely used way of dip metallization globally, and its use is widespread, for example, in in the construction, machinery and steel industries. Products subject to hot galvanizing include metal sheets, pipes, wire rods and steel wires. There are two methods of hot galvanizing. The first is the discontinuous method, where the coating is applied on large-format steel structure elements and on small steel elements, by completely immersing them in a zinc bath. The continuous method is used for galvanizing strips, hoops, sheets and steel wires [1].
The process of obtaining the coatings consists in immersing the wires or elements made of iron and carbon alloys onto the cleaned and prepared surface in a bath, where a layer with pure liquid zinc is applied. According to Marder [2], the first stage of galvanizing after immersion in a bath is wetting the solid metal with a liquid metal with the usage of the atmosphere (gases). The wetting process, which initiates the reaction between the iron and the bath, is a non-equilibrium system, where the interfacial tension decreases by the amount of the energy released as a result of the exothermic reaction [3, 4]. Diffusion occurs during the ongoing reaction between the liquid metal (shell) and the solid (core). The immersed wire, reaching the temperature of the bath (molten zinc), causes the iron to dissolve in the zinc, producing an iron–zinc alloy. It is the next stage of creating the coating. As a result of the bidirectional diffusion of iron and zinc atoms, an alloy layer is formed, which determines the durability of the bond between the base surface and zinc coating [5]. The alloy (diffusion) layer is composed of intermetallic phases, and its structure is made of the elements occurring on the surface of steel wires and the metal bath [6]. The formation of a zinc layer on iron is regarded as a process of diffusive growth of intermetallic Fe–Zn phases and their dissolution in the bath. The formation rate of new phases is determined by the diffusion coefficient of the elements in phases and the kinetics of the reaction at interfacial boundaries [7, 8]. In Fig. 1 the equilibrium diagram Fe–Zn was presented (the diagram was developed using FactStage64TM software).
On the very steel surface, a very thin layer (approx. 3–4 µm thick) is formed, and the layer is called phase α (alpha) [9]. According to Krasilnikow [10], it is a hard solution of zinc in iron, crystallizing in a regular spatially centred lattice. It includes approx. 3% zinc at 200 °C.
Phase Γ (gamma) is described by means of a chemical formula Fe3Zn10 and contains between 20.5 to 28% Fe, and the melting point is 668–782 °C. It is a tight, brittle and hard compound showing high microhardness, causing adhesion of the coating and showing good plastic properties, crystallizing in a regular spatially centred lattice [6].
Phase Γ1 with the chemical formula Fe5Zn21 forms a regular planar centric lattice. It is characterized by the highest microhardness of all phases. At a temperature of 450 °C, the mass share of iron ranges from 17 to 19.5%. According to Gellings [11], this phase is created between the Γ and δ1 phase as a result of the peritectoidal reaction at the temperature of 550 °C.
Phase δ1 contains 7–11.5% Fe has the chemical formula FeZn7 and is coarse-grained. This phase was first presented in a historic speech by Hanson [12] in 1936, where the presence of intermetallic phases was officially proved δ1 and Γ. Schramm [13] suggested the possibility of the existence of the high-temperature phase δ, while Bühler [14] found the existence of separate low-temperature phases with a column structure (δ1 k) and a palisade structure (δ1p).
Later studies [15, 16] eliminated the possibility of phase δ1 division for low and high-temperature, allowing for the distinguishing of the Γ1 phase only.
At a temperature not exceeding 480 °C phase ζ (zeta) increases in the layer of the near-surface coating in places rich in zinc. During the galvanization process, at the temperature of 480–530 °C said phase loses stability and dissolves in the bath during diffusion. It contributes to a large extent to the increase of iron solubility [17]. Above the temperature of 530 °C phase ζ does not exist. This phase has a columnar-fibrous structure, and it is very brittle and not favourable for the zinc coating. It contains 6–6.2% iron and has a lower microhardness than phase δ1.
There is also a mixed layer consisting of phases ζ (zeta) and η (eta) with decreasing microhardness towards the phase η. Phase η (eta), being the outer layer, is practically pure zinc because it contains 0.003% Fe in the solid state at eutectic temperature.
The main factor having a direct impact on the structure of the coating is the temperature of the zinc bath. The temperature range in which steel wires are galvanized is the value of 440–470 is called the traditional temperature range, while a second range called high temperature ranges between 540 and 560 °C [18, 19].
Another factor determining the structure of the coating is the time the wire remains in the zinc bath, known as the galvanizing time. The time that the galvanized material is in the bath at a constant temperature contributes to the iron diffusion into the zinc, building up the zinc coating. The thickness of the coating consists of the process of building intermetallic layers, whose growth depends on the galvanizing time.
The research carried out by Witkin and Tejndl [20] presents the growth kinetics of intermetallic phases at the process temperature of 450 °C for 120 min in a zinc bath. The studies have proven the dynamic growth of phases δ1 and ζ, where phase ζ grew the fastest of all phases, up to 100 min of bathing. After a period of 100 min, the increase of phase ζ has stopped, while phase δ1 continued increasing, ultimately achieving the greatest thickness of this phase. The study also confirmed that the galvanizing time did not affect the thickness of phase Γ. After reaching an optimal thickness of 4–5 µm at an early stage of development phase, δΓ stopped growing, which draws the conclusion that the galvanizing time for phase Γ does not contribute to its expansion. In actual conditions, the process of galvanizing wire rod and wire takes a few seconds and the immersion time in the bath depends on the diameter of the wire. The final coating thickness depends on the development of phases δ1 and ζ. The plasticity of the coating is influenced by the brittle phase ζ, therefore, the wires should be galvanized with the minimum galvanizing time to maintain the proper properties of the coating. The coating thickness depends on the wire diameter. The bigger the diameter of the steel wire on which the coating is applied, the thicker the coating can be produced.
The alloying elements included in the zinc bath also play a very important role in shaping the structure and properties of the zinc coating, which is corroborated, inter alia, by works [21,22,23].
To sum up, depending on the chemical composition of the steel and the parameters of hot galvanizing (surface preparation, time, temperature, etc.), metal products are covered with zinc coatings of certain thickness and properties in accordance with the applicable EN standards [24,25,26,27].
Hot galvanizing is used to apply coatings to final products such as metal sheet, hoop and semi-finished products such as wire rod [28], which is the input for the process of drawing steel wires [29,30,31]. When drawing galvanized steel wire, the diameter of the wire and the thickness of the coating are subject to change. It is due to the operation of the principle of constant volume in the drawing process, according to which reducing the diameter of the wire results in the elongation of the steel core and thinning of the zinc coating. Hence, after the drawing process, not only the mechanical properties of the wire, but also the zinc coating are subject to change. This is corroborated by the authors' previous works [29,30,31,32] which indicated a significant impact of the drawing technology, i.e., the die angle, the method and speed of drawing, on the mechanical properties of the wire and the thickness of the coating. In the process of multi-stage drawing of wires with metallic coatings, the factor determining the correct drawing process is temperature. According to the theory of multi-stage drawing, the drawing speed and the temperature in the surface layer of the wire increase with the passage through the next die. After exceeding a certain critical temperature, depending on the chemical composition of the coating and the distribution of deformations and the geometry of the dies, the coating becomes plastic and its tendency to stick to the die increases. This is confirmed by the research results presented in the work [32], which shows that when drawing a galvanized wire in industrial conditions at a speed of 20 m/s, the phenomenon of grinding the zinc coating on the contact surface of the wire/die with the friction surface between the tool and the material occurs, the zinc coating of the drawing die as well as breaking the wire and interrupting the drawing process. These works focused on the analysis of wire drawing technology, while the analysis of structural changes taking place in the zinc coating during the drawing process was completely neglected. There is little information on this subject in the literature. Hence, basing on SEM and EDX tests and on the measurement of microhardness, this study presents a comprehensive analysis of the drawing process effects on phase transformations and on the changes in the morphology of individual structural components of the zinc coating.
2 Study material
For the analysis of structural changes in the zinc coating, we have selected a C42D steel wire rod. Before drawing, the material was patented and hot galvanized in industrial conditions at the Drumet factory (WireCoWorldGroupBrand). Then, the wire rod was delivered to the “STALEX” s.c. company. The material was drawn using a multistage drum-type wire drawing machine in 7 passes on a wire with a diameter of 2.2 mm. The parameters of the drawing process are shown in Fig. 2 and Table1.
The implementation of the process of heat treatment, galvanizing and drawing of wire rod in industrial conditions with the use of typical, commonly used machines for the production of wire, enables the actual assessment of structural changes taking place in the zinc coating during the drawing process.
3 Results of computer simulation of the tested drawing processes
In the process of drawing steel wires, drawing parameters such as: temperature, wire pressure on the drawing die and drawing stress have a significant impact on the deformation conditions of the thin zinc coating. This is confirmed, among others, by the authors' earlier work [32], which showed the phenomenon of tearing off the zinc coating in the die and the wire sticking to the drums of the drawing machine. Theoretical analysis of the wire drawing process was carried out in the Drawing 2d program. The zinc coating after hot-dip galvanizing consists, depending on the amount of deformation and drawing parameters, of several layers with a thickness of about 2–5 μm. These are thicknesses similar to the roughness of the wire. Therefore, a wire with a zinc coating was adopted for the simulation as a solid material. Boundary conditions in computer simulations, such as: single and total draft, drawing speed and die geometry were adopted in accordance with Fig. 2 and Table 1. The following input data were additionally adopted for the drawing simulation: wire temperature and ambient temperature 20 °C; heat transfer coefficient between the material and the tool–α = 5000 W/Km2; heat transfer coefficient between the material and air–αpow = 100 W/Km2, emissivity coefficient ε = 0.8. The rheology of the material corresponding to the C42D used in program Drawing 2d, where εis true strain:
A constant coefficient of friction was assumed for all drawing variants equal to μ = 0.07. Figure 2 shows the maximum temperature of wires drawn at speeds from 5 to 20 m/s at the angle α = 3–7°, while Fig. 4 shows the temperature distribution on the cross-section of wires drawn at speeds of 20 m/s at the angle α = 5°.
In the multi-stage drawing process, the temperature of the wire increases with the next pass and reaches its maximum values in the last pass. Figure 3 shows that the use of too small a drawing angle causes a significant increase in the temperature on the wire surface, especially at drawing speeds of v = 20 m/s. The differences between α = 3°and α = 7° were over 350 °C. Such a significant temperature increase on the surface of wires drawn with small angles should be associated with an increase in friction forces at the wire/die die interface. As a consequence, it may contribute to the peeling off of the zinc coating in the die, and after leaving the die, its sticking on the drums of the drawing machine. Figure 4 shows, however, that the galvanized wire can heat up to a temperature exceeding 500 °C only in a thin surface layer. Immediately after the wire exits the die, heat is removed from the wire surface to the environment and the wire core. Hence, the noted increase in the temperature of the wire in the layers closer to the axis of the wire and the gradual equalization of the temperature on its cross-section. It was found that in less than 0.01 s the temperature on the wire surface after it exits the die drops from 750 to 350 °C. Therefore, to lower the temperature in the process of drawing galvanized wires, drawing angles should not be too small. On the other hand, an increase in the drawing angle lowering the temperature increases the risk of breaking the soft zinc coating in the die, Fig. 5.
FEM results: a Wire temperature with marked readings; b Temperature distribution T on the cross section of the wire R, where: I temperature distribution of the wire in the calibrating part of the die, II temperature distribution of the wire after leaving the die (v = 20 m/s, α = 5o, draft 7, drawing from 2,46 to 2.2 mm)
The data presented in Fig. 5 show that the increase of the drawing angle from 3 to 7° increases the stress σx by more than 40%. The greatest pressures occur at the entrance of the wire to the die at the point of contact between the wire and the die. In this area, shear stresses can lead to the rupture of the zinc coating. Therefore, when designing the process of drawing tinned wires, optimal values of the angle of the working part of the die should be selected, leveling the heating and tearing of the zinc coating within a certain range. The parameter enabling determination of the optimal drawing angle is the drawing tension, Fig. 6.
The dependence of the drawing stress on the drawing angle and the drawing speed is not a linear function and reaches a minimum at the angle α = 5°, reducing or increasing the angle, regardless of the drawing speed, results in an increase in the stress. Too small a drawing angle means higher friction forces, while too large an increase in deformation resistance. Summarizing the analysis of wire heating and stress analysis σx and σc, in relation to the deformation conditions of the zinc coating, it can be concluded that the optimal drawing angle of galvanized wires is the angle α = 5°.
4 Structural analysis of the zinc coating
The quality of the zinc coating is defined not only by qualifying the weight of the wire's zinc coating to certain classes compliant with the PN-EN 10,244–1 standard. When applying the zinc coating with the use of continuous hot galvanizing method, attention should be paid to the resulting structure. Unlike in the electrolytic method, the coating does not consist solely of pure zinc. This is of key importance for the properties of the coating obtained. One of the main factors describing the coating obtained with the use of hot galvanizing method is the phase equilibrium system of the occurring Fe–Zn alloys. This allows for the identification of individual components of the structure and determination of their place of occurrence in zinc coatings.
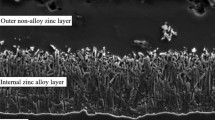
The analysis of zinc coatings on wires is much more complex than in the case of metal sheets. Unlike metal sheets, wires are subjected to plastic processing after hot galvanizing. The thickness of the coating and its morphology change with the increase of total compaction. Hence, it seems advisable to test the zinc coating on the wire rod and on the wires after the drawing process. Figure 7 shows the characteristic phase arrangement on the cross-section of galvanized wire rod.
The zinc coating produced at the traditional temperature by hot galvanizing consists of the outer layer (η) and the diffusion layer, which consists of intermetallic phases: Γ, δ1, ζ, where the distinguishing of the layers is noticeable. The boundary between phase η and phase ζ, shown in Figs. 7 and 8, is characterized by tightly packed crystals perpendicular to the coated surface, separating the diffusion and outer layers. The uniform boundary of the separation in the diffusion layer allows the visualization of the remaining intermetallic phases. To precisely determine the microstructure and chemical composition of the coatings, a test was performed by means of Energy Dispersion Spectroscopy. The chemical composition test was performed on the cross-section of selected samples considered representative with the use of software by Noram Instruments – System six. The test results have been presented in Figs. 8 and 9 and in Table 3.
Spectral X-ray EDS analysis of the micro-areas of the coating marked in Fig. 8
The silicon content in the chemical composition of the wire rod falls within the Sebisty steel limits [6], therefore, the structure of the coating consists of a very thin phase Г adjacent to the substrate. Phase δ1 which has visible cracks, and phase ζ formed by bidirectional diffusion has the dominant thickness in the diffusion layer. The microanalysis of the chemical composition of the zinc coating in the micro-areas allows for the identification of the occurring intermetallic phases with greater precision. We can conclude that in the zinc coating applied to the wire rod (Fig. 8) points 1, 2, 3 represent the pure zinc phase. This is proven by the analysis of the chemical composition in these micro-areas (Table 3), where 100% of the elements are zinc, forming the outer layer of the coating. The diffusion layer begins with tightly packed crystals, whose growth kinetics depends on bidirectional diffusion. Point 4 was applied to the fibrous crystals forming the structure which shows the direction of the diffusive growth of phase ζ [9]. The confirmation of the presence of the phase is the analysis of the chemical composition (Table 3), where the mass content of Fe is 6.2%. This phase is the basic ingredient of hard zinc. 6.8% mass of iron content was found in the diffusion layer in the micro-area of point 5. Such iron content in the Fe–Zn phase corresponds to the stability range ζ and may be its lower zone of occurrence. Due to the hot galvanization of an element with circular cross-section such as wire rod, the interface is not as uniform as for galvanized flat elements. Therefore, point 5 was used to determine the lower limit of phase ζ and for the analysis of the chemical composition. The analyzed micro-area designated as point 6 contains 11.1% mass of iron in the Fe-Zn phase. This iron content in the intermetallic phase corresponds to the range of the coarse phase δ1. Directly adjacent to the steel core, there is a continuous, thin layer with a regular face-centred network. From the analysis of the chemical composition of point 7, where the Fe content is 22.3% mass, it is possible to assign a layer to the intermetallic phase Γ according to the Fe content.
The drawing technology not only changes the thickness of the coating, but also changes the distribution of individual phases in the coating. Hence, the paper analyzes the development of the microstructure of the zinc coating as a function of total compaction. Figure 10 shows the microstructure of the zinc coating of the wire rod and the wires (drawn according to A5 variant) after two different degrees of deformation.
The presented summary of the microstructure shows the mechanism and changes taking place in the zinc coating during plastic working. As the cross-section of the tested wire decreases, the layer of the coating on the wire decreases as well. The noticeable two-layer structure of the coating is maintained from the input material throughout the entire forming process up until the final product. Noticeable up to the value of total compression of 58%, it maintains proportional thicknesses of the outer and diffusion layers, as indicated in Fig. 10b. On the other hand, with the compression greater than 80% (Fig. 10c), there was a decrease in the thickness of the outer layer in relation to the diffusion layer. The decrease is due to unfavourable lubrication conditions, the influence of friction and abrasion of the outer layer while the wire is drawn through the drawing dies.
In Fig. 10a, we observed tightly packed fibrous crystals, corresponding to phase ζ stability range, which was corroborated by the analysis of the chemical composition in the micro-regions (Fig. 8 point 4). Due to the lack of interference of plastic processing, the direction of Fe ↔ Zn diffusion determines the growth kinetics of fibrous crystals, and the inclination of the side walls of the crystals in relation to each other occurs at an angle of 108° and 72° [6]. On the other hand, when the cross-section was lost during the drawing process, it caused visible deformations of the diffusion layer. The cover subject to plastic processing (Fig. 10b) forced the gradual formation of the crystals. The brittleness of fibrous crystals causes splitting cracks during the compaction. The hard particles of those crystals are dispersed during phase η. With higher total densities (Fig. 10) it is impossible to isolate single crystals and clear boundaries of phase ζ separation. It can be concluded that with the compression greater than 80% phase ζ shows no stability and is not present in the diffusion layer after plastic processing. With large compressions during phase ζ, the intermetallic phase δ1forms, increasing its share in the diffusion layer. A typical zinc coating (Fig. 8) at the border with the substrate is characterized by uniform separation of the layers, and phase Г occurs at the point of division, and the separation is noticeable in the remaining intermetallic phases. It was also found that along with the increase in total compaction, there was an increase in the deformation of the contact between the substrate and the diffusion layer, which made it difficult to identify the separating boundary of intermetallic layers. This means that such the coating structure after the drawing process is not typical for this type of coating.
The intensification of the changes taking place in the zinc coating depends, among other things, on die angle and drawing speed. These parameters affect the lubrication conditions, friction and temperature of the wire. This, in turn, should have a significant impact on the formation of the zinc coating in the drawing process. The analysis of the microstructure and of the chemical composition of the coating on wires with a diameter of 2.2 mm drawn at an angle α = 3° carried out herein confirmed the significant impact of the drawing speed on the structural changes of zinc coatings (Fig. 11, 12, 13, Table 4).
After the drawing process at various speeds, the applied zinc coatings showed a shiny and bright appearance on the surface of the wire. This proves the uninterrupted continuity of the applied coating. Based on the analysis of the structures in Figs. 11 and 12, it can be concluded that, regardless of the speed of the drawing, the zinc coating remaining after the plastic working process is composed of an outer layer and a diffusion layer consisting of individual intermetallic phases.
The microanalysis performed for the samples considered representative of the variants A3 and D3 of the chemical composition confirmed the presence of intermetallic phases in the zinc coating. In variant A3 (v = 5 m/s), irregular boundaries of intermetallic phases are noticeable, primarily the phase boundary α and a diffusion layer on the cross section. The irregular shape of the intermetallic boundaries in the zinc coating should be attributed to the drawing process. The jump pattern of the boundary is due to the fact that it forms in a gradually reduced cross-section of the wire, keeping the original length of the boundary. On the other hand, on the longitudinal section, with tensile stresses occurring (Fig. 11b), the linearity of this border is noticeable, which is a normal phenomenon during the drawing process. The crystals of the intermetallic phases on the boundary of diffusion and outer layers crush and remain dispersed in zinc. This is corroborated by an analysis of the chemical composition carried out in points 1 and 2 (Fig. 11), which contain 100%mass Zn (Table 4). Figure 11a (3) indicates the crushed crystals of the diffusion phase. In contrast, points 4 and 5 containing 7% and 8.1% mass of iron are tantamount to phase stability δ1.
The metallographic tests indicated that an increase in the drawing speed results in an increase in the heterogeneity of the zinc coating. The dividing line between the individual layers is more irregular. This is due to the deterioration of lubrication conditions and to an increase of friction. Hence, wires drawn at a speed of 20 m/s are also characterized by a much thinner zinc coating, which is corroborated by a spectral X-ray analysis in the micro-areas of the coating on the cross-section of a galvanized wire drawn at a speed of 20 m/s presented in Fig. 14. In the high-speed drawing process, deterioration of lubrication conditions and increase in friction should be regarded as the cause of unfavourable heating of the wire and the zinc coating to temperatures where zinc coating turns into a thermoplastic state. With no doubt, the above phenomena cause diffusion during the drawing process, which in turn leads to the reconstruction of both the external and internal alloy diffusion layer.
The deterioration of the lubrication conditions during the drawing process at a speed of 20 m/s led to an increase in the contact surfaces of the wire and the die. This, in turn, resulted in the grinding of the zinc surface and the detachment of intermetallic materials in the diffusion phase, which significantly reduced the thickness of the outer layer. The mass content of iron in the crushed intermetallic materials has been specified in point 6 (Fig. 12a) to be at the level of 2.5%. It is assumed that it is a residue of phase ζ, although, as a result of the diffusion process, partially dissolved in pure zinc and lost its phase stability. Additionally, the temperature increase resulting from plastic processing resulted in the phase reconstruction. In variant D3 (v = 20 m/s, α = 3°) a thicker diffusion layer was noted than in variant A3 (v = 5 m/s, α = 3°). The visible cracks and their direction are the result of the impact of heavy tangential stresses caused by the settling of the material during the crushing process in the die. The high drawing speeds have caused the temperature during processing to be so high as a result of friction that it led to another two-way diffusion. This, in turn, has led to the growth and an increase in the content of iron elements in the diffusion layer, which has been proven by the analysis of the chemical composition of the micro-areas. In variant A3, points 4 and 5 (Fig. 11) indicate the mass content of iron to be at a level of 7.0% and 8.1% of the lower part of the diffusion layer, while points 7 and 8 (Fig. 12) indicate that in the upper and middle part of the diffusion layer of variant D3 variant the mass iron content is already higher, i.e., 7.4% and 8.7%, respectively, which suggests stability phase δ1 in different areas. This is also confirmed by point 9 containing 12.9% mass of iron in the micro-area and point 10 containing 27.9% mass of iron, which suggests the stability of phase Г. The study of the chemical composition in selected micro-areas indicates that the drawing speed during plastic working plays a key role in the formation of the zinc coating. At a drawing speed of 5 m/s, one can notice a thick outer layer consisting of pure zinc and a diffusion layer quite thin in relation to the outer layer. As the drawing speed was increased, the thickness of the outer layer decreased due to friction. Moreover, as a result of high temperatures occurring at such high drawing speeds, the diffusion phase expanded and the mass content of iron increased in individual intermetallic phases (Fig. 12).
In addition to the drawing speed, another parameter having a significant impact on the drawing process is the drawing angle. Figures 15, 16, 17 and Table 5 list examples of metallographic tests of the zinc coating on drawn wires at a speed of 5 and 20 m/s in dies with an angle of α = 7°. These studies made it possible to compare the microstructures of wires drawn in dies with an angle α = 3 and 7°.
The microanalysis of the chemical composition for samples considered as representative for variants A7 (v = 5 m/s, α = 7°) and D7 (v = 20 m/s, α = 7°) proved the presence of intermetallic phases. In variant A7, irregular boundaries of the intermetallic phases are noticeable, especially the phase boundary α and the diffusion layer on the cross-section (Fig. 15a). In case of variant A3, there was a similar tendency. It is assumed that deformation of the interface is normal during plastic processing. The difference between variants A3 and A7 is noticeable and relates to the thickness of the coatings and the thickness of the layers. Metallographic tests have shown that the use of low drawing angles in the drawing process increases the thickness of the diffusion layer. The use of dies with an angle α = 7°, on one hand, reduces the contact surface of the wire and the die, and on the other hand, contributes to an increase in shear stresses in the deformation zone. Hence, the crystals of intermetallic phases with a low iron content have cracked during the processing and were dispersed in the pure zinc layer. The only remaining intermetallic phases were those with such iron content that their microhardness allowed to keep the original structure even after the drawing process. The more intensively crushing intermetallic phase of variant A7 (α = 7°) resulted in a thinner diffusion layer compared to variant A3 (α = 3°). At this stage of the analysis, it can be concluded that the angle of the working part of the die at a speed of 5 m/s affects the thickness of the coating, the microstructure of individual layers and the change in the content of iron elements in intermetallic phases. The intensification of the process caused by the speed increase to 20 m/s creates drawing conditions where the wire surface heats up to a temperature exceeding 500 °C, where the outer layer and the diffusion layer with a higher iron content may be rebuilt. An additional factor leading to an increase in the intensity of the occurring phenomena may be the drawing angle in variants D3 and D7. In variant the D3, greater thickness of the zinc coating is caused by the expansion of the diffusion layer and a thinner outer layer as compared to variant D7. It is believed that greater thickness of the diffusion layer is due to the high temperature during drawing, causing bidirectional diffusion and film growth. The contact surface between the wire and the drawing die greater by more than 70% in case of variant D3 was due to the higher temperature generated by friction and a longer time of its transfer from the drawing die to the wire surface, which resulted in a greater growth of the diffusion layer as compared to variant D7. The metallographic analysis in Fig. 12a item 6 shows detached intermetallics with Fe mass content of 2.5% Fe and a diffusion layer with a Fe mass content of 8.7% point 8 in variant D3. The increase in drawing speed to 20 m/s in combination with high tangential stresses in the wires in variant D7 (α = 7°) resulted in the formation of numerous cracks in the cross-section of the zinc coating. As a consequence, the diffusion layer cracked during plastic processing. It is presumed that the higher content of iron elements (Fig. 16a point 6, 13.8% mass Fe) resulted in an increase in the hardness of intermetallicss, with a simultaneous decrease in their plasticity. This led to additional microcracks. Metallographic analysis of the specimens (Fig. 12b and Fig. 16b) indicated the presence of cracks in the diffusion layer on the longitudinal section, regardless of the adopted drawing angle. In the thicker diffusion layer in variant D3, the cracks occur at a certain angle, where in the upper part of the diffusion layer there is the lowest iron content in intermetallics. At higher iron contents in the intermetallic phases, the crack is perpendicular to the core. It is shown in Fig. 12b the chemical analysis of micro-areas of intermetallic phases with iron content of, respectively, 7.4, 12.9, 27.9 mass (point 7, point 8, point 10). In variant D7, the cross-section (Fig. 15b) has a thinner zinc coating with a lower diffusion layer thickness as compared to variant D3. This is due to the breaking of the top layer at the inlet of the wire to the die and the shorter contact area between the die and the wire surface, on which thermal absorption takes place. The two-way phenomenon of diffusion resulting from an increase in the temperature contributes to the growth of the diffusion layer. The smaller area of the deformation zone during drawing in the drawing dies with the angle α = 7° decreased the work of friction forces and the temperature of the wire, which partially inhibited the expansion of the diffusion layer.
In cold plastic processing, the material becomes hardened as a result of plastic deformation. Thus, it can be expected that drawing should change the mechanical properties, not only of the steel core, but also of the soft zinc coating. The literature provides information on the microhardness of zinc coatings, including those after hot galvanizing. However, there is no information on the impact of plastic processing on the microhardness of the zinc coating and of the individual intermetallic phases. As mentioned before, the zinc coating after drawing consists of several very thin, 1–5 µm intermetallic phases distributed unevenly on the longitudinal and cross-section of the wire. Thus, the study attempts to estimate the microhardness of the outer coating consisting of pure zinc (phase η) and the averaged microhardness of the alloy coating located between the pure zinc layer and the steel substrate. Figure 18 shows the results of microhardness of wire rod and φ2.2 mm wires from A5 (α = 5°, v = 5 m/s) and D5 (α = 5°, v = 20 m/s) variants. The data presented in Fig. 18 indicate that the drawing process, as expected, increased the microhardness of the material by over 50%. However, the results for the zinc coating are extremely interesting. One would expect that the cold plastic processing should result in a significant increase in the hardening of the zinc coating. No such relationship was observed. For the outer layer of the coating of the constructed pure zinc phase, about 5% increase in HV was recorded, while for the diffusion layer-17% (on average, including the unalloyed and alloy layers of the coating, it was by approx. 10%).
The slight strengthening of the outer zinc coating, i.e., approx. 5%, should be attributed to the heating of the outer layer of the wire. It is believed that this contributed to the partial recrystallization of the coating and created conditions for the diffusion of iron atoms from the steel core into the diffusion layer. This is corroborated by Figs. 6, 7, 10, 11. Due to the introduction of iron, the intermetallic phases raise the temperature at which the recrystallization processes take place. This is the reason for the near-20% increase of the microhardness of the diffusion layer.
5 Conclusions
Based on the theoretical and experimental research on the production of galvanized steel wires, the following conclusions can be drawn:
-
1.
Numerical analysis of the drawing process showed that the optimal drawing angle of galvanized wires is the angle α = 5o. This angle creates the most favorable conditions for deformation of the soft zinc coating.
-
2.
The drawing technology (die α angle and the drawing speed) affects the thickness of the zinc coating and the morphology of the intermetallic phases. It has been demonstrated that with high total reduction in the place of phase ζ, the intermetallic phase δ1 develops, increasing its share in the diffusion layer.
-
3.
When the wires are drawn at high speeds, structural changes occur in zinc coating. The dividing line between the individual layers is more irregular. In the high speed drawing process, deterioration of lubrication conditions and increased friction should be associated with the unfavourable heating of the wire and the zinc coating to temperatures at which diffusion phenomena occur. This leads to the remodelling of both the outer and inner alloy diffusion layers.
-
4.
The angle of the working part of the die affects the thickness of the coating, the microstructure of the individual layers and the change in the content of iron elements in the intermetallic phases. It has been shown that the use of low drawing angles in the drawing process increases the thickness of the diffusion layer.
-
5.
In the wire drawing process, heterogeneous strengthening of the zinc coating occurs, and the degree of microhardness increase depends on the percentage of iron in individual layers of the coating. For the outer coating of the zinc coating consisting of pure zinc, the microhardness increased by several percent, while for the inner/diffusion layer the differences amounted to almost 20%. The increase in temperature in the outer layer of the wire contributed to a partial recrystallization of the coating.
Data availability
The datasets and material generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Wartacz R. Analiza teoretyczno-doświadczalna ciągnienia wielostopniowego drutów ocynkowanych ze stali C42D, praca doktorska (Theoretical and experimental analysis of the multistage drawing of galvanized wires from C42D steel, doctoral thesis). Częstochowa: Czestochowa University of Technology; 2019.
Marder AR. The Metallurgy of zinc-coated steel. Prog Mater Sci. 2000;45:191–271.
Chang S, Shin JC. The Effect of antymony additions on hot dip galvanized coatings. Corr Sci. 1994;36:1425–36.
F. Schneider, G. Lang, Stalhdraht Herstellung und Anwendung VEB, Leipzing 1973.
P. Liberski, Fizykochemiczne podstawy racjonalnego kształtowania zanurzeniowych powłok aluminiowych na żelazie. Zeszyty naukowe Politechniki Śląskiej, Hutnictwo 6, Gliwice 2002 (in Polish).
P. Liberski, Antykorozyjne Powłoki Zanurzeniowe. Wydawnictwo Politechniki Śląskiej, Gliwice 2013 (in Polish).
Culcasi JD, Seré PR, Elsner CI, Di Sarli AR. Control of the growth of zinc–iron phases in the hot-dip galvanizing process. Surf Coat Technol. 1999;122(1):21–3. https://doi.org/10.1016/S0257-8972(99)00404.
S. Tkaczyk, B. Balcerowska, W. Ozgowicz, H. Rydarowski, J. Szota, Powłoki ochronne. Skrypt Politechniki Śląskiej, nr 2024, Wydawnictwo Politechniki Śląskiej, Gliwice 1997 (in Polish).
K. Kurski, Cynkowanie Ogniowe. WNT Warszawa 1971 (in Polish).
I.A. Krasilnikov, Cynkowanije, łużenije i łatynirowanije stalnoj pro wołoki. Izd. Mietałłurgija, Moskwa 1968.
Gellings PJ, Gierman G, Koster D, Kuit J. Synthesis and characterization of homogeneus intermetalic Fe-Zn compounds. Phase Diag. 2008;71:70–5.
Hansen M. Der Aufbau von Zweistofflegierungen. Berlin: Springer Verlag; 1936.
J. Schramm, Z. Metallkunde (1938) 30. 327: 122–131.
Bühler HE, Jackel G, Mayer L, Baumgartl S. Microchimica Acta. Suppl Vienna. 1970;11:75–86.
Bastin GF, Van Loo JJ, Rieck GD. A new compound in the iron-zinc system. Z Metallkunde. 1974;65:656–60.
Bastin GF, Van Loo JJ, Rieck GD. On the texture in the Delta (Fe-Zn) Layer Formed During Hot-Dpi Galvanizing. Int J Mater Res. 1976;67:694–8.
H. Kania, 2002 Podstawy technologii wytwarzania powłok na stopach żelaza w procesie wysokotemperaturowego cynkowania zanurzeniowego. Praca doktorska, Katowice (doctoral thesis, in Polish).
Foct J, Perrot P, Reumont G. Interpretation of role of silicon on the galvanizing reactions based on kinetics, morphology and thermodynamics. Scr Metall Mater. 1993;28:1195–200.
Kania H, Mendala J, Kozuba J, Saternus M. Development of bath chemical composition for batch hot-dip galvanizing. Rev Mater. 2020;13:4168. https://doi.org/10.3390/ma13184168.
Hawryluk M, Rychlik M, Ziemba J, Jasiak K. Filip Lewandowski and Łukasz Dudkiewicz Analysis of the production process of the forked forging used in the excavator drive system in order to improve the currently implemented technology by the use of numerical modelling. Mater Sci-Pol. 2021. https://doi.org/10.2478/msp-2021-0020.
Kania H, Saternus M, Kudláček J. Structural aspects of decreasing the corrosion resistance of zinc coating obtained in baths with Al. Ni, and Pb addit Mater. 2020;13(2):385. https://doi.org/10.3390/ma13020385.
Grandhi S, Raja VS, Parida S. Effect of manganese addition on the appearance, morphology, and corrosion resistance of hot-dip galvanized zinc coating. Surf Coat Technol. 2021;421:127377. https://doi.org/10.1016/j.surfcoat.2021.127377.
Järvinen H, Honkanen M, Patnamsetty M, Järn S, Heinonen E, Jiang H, Peura P. Press hardening of zinc-coated boron steels: Role of steel composition in the development of phase structures within coating and interface regions. Surf Coat Technol. 2018;352:378–91. https://doi.org/10.1016/j.surfcoat.2018.08.040.
Kania H, Sipa J. Microstructure characterization and corrosion resistance of zinc coating obtained on high-strength grade 109 bolts using a new thermal diffusion process. Materials. 2019;12:1400. https://doi.org/10.3390/ma12091400.
Jabłońska M, Jasiak K, Kowalczyk K, Bednarczyk I, Skwarski M, Tkocz M, Gronostajski Z. Deformation behaviour of high-manganese steel with addition of niobium under quasi-static tensile loading. Mater Sci-Pol. 2022;40:1–11. https://doi.org/10.2478/msp-2022-0029.
Królicka A, Janik A, Żak A, Radwański K. The qualitative–quantitative approach to microstructural characterization of nanostructured bainitic steels using electron microscopy methods. Mater Sci-Pol. 2021;39:188–99. https://doi.org/10.2478/msp-2021-0017.
Kubaszek T, Góral M, Pędrak P. Influence of air plasma spraying process parameters on the thermal barrier coating deposited with micro- and nanopowders. Mater Sci-Pol. 2022;40:80–92. https://doi.org/10.2478/msp-2022-0034.
Górnik M, Jonda E, Łatka L, Nowakowska M, Godzierz M. Influence of spray distance on mechanical and tribological properties of HVOF sprayed WC-Co-Cr coatings. Mater Sci-Pol. 2021;39:545–54. https://doi.org/10.2478/msp-2021-0047.
Suliga M, Wartacz R. The influence of the angle of working part of die on the zinc coating thickness and mechanical properties of medium carbon steel wires. Arch Metal Mater. 2019;64:1295–9. https://doi.org/10.24425/amm.2019.130093.
Roger N. Wright. Wire technology. Process Engineering and Metallurgy: Elsevier; 2011.
Golis B. Methods of assessing selected properties of galvanized and non-galvanized linear wires (in Polish). Metal prod Res Develop Center Metal Prod Indus Krak,. 1984;2:1–30.
Suliga M, Wartacz R, Michalczyk J. High speed multi-stage drawing process of hot-dip galvanised steel wires. Int J Adv Manuf Technol. 2022;120:7639–55. https://doi.org/10.1007/s00170-022-09277-y.
Funding
No funding was received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest. The authors have no conflicts of interest/competing interests to declare that are relevant to the content of this article.
Ethical approval
The article follows the guidelines of the Committee on Publication Ethics (COPE) and involves no studies on human or animal subjects.
Consent to participate
Applicable.
Consent to publication
Applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Suliga, M., Wartacz, R. & Hawryluk, M. Evolution of zinc coatings during drawing process of steel wires. Archiv.Civ.Mech.Eng 23, 120 (2023). https://doi.org/10.1007/s43452-023-00669-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43452-023-00669-9