Abstract
Ishige okamurae Yendo 1907, Fucaceae, a brown alga, has garnered attention for its antipyretic, anti-inflammatory, and anti-edema properties. While numerous studies have explored the effects of a 95% ethanol extract of I. okamurae, limited research has been conducted on its 70% ethanol extract. This study focuses on diphlorethohydroxycarmalol, a bioactive compound identified in the 70% ethanol extract of I. okamurae. Diphlorethohydroxycarmalol has garnered significant interest due to its potential health benefits, including antioxidant, anti-inflammatory, antiviral, and anticancer properties. We investigate the impact of the 70% ethanol extract of I. okamurae and diphlorethohydroxycarmalol on atopic dermatitis using human keratinocyte cell lines (HaCaT) and atopic dermatitis mouse models induced by dinitrochlorobenzene and house dust mite. Treatment with extract of I. okamurae effectively reduced dinitrochlorobenzene/house dust mite -induced ear edema, ear thickness, mast cell infiltration, as well as levels of immunoglobulin (Ig) E, IgG1, IgG2a, cytokines, and chemokines in atopic dermatitis mice. In HaCaT cells, extract of I. okamurae also suppressed TNF-α/IFN-γ-induced cytokine and chemokine production. To further understand the anti-atopic and anti-inflammatory properties of extract of I. okamurae, we evaluated the effects of diphlorethohydroxycarmalol on atopic dermatitis mice and HaCaT cells. In atopic dermatitis mice, diphlorethohydroxycarmalol demonstrated the ability to reduce the inflammatory response induced by TNF-α/IFN-γ. These findings highlight the potential of extract of I. okamurae as an anti-inflammatory agent for inflammatory skin disorders and suggest that diphlorethohydroxycarmalol may represent a promising therapeutic approach for the treatment of inflammatory skin diseases.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Skin diseases represent a significant global health challenge, affecting individuals of all ages and genders due to a combination of intrinsic and extrinsic factors (Hay and Fuller 2011). Atopic dermatitis, the most prevalent inflammatory skin condition worldwide, manifests through various symptoms including erythema, dryness, edema, and pruritus (Gutermuth et al. 2004; Nutten 2015). Allergens are a key contributor to the development of atopic dermatitis lesions.
Among allergens, house dust mites (HDM) are the predominant airborne allergens in Asian countries, commonly found in household dust and bedding materials such as mattresses, pillows, and stuffed animals. Activated T helper type (Th) 2 cells trigger the production of HDM allergen-specific immunoglobulin (Ig) E and the recruitment of inflammatory cells, thus inducing HDM-induced allergies. This immune response leads to structural changes in the skin (Thomas and Hales 2007). To counter inflammation, resident cells like keratinocytes and macrophages, as well as recruited cells such as neutrophils, eosinophils, and lymphocytes, produce a range of cytokines and chemokines. In particular, keratinocytes in atopic patients tend to overexpress cytokines such as interleukin (IL)-1β, IL-6, and IL-8, along with inflammation-sustaining chemokines like CCL17 and CCL22 (Hänel et al. 2013; Pivarcsi and Homey 2005). Thus, an effective therapeutic strategy for atopic dermatitis focuses on suppressing the aberrant activation of keratinocytes in affected skin areas (Trautmann et al. 2001).
Seaweed is a natural source of bioactive compounds, including proteins, sterols, polysaccharides, and polyphenols, known for their diverse bioactivities, such as anti-inflammatory, anti-obesity, antioxidant, and anti-aging effects (Kim and Kong 2010; Lee et al. 2015; Fernando et al. 2018; Wang et al. 2018a, 2018b). Certain seaweeds, including Hizikia fusiforme, Sargassum fulvellum, Undaria pinnatifida, and Ishige okamurae, have a long history of use as food or medicinal ingredients in Asian countries (Lee et al. 2015; Kang et al. 2019; Wang et al. 2018a, 2018b).
Ishige okamurae Yendo 1907, Fucaceae, an edible brown algae, thrives along the southern coast, Jeju Island, Japan, and China, predominantly in tranquil intertidal zones. Diphlorethohydroxycarmalol (1) ranks among the most abundant bioactive compounds in I. okamurae. Previous research has highlighted diphlorethohydroxycarmalol’s potent anti-inflammatory, anti-diabetic, antioxidant, and anti-melanogenic properties (Heo et al. 2008, 2009, 2010). While diphlorethohydroxycarmalol studies have been actively pursued, research regarding its impact on dermatitis remains insufficient.

Hence, this study aims to investigate the effects of 70% ethanol extract of I. okamurae and diphlorethohydroxycarmalol on atopic dermatitis across various applications. Through this investigation, we unveil the potential of extract of I. okamurae and diphlorethohydroxycarmalol as materials possessing activity against atopic dermatitis.
Materials and Methods
General Experimental Procedures
1H, 13C and 2D NMR spectra were generated using a JEOL JNM-ECA 400 and JEOL JNM-ECA600 instruments (JEOL, Tokyo, Japan) using TMS as an internal standard. High resolution election spray ionization mass spectrometry (HRESIMS) data were acquired using a Waters SYNAPT G2-Si HDMS spectrometer (Waters, Milford, MA, USA). The aforementioned instruments were analyzed at the Center for University-Wide Research Facilities, Jeonbuk National University (Jeonju, Korea). Column Chromatography (C.C) was performed using Silica gel (Kieselgel 60, 200–400 mesh, Merck, Darmstadt, Germany). Each fraction was monitored by TLC profiling using silica gel 60 F254 and RP-18 F254s (Merck, Burlington, USA), and medium-pressure liquid chromatography (MPLC, CombiFlash RF, Teledyne lsco, Lincoln, NE, USA) was used to separate the fractions of the extract. Semipreparative high-speed liquid chromatography (semipreparative HPLC) was conducted using a Shimadzu LC-6AD instrument (Shimadzu Corp., Tokyo, Japan) equipped with an SPD-20A detector and Phenomenex Luna C18 (21.2 mm × 250 mm, 5 µm) column. All fractions and compounds were analyzed using an Agilent 1200 series HPLC system (Agilent, Santa Clara, USA).
Plant Material and Extraction
Ishige okamurae Yendo 1907, Fucaceae, were collected from the coast of Jeju island, Korea, between March and June 2021, from the area known as para Jeju. The samples were washed five times with tap water and dried in a constant-temperature room for 1 week. The dried I. okamurae (3 kg) were extracted with 70% EtOH (20 l) at 70 ℃ (5 h, × 20) and filtered (No. 10, 600 mm, Hyundai Micro Co., Seoul, South Korea). The filtrates were then concentrated under reduced pressure to obtain 100 g of extract. The residue (100 g) was suspended in distilled water (2 l), and the aqueous layer was partitioned with hexane, EtOAc and BuOH. The EtOAc layer (16.05 g) was subjected to silica gel column chromatography eluted with chloroform:methanol (1:0 → 0:1, v/v), to obtain 16 fractions (EA1 ~ EA16). Fraction EA2 (1.8 g) and EA8 (1.02 g) was subjected to MPLC [column: ODS RediSepRf (40 g); mobile phase: water:MeOH (1:0 → 0:1, v/v)] to yield 7 subfractions (EA2-1∼7) and 7 subfractions (EA8-1∼7). Fraction EA8-3 (20.7 mg) was further purified by preparative HPLC (Phenomenex Luna C18 column 250 × 21.2 mm, 5 μm) and isocratic elution with 30% CH3CN in H2O, resulting in the isolation of compound 1 (4.5 mg, Fig S3). For further research, diphlorethohydroxycarmalol was purchased from ChemNorm Biotech Co., Ltd (> = 98% (HPLC), Lot No. TFS20210619, Wuhan, China).
Diphlorethohydroxycarmalol (1): brownish yellow amorphous powder; ESI–MS m/z 512.1 [M + H]+; 1H NMR (DMSO-d6, 500 MHz, Fig. S1) δH 6.06 (1H, s), 5.87 (2H, s), 5.78 (1H, t, J = 2 Hz), 5.69 (1H, s), 5.67 (2H, d, J = 2 Hz); 13C NMR (DMSO-d6, 125 MHz, Fig. S2) δC 160.1, 158.9, 154.9, 151.3, 146.0, 143.0, 139.6, 138.8, 135.1, 133.8, 130.7 126.4, 125.5, 124.1, 122.9, 96.1, 95.0, 94.3, 93.7, 92.4 (Zou et al. 2008).
Cell Culture
The human keratinocyte cell line, HaCaT, was cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% heat-inactivated fetal bovine serum and 1% penicillin–streptomycin. The cells were incubated at 37 °C in a humidified atmosphere of 90–95% and 5% CO2.
Cell Viability
Cell viability was assessed using the 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) assay. The HaCaT cells were seeded at a density of 3 × 104 cells/ well in 200 μL culture medium in 96-well plates and incubated for 24 h at 37 °C. The cells were treated with ethanol extract of I. okamurae or diphlorethohydroxycarmalol (1) at various concentrations: 1, 3, 6, 10, 30, 60, and 100 μg/ml of extract of I. okamurae and, 1, 3, 6, 10, 30, 60, and 100 μM 1. After 24 h of incubation, MTT solution was added to the plates, and the cells were further incubated 3 h at 37 °C. The results formazan crystals were dissolved in DMSO, and the absorbance was read at 540 nm using a microplate reader.
Real-Time Quantitative Polymerase Chain Reaction
Total RNA was isolated from HaCaT cells treated with extract of I. okamurae, diphlorethohydroxycarmalol or cyclosporine and/or tumor necrosis factor (TNF)-α (50 ng/mL)/ interferon (IFN)-γ (50 ng/ml) as well as from ear tissues from each group, using TRIzol reagent. First-strand complementary DNA (cDNA) was synthesized using a PrimeScript 1st Strand cDNA Synthesis Kit (Takara Bio Inc., Shiga, Japan), and quantitative PCR was performed using a Bio-Rad T100 thermal cycler (Bio-Rad, Hercules, CA, USA) following the manufacturer’s protocol. Real-time PCR was carried out with a StepOnePlus Real-Time PCR System using TaqMan probes and TaqMan Real-Time PCR Master mix (Applied Biosystems, Foster City, CA, USA). For normalization, 18 s rRNA or GAPDH was used as an endogenous control. The following TaqMan primers and probes were purchased from Applied Biosystems, Thermo Fisher Scientific: TNF-α (Hs01113624_g1, Mm00443258_m1), interleukin (IL)-1β (Hs01555410_m1, Mm00434228_m1), IL-6 (Hs00174131_m1, Mm00446190_m1), IL-8 (Hs00174103_m1), CCL17 (Hs00171074_m1, Mm01244826_g1), and CCL22 (Hs01574247_m1, Mm00436439_m1).
Enzyme-Linked Immunosorbent Assay
Serum IgE, IgG1, IgG2a, cytokine (IL-1β, IL-6, IL-8, and TNF-α), and chemokine (CCL17/TARC, CCL22/MDC) levels in the cell culture medium were determined using ELISA kits (Thermo Fisher Scientific, Waltham, MA, USA; BD Biosciences, San Diego, CA, USA; R&D Systems, Minneapolis, MN, respectively) according to the manufacturer’s instructions. The absorbance was measured at 450 nm using a microplate reader.
Western Blotting
Total protein was extracted as previously described HaCaT cells (1 × 106 cells/well in 6-well plates) were pretreated with extract of I. okamurae, 1 or cyclosporine for 1 h and then stimulated with TNF-α/IFN-γ for 30 min. The cells were lysed with 100 μL of cell lysis buffer (Cell Signaling Technology, Danvers, MA, USA), and the lysed samples were vortexed, incubated for 30 min on ice and centrifuged at 17,949 × g for 10 min at 4 °C. The supernatants were collected and quantified using a DC protein assay kit (Bio-Rad, Contra Costa County, CA, USA). Equal amounts of protein lysate were subjected to electrophoresis on an 8–12% SDS-PAGE gel and then transferred onto a polyvinylidene fluoride (PVDF) membrane. After blocking with 5% bovine serum albumin in tris-buffered saline, the membrane was incubated with primary antibody against the target protein, washed, and subsequently incubated with horseradish peroxidase-conjugated IgG secondary antibody. The bands were developed using a West-Queen RTS Western Blot Detection Kit (iNtRON Bio, Seongnam, Korea). The following antibodies were purchased from Cell Signaling Technology: phosphor(p)-p38 (#4511S, rabbit monoclonal, 1:1000), p38 (#8690S, rabbit monoclonal, 1:1000), p-ERK (#9101S, rabbit monoclonal, 1:1000), ERK (#4348S, rabbit monoclonal, 1:1000), actin (#4967S, rabbit monoclonal, 1:1000), and p-NF-κB (#3033S, rabbit polyclonal, 1:1000). The following antibodies were purchased from Santa Cruz: p-JNK (#sc-6254, mouse polyclonal, 1:1000), JNK (#sc-7345, mouse polyclonal, 1:1000), and p-Stat1 (#sc-8394, mouse polyclonal, 1:1000).
Animals
Female BALB/c mice (6 weeks) were purchased from Samtako (Osan, Korea). All animals had ad libitum access to standard rodent chow and filtered water during the study. The animals were housed (5 per cage) in a laminar air flow room maintained at a temperature of 22 ± 2 °C, relative humidity of 55 ± 5%, on a 12-h light/dark cycle throughout the study. Animal care and treatment protocols were conducted in accordance with the guidelines established by the Public Health Service Policy on the Humane Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the Korea Research Institute of Bioscience and Biotechnology (KRIBB-AEC-21103).
Generation of DNCB/HDM-Induced Atopic Dermatitis-Like Lesions
A total of 25 mice were divided into five groups (n = 5): the vehicle phosphate-buffered saline (PBS), DNCB/HDM vehicle (PBS), DNCB/HDM and extract of I. okamurae (50 and 100 mg/kg), and DNCB/HDM and dexamethasone (DX) (1 mg/kg) groups. During the first week of induction DNCB (2%, 20 µl/ear) was applied once to each ear for sensitization. Then, both ears of each BALB/c mouse were challenged with DNCB/HDM (DNCB: 1%, 20 µl/ear, HDM: 10 mg/ml, 20 µµl/ear) for 3 weeks. Ethanol extract of I. okamurae (50 and 100 mg/kg) or DX (1 mg/kg) was orally administered by gavage for 5 consecutive days per week at the time of the DNCB/HDM challenge. Analysis of DPHC (25 mg/kg) was performed in the AD model, and diphlorethohydroxycarmalol was administered instead of extract of I. okamurae.
Histological Assay
Mouse ear tissues were fixed in 4% (w/v) paraformaldehyde in PBS, (pH 7.4). The fixed tissues were embedded in paraffin and cut into 4-μm-thick sections, which were stained with hematoxylin and eosin (H&E) and toluidine blue.
Statistical Analysis
Statistical analysis was performed using Prism 5 software (GraphPad Software, San Diego, CA, USA). The data are presented as the mean ± SD of nine individual experiments. Statistical significance was determined by one-way ANOVA followed by Tukey’s multiple comparisons test.
Results and Discussion
Effect of Extract on Cell Viability and Expression of Pro-inflammatory Genes
HaCaT cells were exposed to the prescribed amount of the ethanol extract of I. okamurae to study its effect on the viability of HaCaT cells. Then, the MTT assay was used to determine cell vitality after 24 h. For doses of 1, 3, 6, 10, and 30 μg/ml, the adverse effect was insignificant (Fig. 1A). In the context of inflammatory skin diseases, TNF-αNF-he context of CCL17/TARC and CCL22/MDC synthesis in epidermal keratinocytes (Esche et al. 2004). In order to investigate the pro-inflammatory cytokines and chemokines expression in HaCaT human keratinocytes under TNF-α/IFN-γ (each of 50 ng/ml)-induced inflammatory conditions. Before stimulation, HaCaT cells were pretreated for 1 h with various concentrations of the extract (3, 10, and 30 μg/ml). TNF-α/IFN-γ-stimulated HaCaT cells produced a significant level of proinflammatory factors, chemokines (IL-1β, IL-6, IL-8, TNF-α, and CCL17/TARC, CCL22/MDC). Pretreatment with the extract, on the other hand, inhibited TNF-α/IFN-γ-stimulated proinflammatory gene expression and production in a concentration-dependent manner (Fig. 1B and C). Our data suggest that extract of I. okamurae inhibits pro-inflammatory gene expression in HaCaT cells. Further research is necessary to explore the molecular mechanisms underlying its anti-inflammatory effects in HaCaT cells.
Effect of ethanol extract of Ishige okamurae on proinflammatory factor gene expression and production in TNF-α/IFN-γ-stimulated HaCaT cells. A Cell viability of HaCaT cells treated with various concentrations of ethanol extract of I. okamurae. B The levels of proinflammatory cytokines and chemokines were measured by real-time qPCR. C The levels of secreted proinflammatory cytokines and chemokines were measured by ELISA. CyA, an immune suppressor, was used as a positive control drug. Results are presented as average ± SD (n = 3). *p < 0.05 compared with the TNF-α/IFN-γ-stimulated group
Effect of Extract on Intracellular Signaling
Signaling of MAPKs, STAT-1, and NF-κB is essential for the pathogenesis of several inflammatory responses, including atopic dermatitis (Choi et al. 2013). The study showed that extract of I. okamurae suppressed the phosphorylation of signaling molecules in HaCaT cells stimulated with TNF-α/IFN-γ. Specifically, the extract was found to significantly suppress the phosphorylation of MAPKs and NF-κB. The phosphorylation of signaling molecules increased in TNF/IFN-stimulated HaCaT cells. Nevertheless, pretreatment with the extract inhibited TNF/IFN-induced phosphorylation of signaling molecules (Fig. 2). Additionally, the greatest dose substantially inhibited MAPK and NF-κB phosphorylation. Our findings suggest that extract of I. okamurae substantially governs atopic skin lesions by modulating keratinocyte inflammatory responses.
Effect of ethanol extract of Ishige okamurae on intracellular signal transduction in TNF-α/IFN-γ-stimulated HaCaT cells. HaCaT cells were pretreated with 3, 30 μg/ml extract of I. okamurae for 1 h before stimulation with TNF-α/IFN-γ for 30 min, and total protein was extracted using Thermo Fisher total protein extraction reagent. Results are presented as average ± SD (n = 3). *p < 0.05 compared with the TNF-α/IFN-γ-stimulated group
Effect of Extract on Atopic Dermatitis
The DNCB/HDM-induced atopic dermatitis animal model was utilized to assess the anti-atopic effects of extract of I. okamurae. DNCB/HDM are potent agents for inducing atopic lesions. Atopic skin lesions were greatly worsened in DNCB/HDM-treated mice, as seen in Fig. 3A. Histopathological examination with HE staining revealed an increase in ear edema and immune cell infiltration (Fig. 3A, middle panel). Moreover, toluidine blue staining revealed an increase in mast cell infiltration in the ear dermis (Fig. 3A, lower panel). Further, DNCB/HDM therapy enhanced ear thickness (Fig. 3B). Oral treatment of extract of I. okamurae, on the other hand, reduced DNCB/HDM induced atopic skin lesions (Fig. 3A and B). Immunoglobulins such as IgE, IgG1, and IgG2a are associated with Th1 and Th2 responses in atopic dermatitis (Matsuoka et al. 2003; Bak et al. 2022). IgE and IgG1 are involved with Th2 responses, which play roles in T cell differentiation and development (Matsuoka et al. 2003). Th1 responses are influenced by IgG2a (Jo et al. 2018). Serum IgE, IgG1, and IgG2a levels were considerably higher in the DNCB/HDM-treated group. Oral dose, on the other hand, reduced blood immunoglobulin levels (Fig. 4A). Inflammatory mediators and cytokines exacerbate the inflammatory response to skin lesions in skin diseases such as atopic dermatitis (Ahn et al. 2016). In atopy, macrophages activated by foreign substances secrete proinflammatory cytokines such as TNF-α and IFN-γ (Jin et al. 2015).
Effect of ethanol extract of Ishige okamurae on skin lesions and histological analysis of DNCB/HDM-induced atopic dermatitis mouse ear. A Skin lesions on the ears of mice with DNCB/HDM-induced atopic dermatitis. Ear tissue sections were stained with hematoxylin and eosin (H&E, middle panel) or toluidine blue (lower panel). The sections of ear tissue were evaluated at original magnification of 200 × , scale bar = 100 μm. B Ear thickness was measured using a dial thickness gauge at 24 h after induction by DNCB or HDM. Results are presented as average ± SD (n = 5). *p < 0.05 compared with the DNCB/HDM-treated group
Effect of ethanol extract of Ishige okamurae on serum IgE, IgG1, and IgG2a levels in the DNCB/HDM-induced atopic dermatitis mouse model. A Serum IgE, IgG1, and IgG2a levels were measured by ELISA. B Total RNA was extracted from ear tissues, and the levels of proinflammatory cytokines and chemokines were measured by real-time qPCR. Results are presented as average ± SD (n = 5). *p < 0.05 compared with the DNCB/HDM-treated group
Damage to the skin layer is induced by secreted inflammatory cytokines, leading to inflammatory responses such as the expression of inflammatory cytokines and chemokines (Kasraie and Werfel 2013). Damaged epithelium cells and keratinocytes in atopic dermatitis release proinflammatory cytokines and chemokines such as TNF-α, IL-1β, IL-4, IL-6, IL-8, CCL17/TARC, and CCL22/MDC. The gene expression of pro-inflammatory cytokines and chemokines in ear tissues was measured to examine the anti-inflammatory effect of the extract of I. okamurae in atopic dermatitis. The DNCB/HDM-treatment group had significantly higher levels of proinflammatory cytokines and chemokines, such as IL-1β, IL-4, IL-6, TNF-α, CCL17/TARC, and CCL22/MDC. However, oral administration lowered the expression levels of these genes (Fig. 4B). Our data indicated that the extract of I. okamurae attenuates Th2-mediated AD-related skin lesions.
Diphlorethohydroxycarmalol Effect on Cell Viability and the Expression of Proinflammatory Genes
Ishige okamurae contains several compounds, and these compounds have been reported to exert various pharmacological effects (Choi et al. 2013; Jiang et al. 2014; Mohammed and Kobayashi 2019; Rojasawasthien et al. 2021). However, diphlorethohydroxycarmalol (1) is isolated from and is a new indicator component for I. okamurae (Fig. 5A). As a result, we conducted an experiment to validate the activity of diphlorethohydroxycarmalol in HaCaT cells. To investigate the effect of diphlorethohydroxycarmalol on HaCaT cell viability, we first treated cells with the stated doses of diphlorethohydroxycarmalol and the evaluated cell vitality after 24 h using the MTT test. At concentrations of 10, 30, and 60 μM, diphlorethohydroxycarmalol had no notable harmful effects (Fig. 5B). During 1 h before stimulation with TNF-α/IFN-γ, HaCaT cells were pretreated with different dose of diphlorethohydroxycarmalol (10, 30, and 60 μM). TNF-α/IFN-γ-stimulation increased gene expression and the generation of inflammatory factors such as proinflammatory cytokines and chemokines in HaCaT cells. However, pretreatment with diphlorethohydroxycarmalol inhibited TNF-α/IFN-γ-induced proinflammatory factor gene expression and production in a concentration-dependent manner (Fig. 5C and D). Moreover, the greatest dose of diphlorethohydroxycarmalol inhibited the effect of the positive control drug, cyclosporine A. DPHC substantially regulates inflammatory gene expression in keratinocytes according to our results.
Effect of diphlorethohydroxycarmalol (1) on proinflammatory gene expression and production levels in TNF-α/IFN-γ stimulated HaCaT cells. A Cell viability of HaCaT cells treated with various concentrations of diphlorethohydroxycarmalol. B The level of proinflammatory cytokines and chemokines were measured by real-time qPCR. C The levels of secreted proinflammatory cytokines and chemokines were measured by ELISA. Results are presented as average ± SD (n = 3). *p < 0.05 compared with the TNF-α/IFN-γ-stimulated group
Diphlorethohydroxycarmalol Effect on Intracellular Signaling
The intracellular signaling pathway induced by TNF-α/IFN-γ was investigated to better understand the mechanism of action of diphlorethohydroxycarmalol. TNF-α/IFN-γ-stimulated increased phosphorylation of signaling molecules in HaCaT cells. However, pretreatment with diphlorethohydroxycarmalol blocked the TNF-α/IFN-γ-induced phosphorylation of signaling molecules (Fig. 6). Additionally, the greatest quantity of ethanol extract of I. okamurae strongly inhibited MAPKs, STAT-1, and NF-κB phosphorylation. Our data indicated that diphlorethohydroxycarmalol substantially governs atopic skin lesions by modulating keratinocyte inflammatory responses.
Effect of diphlorethohydroxycarmalol (1) on intracellular signal transduction in TNF-α/IFN-γ stimulated HaCaT cells. HaCaT cells were pretreated with 10, 60 μg/ml diphlorethohydroxycarmalol for 1 h before stimulation with TNF-α/IFN-γ for 30 min, and total protein was extracted using Thermo Fisher total protein extraction reagent. Results are presented as average ± SD (n = 3). *p < 0.05 compared with the TNF-α/IFN-γ-stimulated group
Diphlorethohydroxycarmalol Effect on Atopic Dermatitis
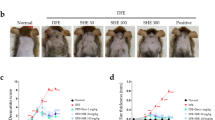
An experiment was carried out to determine if anti-atopic effects were attributable to diphlorethohydroxycarmalol. Ear edema, ear thickness, immune cell and mast cell infiltration, and other atopic skin lesions were considerably exacerbated in the DNCB/HDM-treated AD mice. Oral administration of diphlorethohydroxycarmalol, on the other hand, reduced the DNCB/HDM-treated atopic skin lesions (Fig. 7). In addition, DNCB/HDM-therapy elevated blood IgE, IgG1, and IgG2a levels considerably. However, diphlorethohydroxycarmalol decreased serum IgE, IgG1, and IgG2a levels (Fig. 8A). In particularly, the dosage of diphlorethohydroxycarmalol clearly restricted the usual control level. Our data indicated that diphlorethohydroxycarmalol strongly attenuates AD-related skin lesions in vivo. Damaged epithelium cells and keratinocytes in atopic dermatitis release proinflammatory cytokines and chemokines such as TNF-α, IL-1β, IL-4, IL-6, IL-8, CCL17/TARC, and CCL22/MDC. The gene expression of proinflammatory cytokines and chemokines in ear tissues was measured to examine the anti-inflammatory impact of DPHC in atopic dermatitis. The DNCB/HDM-treatment group had significantly higher levels of proinflammatory cytokines and chemokines, such as IL-1β, IL-4, IL-6, TNF-α, CCL17/TARC, and CCL22/MDC. However, oral administration of diphlorethohydroxycarmalol decreased the expression levels of these genes (Fig. 8B). Our data indicated that diphlorethohydroxycarmalol attenuates Th2-mediated AD-related skin lesions.
Effect of diphlorethohydroxycarmalol (1) on skin lesions and histological analysis of DNCB/HDM-induced atopic dermatitis mouse ear. A Skin lesions on the ears of mice with DNCB/HDM-induced atopic dermatitis. Ear tissue sections were stained with hematoxylin and eosin (H&E, middle panel) or toluidine blue (lower panel). The sections of ear tissue were evaluated at original magnification of 200 × , scale bar = 100 μm. B Ear thickness was measured using a dial thickness gauge at 24 h after induction DNCB or HDM. Results are presented as average ± SD (n = 5). *p < 0.05 compared with the DNCB/HDM-treated group
Effect of diphlorethohydroxycarmalol (1) on serum IgE, IgG1, and IgG2a levels in the DNCB/HDM-induced atopic dermatitis mouse model. A Serum IgE, IgG1, and IgG2a levels were measured by ELISA. B Total RNA was extracted from ear tissues, and the levels of proinflammatory cytokines and chemokines were measured by real- time quantitative PCR. Results are presented as average ± SD (n = 5). *p < 0.05 compared with the DNCB/HDM-treated group
Conclusion
In conclusion, this study underscores the promise of ethanol extract of I. okamurae and diphlorethohydroxycarmalol as prospective therapeutic interventions for skin disorders associated with inflammation, particularly atopic dermatitis. The findings indicate that extract of I. okamurae and diphlorethohydroxycarmalol may effectively mitigate the expression of inflammatory cytokines and chemokines, while also inhibiting key signaling pathways such as MAPKs, STAT-1, and NF-κB, which play pivotal roles in the pathogenesis of atopic dermatitis. Additionally, this research underscores the significance of natural products in the development of novel therapeutic modalities for dermatological conditions. The discovery and isolation of diphlorethohydroxycarmalol from extract of I. okamurae as a novel bioactive component, along with its demonstrated inhibitory effects on chemokine and cytokine production, provide valuable insights into the pharmacological potential of Ishige okamurae extract. Taken together, these results suggest that extract of I. okamurae and diphlorethohydroxycarmalol hold promise for future development as both preventative and therapeutic agents for inflammation-related skin ailments. Nonetheless, further research is imperative to substantiate the efficacy and safety of extract of I. okamurae and diphlorethohydroxycarmalol, as well as to gain a deeper understanding of their mechanisms of action.
References
Ahn S, Siddiqi MH, Aceituno VC, Simu SY, Zhang J, Perez ZEJ, Kim Y-J, Yang D-C (2016) Ginsenoside Rg5: Rk1 attenuates TNF-α/IFN-γ-induced production of thymus-and activation-regulated chemokine (TARC/CCL17) and LPS-induced NO production via downregulation of NF-κB/p38 MAPK/STAT1 signaling in human keratinocytes and macrophages. In Vitro Cell Dev-an 52:287–295. https://doi.org/10.1007/s11626-015-9983-y
Bak SG, Lim HJ, Won YS, Lee S, Cheong SH, Lee SJ, Bae EY, Lee SW, Lee SJ, Rho MC (2022) Regulatory effects of Lycium barbarum extract and isolated scopoletin on atopic dermatitis-like skin inflammation. Biomed Res Int 2022:2475699. https://doi.org/10.1155/2022/2475699
Choi JK, Oh H-M, Lee S, Park J-W, Khang D, Lee SW, Lee WS, Rho M-C, Kim S-H (2013) Oleanolic acid acetate inhibits atopic dermatitis and allergic contact dermatitis in a murine model. Toxicol Appl Pharmacol 269:72–80. https://doi.org/10.1016/j.taap.2013.03.001
Esche C, de Benedetto A, Beck LA (2004) Keratinocytes in atopic dermatitis: inflammatory signals. Curr Allergy Asthma Rep 4:276–284. https://doi.org/10.1007/s11882-004-0071-8
Fernando IS, Jayawardena TU, Sanjeewa KA, Wang L, Jeon Y-J, Lee WW (2018) Anti-inflammatory potential of alginic acid from Sargassum horneri against urban aerosol-induced inflammatory responses in keratinocytes and macrophages. Ecotoxicol Environ Saf 160:24–31. https://doi.org/10.1016/j.ecoenv.2018.05.02
Gutermuth J, Ollert M, Ring J, Behrendt H, Jakob T (2004) Mouse models of atopic eczema critically evaluated. Int Arch Allergy Immunol 135:262–276. https://doi.org/10.1159/000082099
Hänel KH, Cornelissen C, Lüscher B, Baron JM (2013) Cytokines and the skin barrier. Int J Mol Sci 14:6720–6745. https://doi.org/10.3390/ijms14046720
Hay RJ, Fuller LC (2011) The assessment of dermatological needs in resource-poor regions. Int J Dermatol 50:552–557. https://doi.org/10.1111/j.1365-4632.2011.04953.x
Heo S-J, Hwang J-Y, Choi J-I, Han J-S, Kim H-J, Jeon Y-J (2009) Diphlorethohydroxycarmalol isolated from Ishige okamurae, a brown algae, a potent α-glucosidase and α-amylase inhibitor, alleviates postprandial hyperglycemia in diabetic mice. Eur J Pharmacol 615:252–256. https://doi.org/10.1016/j.ejphar.2009.05.017
Heo S-J, Kim J-P, Jung W-K, Lee N-H, Kang H-S, Jun E-M, Park S-H, Kang S-M, Lee Y-J, Park P-J (2008) Identification of chemical structure and free radical scavenging activity of diphlorethohydroxycarmalol isolated from a brown alga, Ishige okamurae. J Microbiol Biotechnol 18:676–681
Heo S-J, Ko S-C, Kang S-M, Cha S-H, Lee S-H, Kang D-H, Jung W-K, Affan A, Oh C, Jeon Y-J (2010) Inhibitory effect of diphlorethohydroxycarmalol on melanogenesis and its protective effect against UV-B radiation-induced cell damage. Food Chem Toxicol 48:1355–1361. https://doi.org/10.1016/j.fct.2010.03.001
Jiang Y, Zeng K-W, David B, Massiot G (2014) Constituents of Vigna angularis and their in vitro anti-inflammatory activity. Phytochemistry 107:111–118. https://doi.org/10.1016/j.phytochem.2014.08.011
Jin SE, Lim H-S, Kim Y, Seo C-S, Yoo S-R, Shin H-K, Jeong S-J (2015) Traditional herbal formula banhasasim-tang exerts anti-inflammatory effects in RAW 264.7 macrophages and HaCaT keratinocytes. Evid Based Complement Alternat Med 2015:728380. https://doi.org/10.1155/2015/728380
Jo G-H, Kim S-N, Kim M-J, Heo Y (2018) Protective effect of Paeoniae radix alba root extract on immune alterations in mice with atopic dermatitis. J Toxicol Environ Health A 81:502–511. https://doi.org/10.1080/15287394.2018.1460785
Kang M-C, Ding Y, Kim H-S, Jeon Y-J, Lee S-H (2019) Inhibition of adipogenesis by diphlorethohydroxycarmalol (DPHC) through AMPK activation in adipocytes. Mar Drugs 17:44. https://doi.org/10.3390/md17010044
Kasraie S, Werfel T (2013) Role of macrophages in the pathogenesis of atopic dermatitis. Mediators Inflamm 2013:942375. https://doi.org/10.1155/2013/942375
Kim S-K, Kong C-S (2010) Anti-adipogenic effect of dioxinodehydroeckol via AMPK activation in 3T3-L1 adipocytes. Chem Biol Interact 186:24–29. https://doi.org/10.1016/j.cbi.2010.04.003
Lee S-H, Kang S-M, Sok CH, Hong JT, Oh J-Y, Jeon Y-J (2015) Cellular activities and docking studies of eckol isolated from Ecklonia cava (Laminariales, Phaeophyceae) as potential tyrosinase inhibitor. Algae 30:163–170. https://doi.org/10.4490/algae.2015.30.2.163
Matsuoka H, Maki N, Yoshida S, Arai M, Wang J, Oikawa Y, Ikeda T, Hirota N, Nakagawa H, Ishii A (2003) A mouse model of the atopic eczema/dermatitis syndrome by repeated application of a crude extract of house-dust mite Dermatophagoides farinae. Allergy 58:139–145. https://doi.org/10.1034/j.1398-9995.2003.23790.x
Mohammed MM, Kobayashi N (2019) Anti-Influenza a virus of a new oligosaccharide citric acid derivative isolated from Vigna angularis (Ohwi et Ohashi. var. dainagon) seeds. J Carbohydr Chem 38:234–245. https://doi.org/10.1080/07328303.2019.1615499
Nutten S (2015) Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab 66:8–16. https://doi.org/10.1159/000370220
Pivarcsi A, Homey B (2005) Chemokine networks in atopic dermatitis: traffic signals of disease. Curr Allergy Asthma Rep 5:284–290. https://doi.org/10.1007/s11882-005-0068-y
Rojasawasthien T, Shirakawa T, Washio A, Tsujisawa T, Matsubara T, Inoue A, Takahama U, Nakashima K, Kokabu S (2021) Vignacyanidin polyphenols isolated from Vigna angularis bean promote osteoblast differentiation. In Vivo 35:883–888. https://doi.org/10.21873/invivo.12328
Thomas WR, Hales BJ (2007) T and B cell responses to HDM allergens and antigens. Immunol Res 37:187–199. https://doi.org/10.1007/BF02697369
Trautmann A, Akdis M, Schmid-Grendelmeier P, Disch R, Bröcker E-B, Blaser K, Akdis CA (2001) Targeting keratinocyte apoptosis in the treatment of atopic dermatitis and allergic contact dermatitis. J Allergy Clin Immunol 108:839–846. https://doi.org/10.1067/mai.2001.118796
Wang L, Lee W, Oh JY, Cui YR, Ryu B, Jeon Y-J (2018a) Protective effect of sulfated polysaccharides from celluclast-assisted extract of Hizikia fusiforme against ultraviolet B-induced skin damage by regulating NF-κB, AP-1, and MAPKs signaling pathways in vitro in human dermal fibroblasts. Mar Drugs 16:239. https://doi.org/10.3390/md16070239
Wang L, Park Y-J, Jeon Y-J, Ryu B (2018b) Bioactivities of the edible brown seaweed, Undaria pinnatifida: a review. Aquaculture 495:873–880. https://doi.org/10.1016/j.aquaculture.2018.06.079
Zou Y, Qian Z-J, Li Y, Kim M-M, Lee S-H, Kim S-K (2008) Antioxidant effects of phlorotannins isolated from Ishige okamurae in free radical mediated oxidative systems. J Agric Food Chem 56:7001–7009. https://doi.org/10.1021/jf801133h
Funding
This research was a part of the project titled [Development and advancement of mass production process for Ishige okamurae a functional material for improving sensitive skin condition], funded by the Ministry of Oceans and Fisheries, Korea, and the KRIBB Research Initiative Program (KGM5242322). This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2022R1A6A3A01087055).
Author information
Authors and Affiliations
Contributions
SGB take charge of the experimental design and article writing. HJL, YSW, and EJP are responsible for finishing related experiments. SWL participated in part of the in vitro study. SHC contributed to the statistical analysis. SJL has planned overall study and responsible for revision.
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bak, S.G., Lim, H.J., Park, E.J. et al. Ishige okamurae Extract: Diphlorethohydroxycarmalol with Effect of Atopic Dermatitis-Like Skin Inflammation. Rev. Bras. Farmacogn. 34, 338–349 (2024). https://doi.org/10.1007/s43450-023-00488-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43450-023-00488-2