Abstract
Hydroxysafflor yellow A is extracted from Carthamus tinctorius L., Asteraceae, and has extensive pharmacological properties. In this study, interleukin-1 beta was used to establish the osteoarthritis model in vitro, and the impacts of hydroxysafflor yellow A on the cell model were analyzed. CCK8 was used to measure cell viability, and flow cytometry was used to evaluate apoptosis and reactive oxygen species. An enzyme-linked immunosorbent assay was performed to calculate the release of inflammatory cytokines and oxidative stress index. Western blotting was performed to measure the expression of collagen-related proteins. The protein levels in the HIF-1α/JAK/STAT3 signaling pathway were also measured. The results showed that hydroxysafflor yellow A promoted cell viability and inhibited apoptosis and oxidative stress. In addition, quinochalcone C-glycoside upregulated the expression of collagen II and Sry-related HMG box-9, while downregulating the expression of matrix metalloproteinase-13. Interleukin-1 beta induced high levels of interleukin-6 and tumor necrosis factor-α that were inhibited by hydroxysafflor yellow A. Meanwhile, hydroxysafflor yellow A inhibited the interleukin-1 beta–induced high levels of reactive oxygen species and malondialdehyde and enhanced the interleukin-1 beta–induced low levels of superoxide dismutase and glutathione peroxidase. Furthermore, hydroxysafflor yellow A downregulated the mRNA expression of HIF-1α, JAK, STAT3, and interleukin-6 as well as the protein expression of HIF-1α, p-JAK, and p-STAT3. The results suggest that hydroxysafflor yellow A inhibited the JAK/STAT3 signaling pathways by inhibiting HIF-1α. Therefore, hydroxysafflor yellow A regulates the inflammatory response and oxidative stress in vitro.
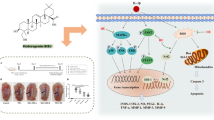
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis is a common disease that often causes pain, stiffness, and disability among older adults (O'Neill et al. 2018), imposing a considerable economic burden on families and society. The main pathological features of osteoarthritis are articular cartilage degeneration and inflammation (Thomas et al. 2017). Osteoarthritis produces synovia, menisci, and ligaments in the bone. Briefly, osteoproliferation leads to joint space narrowing, subchondral bone sclerosis leads to meniscal loss of function, and inflammation leads to synovitis and ligament stiffness (O'Neill et al. 2018). As a complex disease, the risk factors for osteoarthritis include age, gender, body mass index, physical activity, genetics, and joint-level factors (Palazzo et al. 2016).
Multiple studies have demonstrated that oxidative stress is a risk factor for osteoarthritis (Marchev et al. 2017; Feng et al. 2019), which is closely associated with cartilage inflammation, fibrosis, and apoptosis (Xu et al. 2022). Moreover, inflammatory processes have been found in the tissues of the synovium, chondrocytes, and synovial fluid in osteoarthritis (Pelletier et al. 2001; O'Neill et al. 2018). Inflammation in osteoarthritis is chronic, low-grade, and mediated by the innate immune system (Robinson et al. 2016). Studies have suggested that the inflammatory response regulates chondrocyte catabolism and causes low-grade synovitis (Scanzello 2017). However, inflammatory cytokines can activate the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) signaling pathway, thereby regulating oxidative stress, apoptosis, and inflammation during osteoarthritis (Malemud 2017; Zhou et al. 2022). Additionally, the JAK/STAT signaling pathway is involved in cartilage remodeling (Huang et al. 2011). Thus, the JAK/STAT signaling pathway may be a therapeutic target for managing osteoarthritis.
Hydroxysafflor yellow A (1), which has a quinochalcone C-glycosidic structure, is one of the main components extracted from Carthamus tinctorius L., Asteraceae (Asgarpanah and Kazemivash 2013). It has various pharmacological properties including anti-inflammatory, antioxidative, antitumor, immunosuppressive, analgesic, and antidiabetic (Asgarpanah and Kazemivash 2013; Delshad et al. 2018). Yang et al. (2016) reported that hydroxysafflor yellow A modulates inflammatory cytokine release via the TLR4-Myd88 and MAPK-NF-κB signaling pathways. Compound 1 was also found to regulate cholesterol metabolism and prevent fatty liver disease in hyperlipidemic mice (Bao et al. 2015). However, whether hydroxysafflor yellow A regulates the JAK/STAT3 signaling pathway to alleviate osteoarthritis has not yet been studied.

Given the high costs of joint replacement surgery, pharmacological treatments became increasingly important in the treatment of osteoarthritis. Paracetamol, NSAIDs, corticosteroid injections, and tramadol are recommended by the guidelines for the treatment of osteoarthritis. However, these drugs often have adverse effects (Kloppenburg and Berenbaum 2020). For example, NSAIDs can cause gastrointestinal discomfort and corticosteroid injections can cause osteoporosis (Wang et al. 2022). Currently, the inhibition of osteoarthritis-relevant signaling pathways is a new treatment strategy. A recent study reported that inhibiting the JAK/STAT3 signaling pathway can attenuate osteoarthritis (Sun and Xue 2022). Therefore, this study explored whether hydroxysafflor yellow A could act as an inhibitor of the HIF-1α/JAK/STAT3 signaling pathway and alleviate the symptoms of osteoarthritis by inhibiting inflammation and oxidative stress.
Materials and Methods
Regents
Hydroxysafflower yellow A (1) was purchased from Chengdu Must Bio-Technology Co., Ltd. (≥ 98% purity, batch number: 200314; Chengdu, China). The Cell Counting Kit-8 was purchased from Vazyme Corporation (Nanjing, China). IL-1β was obtained from Novoprotein Scientific Inc. (Shanghai, China). The RIPA lysis buffer was purchased from Beyotime (Shanghai, China). Primary antibodies against collagen II (Col2) (1:1000), Sry-related HMG box-9 (SOX9) (1:1000), matrix metalloproteinase-13 (MMP13) (1:5000), HIF-1α (1:2000; cat. no. A7684), JAK (1:2000; cat. no. A11963), P-JAK (1:2000), p-STAT3 (1:2000), STAT3 (1:2000), second antibody goat anti-rabbit (1:5000; cat. no. ab6721), and second antibody goat anti-mouse (1:5000) were purchased from Abcam (Cambridge, UK). β-Actin (1:100000) was obtained from Abclonal (Woburn, MA, USA).
Cell Culture and Treatment
SW1353 cells (Chinese Academy of Sciences Cell Bank, Shanghai, China) were cultured in Dulbecco’s modified Eagle medium (Gibco, USA) supplemented with 10% FBS (Gibco, Australia), 100 units/ml penicillin, and 100 μg/ml streptomycin and incubated at 37 °C with 5% CO2. To assess the effects of hydroxysafflower yellow A on cell viability, SW1353 cells were cultured in 96 wells overnight and then treated with increasing amounts of hydroxysafflor yellow A (0, 20, 40, 60, 80, 100, and 200 μM) for 24 h. Next, 10 μl of CCK-8 reagent was added to each well for further incubation of 2 h. IL-1β was used to induce the osteoarthritis cell model in vitro. After the cells were cultured in 96 wells overnight, they were treated with different doses of IL-1β (0, 2, 4, 6, 8, and 10 μM) to determine the optimum concentration. For the protective effect of hydroxysafflor yellow A, the SW1353 cells were treated with 6 μM IL-1β for 24 h, before the treatment with or without 40 μM of hydroxysafflor yellow A for 2 h. Then, they were treated with a CCK-8 reagent and incubated for 2 h. The absorbance was assessed by a microplate reader (BioTek Epoch, USA) at 450 nm. The test was repeated thrice.
Flow Cytometry
We measured the effects of hydroxysafflower yellow A (the test sample) on cell apoptosis using flow cytometry. The cells were divided into control, IL-1β, test sample (40 μmol/l), and IL-1β + test sample groups. The cells were pretreated as part of the protective effect of the test sample and then digested with trypsin. After centrifugation and resuspension in the buffer, the cells were stained with the Annexin V-FITC/PI kit (Invitrogen, USA) for apoptosis detection and with the reactive oxygen species detection kit (Beyotime, China) for ROS detection. The data were collected using a flow cytometer (Beckman, USA).
Enzyme-Linked Immunosorbent Assay
The levels of C-reactive protein (CRP), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), malondialdehyde (MDA), superoxide dismutase (SOD), and glutathione peroxidase (GSH-Px) in the conditioned medium (collected from cultural supernatant) were measured with CRP ELISA kits (ZC-31853, ZCi Bio, China), TNF-α ELISA kits (ZC-35733, ZCi Bio, China), IL-6 ELISA kits (ZC-32446, ZCi Bio, China), MDA ELISA kits (ZC-S0343, ZCi Bio, China), SOD ELISA kits (ZC-32619, ZCi Bio, China), and GSH-Px ELISA kits (ZC-33141, ZCi Bio, China). The experimental procedures were performed in accordance with the manufacturer’s instructions.
Co-immunoprecipitation
Co-immunoprecipitation experiments on SW1353 cell lysates were performed using a Pierce co-immunoprecipitation kit (Thermo Scientific). Antibodies HIF-1α or STAT3 were used for column binding, and IgG antibody (A7016, Beyotime, China) as a control. After incubation with the antibody/lysate mixture for 1 h at room temperature, the columns were washed for 40 min. The retained proteins were eluted using 40 μl of buffer (Thermo Fisher) at a pH value of 3. Finally, the protein complexes were detected by western blotting.
Western Blot Analysis
RIPA buffer containing 0.1% protease inhibitor was used to homogenize the cell samples, and the lysates from cells were centrifuged at 8496 × g for 15 min at 4 °C; then, the supernatants were collected for protein detection. The total protein concentrations were measured using a BCA kit (Thermo Scientific, Rockford, IL, USA). Equal amounts of protein (20 μg per lane for cell samples) were separated by sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) and then transferred onto PVDF membranes. The membranes were blocked in TBS-T (20 mM Tris-HCl pH 7.6, 150 mM NaCl, and 0.1% Tween-20) at room temperature for 2 h with 5% skim milk or 5% BSA. After being washed in TBS-T three times, the immunoblots were incubated (3% BSA dilution) with the Col2, SOX9, MMP13, HIF-1α, JAK, p-JAK, STAT3, and p-STAT3 primary antibodies overnight at 4 °C. The membranes were incubated with secondary antibodies (1% BSA dilution) for 2 h at room temperature. The membranes were washed again with TBST and visualized using ECL. The band sizes were quantified using Scion Image 4.0 software (Scion Corporation, Frederick, MD, USA). The sample loading was normalized relative to β-actin as a reference standard. The results are shown as “fold changes” in comparison with the control group.
Real-Time Quantitative PCR
TRIzol reagent (Invitrogen, USA) was used to extract the total RNA from SW1353 cells. To quantify the mRNA expression of HIF-1α, JAK, IL-6, and STAT3, a SYBR Green assay (Vazyme, China) was performed on samples based on the manufacturer’s protocol. β-Actin acted as a control and the 2−ΔΔCt method was used to quantify the relative gene expression level. The primers used are listed in Table 1.
Statistical Analyses
GraphPad Prism 9.1.2 (GraphPad Software, Inc.) was used to analyze the data and generate the charts in this experiment. All data are presented as the mean ± standard deviation, and all tests were performed three times. Unpaired, two-tailed Student’s t-test was used to statistically analyze the differences between the two groups. Differences among the three groups were analyzed by one-way analysis of variance followed by Dunnett’s post hoc test. For all experiments, p < 0.05 was considered statistically significant.
Results and Discussion
Cell Viability
SW1353 cells were treated with increasing concentrations of compound 1 to test its effects on cell viability. As shown in Fig. 1A (F = 24.61), compound 1 had no cytotoxicity when the dose was below 40 μM, while it significantly inhibited cell viability when the dose was over 40 μM. IL-1β was used to induce osteoarthritis cell models in vitro (Fig. 1). The results showed that the inhibition ratio of SW1353 increased with increasing levels of IL-1β (F = 455.5). When the concentration of IL-1β was 6 μM, the inhibition ratio was approximately 50%. Therefore, we used 6 μM IL-1β to establish the osteoarthritis cell model.
Effects of hydroxysafflor yellow A on SW1353 cell proliferation and apoptosis. A The cell viability of cells treated with 20, 40, 60, 80, 100, and 200 μM HSYA for 48 h, respectively, measured by CCK-8. B Two, 4, 6, 8, 10 μM IL-1β were used to establish the in vitro model of osteoarthritis, respectively, measured by CCK-8. C A total of 6μM IL-1β was used to treat with SW1353 cells for 48 h, before treatment with or without 40μM HSYA for 2 h, and the con group with no treatment. Measured by CCK-8. D–E Analysis of cell apoptosis by flow cytometry assay. All experiments performed three times at last, and data are presented as means ± SD. **p < 0.01, ***p < 0.001 compared with the con group; ###p < 0.001 compared with the IL-1β group
Apoptosis
Chondrocyte degradation is a classical characteristic of osteoarthritis. To discover whether compound 1 can inhibit this progress, the 6 μM IL-1β induced cells were treated with 40 μM of this test sample. As shown in Fig. 1C, it is apparent that 6 μM IL-1β suppressed cell viability (F = 142.4). However, this effect was attenuated with compound 1 treatment. The results showed that 40 μM remarkably upregulated the cell viability of SW1353. Flow cytometry was performed to measure the effects of compound 1 on apoptosis (Fig. 2D, E) (F = 553.8). The results showed that compound 1 markedly downregulated the IL-1β induced apoptosis rate.
Effects of hydroxysafflor yellow A on extracellular matrix degradation of SW1353 cell in the osteoarthritis cell model. SW1353 cells were treated with 6 μM IL-1β for 48 h, before treatment with or without 40 μM HSYA for 2 h, and the con group with no treatment. A The protein bands of Col2, SOX9, and MMP13 were analyzed by western blot. B Analysis of Col2. C Analysis of SOX9. D Analysis of MMP13. All experiments performed three times at last, and data are presented as means ± SD. **p < 0.01 , ***p < 0.001 compared with the con group;#p < 0.05, ##p < 0.01,compared with the IL-1β group
Levels of Collagen-Relevant Protein
To detect the effects of compound 1 on the levels of collagen-related protein in the osteoarthritis cell model, the protein expression levels of Col2 (F = 17.39), SOX9 (F = 89.74), and MMP13 (F = 178.4) were analyzed (Fig. 2A–D). Compared with the model group (IL-1β group), compound 1 increased the levels of Col2 and SOX9 and decreased the expression of MMP13, which was induced by IL-1β. However, Col2, SOX9, and MMP13 showed no changes when treated with 1 separately in SW1353 cells. These results indicate that compound 1 promoted cell viability and inhibited apoptosis.
Release of Inflammatory Cytokines
Cytokine release is the primary cause of osteoarthritis development. A vital therapy for controlling this disease is inhibition of the release of inflammatory cytokines. Thus, we measured the expression of IL-6 (F = 303.0), TNFα (F = 27.39), and CRP (F = 218.1) with the compound 1 treatment (Fig. 3A–C). Compound 1 successfully inhibited the expression of IL-6, TNFα, and CRP when compared with the IL-1β-induced cell model group.
Inflammatory cytokines and oxidative stress in response to hydroxysafflor yellow A. SW1353 cells were treated with 6μM IL-1β for 48 h, before treatment with or without 40 μM HSYA for 2 h, and the con group with no treatment. The doses of TNFα (A), IL-6 (B), and CRP (C) were measured by ELISA. D–E The doses of MDA, SOD, and GSH-Px were measured by ELISA. F–G ROS was measured by flow cytometry assay. All experiments performed three times at last, and data are presented as means ± SD. *p < 0.05, ***p < 0.001 compared with the con group; #p < 0.05, ##p < 0.01. ###p < 0.001 compared with the IL-1β group
Oxidative Stress
Abnormal oxidative stress levels have also been observed in osteoarthritis. The ROS (Fig. 3F, G) (F = 35.23), MDA (F = 58.96), SOD (F = 25.23), and GSH-Px (F = 48.56) levels were also measured (Fig. 3D, E). In comparison with the IL-1β group, the ROS, SOD, and GSH-Px levels were significantly elevated. The MDA levels were inhibited by the compound 1 treatment. These results demonstrate the effects of compound 1 on the regulation of oxidative stress.
HIF-1α/JAK/STAT Signaling Pathways
To explore the molecular mechanism involved in osteoarthritis, the expression of mRNA and proteins in the HIF-1α/JAK/STAT signaling pathways were analyzed. RT-qPCR analysis was performed for HIF-1α, JAK, IL-6, and STAT3 (Fig. 4A–D). The IL-1β-induced expression of HIF-1α (F = 58.25), JAK (F = 12.00), IL-6 (F = 68.36), and STAT3 (F = 37.74) was inhibited. Western blotting analysis of the HIF-1α expression and the p-JAK/JAK and p-STAT/STAT ratios was performed (Fig. 4E–H). The HIF-1α level (Fig. 4F) (F = 217.2), p-JAK/JAK ratio (Fig. 4G) (F = 149.9), and p-STAT/STAT ratio (Fig. 4H) (F = 98.22) had a similar trend in that they were inhibited by compound 1. Therefore, hydroxysafflower yellow A downregulated the activation of the HIF-1α/JAK/STAT signaling pathways.
Effects of hydroxysafflor yellow A on HIF-1α/JAK/STAT3 signaling pathway. SW1353 cells were treated with 6μM IL-1β for 48 h, before treatment with or without 40 μM HSYA for 2 h, and the con group with no treatment. qRT-PCR was performed to measure the mRNA expression levels of HIF-1α (A), IL-6 (B), JAK (C), and STAT3 (D). WB (E) was performed to measure the levels of HIF-1α (F), p-JAK/JAK(G), and p-STAT3/STAT3 (H). All experiments performed three times at last, and data are presented as means ± SD. **p < 0.01, ***p < 0.001 compared with the con group; #p < 0.05, ##p < 0.01. ###p < 0.001 compared with the IL-1β group
We hypothesized that compound 1 targets the HIF-1α gene to regulate the JAK/STAT signaling pathway. Consequently, co-immunoprecipitation was carried out to detect the interaction of STAT3 and HIF-1α, while IgG was used as a control. HIF-1α was directly bound to STAT3 during co-immunoprecipitation (Fig. 5A–D).
As a chronic disease, osteoarthritis is one of the main causes of physical disability and reduces the quality of life among adults worldwide (Nelson 2018). The mechanism underlying the development of osteoarthritis is complex, and many factors contribute to its progression. Therefore, further understanding of its pathogenesis and identification of effective and low-side-effect medicines are urgently needed. Hydroxysafflower yellow A (1), a natural product extracted from the Chinese C. tinctorius, has various pharmacological properties. In this study, we evaluated its pharmacological functions in osteoarthritis cell models to identify an alternative therapy.
To maintain the integrity and physiology of the joints, it is essential to maintain an intact structure of the articular cartilage and subchondral bone. However, chondrocyte death and matrix loss have been observed during osteoarthritis development (Hwang and Kim 2015). In addition, the decreased expression of collagen II and SOX9 and increased expression of MMP13 in osteoarthritis have been reported (Ouyang et al. 2019). Interestingly, several recent studies have shown that flavonoids, such as fisetin and scutellarin, modulate this process. The elevated expression of MMP13 and decreased levels of collagen II and SOX9 are inhibited by these plant polyphenols (Zheng et al. 2017; Wang et al. 2019). In this study, hydroxysafflower yellow A (1) had a similar function that inhibited the IL-1β-induced high expression of MMP13 and increased the expression of collagen II and SOX9. In addition, compound 1 significantly increased cell viability and decreased the apoptosis ratio of SW1353 cells. Therefore, these results indicate that compound 1 may have a protective function by deterring the degradation of cartilage cells; however, this extrapolation needs to be further studied in vivo.
In affected joints and other tissues damaged by osteoarthritis, inflammatory cytokines are released, promoting the inflammatory response in chondrocytes (Lieberthal et al. 2015). Several cytokines such as IL-1β, TNFα, IL-6, IL-15, IL-17, and many other cytokines are associated with the pathogenesis of osteoarthritis. Research showed that compound 1 reduced the levels of hs-CRP, IL-1β, and IL-6 in the serum of acute myocardial infarction (Zhou 2013). These results agree with the findings of Jin et al. (2013), who showed that the administration of compound 1 significantly inhibited the expression of TNFα, IL-1β, and IL-6 in mice with acute lung injury (Jin et al. 2013). Furthermore, the CRP levels are significantly associated with the progression of osteoarthritis (Mao et al. 2016; Kondo et al. 2021). In the present study, the inhibitory effects of compound 1 on the release of IL-6, TNFα, and CRP were demonstrated. Therefore, it could be suggested that hydroxysafflower yellow A (1) could alleviate inflammation during osteoarthritis progression.
Numerous studies have reported high levels of oxidative stress in osteoarthritis patients (Devi et al. 2021; Pal et al. 2023), and it has been regarded as a risk factor for initiating this disease (Wang et al. 2020; Peng et al. 2021). The overproduction of ROS is inexorable in osteoarthritis. Antioxidant enzymes such as SOD and GSH-PX usually scavenge ROS (Wu et al. 2012; Shen et al. 2019). Moreover, excess ROS can react with membrane lipids to generate MDA (Wang et al. 2014). Thus, high levels of ROS and MDA and low levels of SOD and GSH-PX mark high levels of oxidative stress. In this study, compound 1 decreased the oxidative stress induced by IL-1β. Importantly, the chemical structure of hydroxysafflower yellow A (1), an active redox agent, is associated with the keto-enol tautomerism of the quinochalcone moiety (Feng et al. 2013).
Many signaling pathways are involved in the development of osteoarthritis, including the NF-κB and PI3K/Akt/mTOR signaling pathways (Chen et al. 2013; Woo et al. 2017). Furthermore, previous studies have noted the importance of the JAK/STAT3 signaling pathway in chondrocyte-intrinsic inflammatory responses (van Beuningen et al. 2014; Malemud 2017). During the development of osteoarthritis, high levels of IL-6 can stimulate the activation of the JAK/STAT3 signaling pathway and thus promote cartilage degeneration (Legendre et al. 2003). Moreover, JAK inhibitors are regarded as therapies for osteoarthritis (Chiu et al. 2021). The results of the present study showed that the levels of HIF-1α, p-JAK, and p-STAT3 decreased with the compound 1 treatment. Additionally, our results suggest that hydroxysafflower yellow A (1) modulates the JAK/STAT3 signaling pathway in osteoarthritis. Furthermore, our results indicate that HIF-1α may be a target of compound 1 during the treatment of osteoarthritis.
Conclusion
This study demonstrated that hydroxysafflower yellow A (1) promoted chondrocyte viability and inhibited chondrocyte apoptosis. This quinochalcone inhibited the release of inflammatory cytokines IL-6 and TNFα and inhibited oxidative stress via the HIF-1α/JAK/STAT3 signaling pathway. This indicates that hydroxysafflower yellow A (1) is a potential drug for the treatment of osteoarthritis. However, in vivo studies are required to determine the exact side effects and therapeutic dosage.
References
Asgarpanah J, Kazemivash N (2013) Phytochemistry, pharmacology and medicinal properties of Carthamus tinctorius L. Chin J Integr Med 19:153–159. https://doi.org/10.1007/s11655-013-1354-5
Bao LD, Wang Y, Ren XH, Ma RL, Lv HJ, Agula B (2015) Hypolipidemic effect of safflower yellow and primary mechanism analysis. Genet Mol Res 14:6270–6278. https://doi.org/10.4238/2015.June.9.14
Chen J, Crawford R, Xiao Y (2013) Vertical inhibition of the PI3K/Akt/mTOR pathway for the treatment of osteoarthritis. J Cell Biochem 114:245–249. https://doi.org/10.1002/jcb.24362
Chiu YS, Bamodu OA, Fong IH, Lee WH, Lin CC, Lu CH, Yeh CT (2021) The JAK inhibitor tofacitinib inhibits structural damage in osteoarthritis by modulating JAK1/TNF-alpha/IL-6 signaling through Mir-149-5p. Bone 151:116024. https://doi.org/10.1016/j.bone.2021.116024
Delshad E, Yousefi M, Sasannezhad P, Rakhshandeh H, Ayati Z (2018) Medical uses of Carthamus tinctorius L. (safflower): a comprehensive review from traditional medicine to modern medicine. Electron Physician 10:6672–6681. https://doi.org/10.19082/6672
Devi A, Dwibedi V, Khan ZA (2021) Natural antioxidants in new age-related diseases. Rev Bras Farmacogn 31:387–407. https://doi.org/10.1007/s43450-021-00175-0
Feng K, Chen Z, Pengcheng L, Zhang S, Wang X (2019) Quercetin attenuates oxidative stress-induced apoptosis via SIRT1/AMPK-mediated inhibition of ER stress in rat chondrocytes and prevents the progression of osteoarthritis in a rat model. J Cell Physiol 234:18192–18205. https://doi.org/10.1002/jcp.28452
Feng ZM, He J, Jiang JS, Chen Z, Yang YN, Zhang PC (2013) NMR solution structure study of the representative component hydroxysafflor yellow A and other quinochalcone C-glycosides from Carthamus tinctorius. J Nat Prod 76:270–274. https://doi.org/10.1021/np300814k
Huang CY, Lai KY, Hung LF, Wu WL, Liu FC, Ho LJ (2011) Advanced glycation end products cause collagen II reduction by activating Janus kinase/signal transducer and activator of transcription 3 pathway in porcine chondrocytes. Rheumatol 50:1379–1389. https://doi.org/10.1093/rheumatology/ker134
Hwang HS, Kim HA (2015) Chondrocyte apoptosis in the pathogenesis of osteoarthritis. Int J Mol Sci 16:26035–26054. https://doi.org/10.3390/ijms161125943
Jin M, Sun CY, Pei CQ, Wang L, Zhang PC (2013) Effect of safflor yellow injection on inhibiting lipopolysaccharide-induced pulmonary inflammatory injury in mice. Chin J Integr Med 19:836–843. https://doi.org/10.1007/s11655-012-1151-6
Kloppenburg M, Berenbaum F (2020) Osteoarthritis year in review 2019: epidemiology and therapy. Osteoarthr Cartil 28:242–248. https://doi.org/10.1016/j.joca.2020.01.002
Kondo F, Takegami Y, Ishizuka S, Hasegawa Y, Imagama S (2021) The association of the progression of knee osteoarthritis with high-sensitivity CRP in community-dwelling people-the Yakumo study. Clin Rheumatol 40:2643–2649. https://doi.org/10.1007/s10067-020-05541-2
Legendre F, Dudhia J, Pujol JP, Bogdanowicz P (2003) JAK/STAT but not ERK1/ERK2 pathway mediates interleukin (IL)-6/soluble IL-6R down-regulation of type II collagen, aggrecan core, and link protein transcription in articular chondrocytes. Association with a down-regulation of SOX9 expression. J Biol Chem 278:2903–2912. https://doi.org/10.1074/jbc.M110773200
Lieberthal J, Sambamurthy N, Scanzello CR (2015) Inflammation in joint injury and post-traumatic osteoarthritis. Osteoarthr Cartil 23:1825–1834. https://doi.org/10.1016/j.joca.2015.08.015
Malemud CJ (2017) Negative regulators of JAK/STAT signaling in rheumatoid arthritis and osteoarthritis. Int J Mol Sci 18:484. https://doi.org/10.3390/ijms18030484
Mao Y, Xu W, Xie Z, Dong Q (2016) Association of irisin and CRP levels with the radiographic severity of knee osteoarthritis. Genet Test Mol Biomarkers 20:86–89. https://doi.org/10.1089/gtmb.2015.0170
Marchev AS, Dimitrova PA, Burns AJ, Kostov RV, Dinkova-Kostova AT, Georgiev MI (2017) Oxidative stress and chronic inflammation in osteoarthritis: can NRF2 counteract these partners in crime? Ann N Y Acad Sci 1401:114–135. https://doi.org/10.1111/nyas.13407
Nelson AE (2018) Osteoarthritis year in review 2017: clinical. Osteoarthr Cartil 26:319–325. https://doi.org/10.1016/j.joca.2017.11.014
O'Neill TW, McCabe PS, McBeth J (2018) Update on the epidemiology, risk factors and disease outcomes of osteoarthritis. Best Pract Res Clin Rheumatol 32:312–326. https://doi.org/10.1016/j.berh.2018.10.007
Ouyang Y, Wang W, Tu B, Zhu Y, Fan C, Li Y (2019) Overexpression of SOX9 alleviates the progression of human osteoarthritis in vitro and in vivo. Drug Des Devel Ther 13:2833–2842. https://doi.org/10.2147/DDDT.S203974
Pal R, Kumar L, Anand S, Bharadvaja N (2023) Role of natural flavonoid products in managing osteoarthritis. Rev Bras 4: in press. https://doi.org/10.1007/s43450-023-00387-6
Palazzo C, Nguyen C, Lefevre-Colau MM, Rannou F, Poiraudeau S (2016) Risk factors and burden of osteoarthritis. Ann Phys Rehabil Med 59:134–138. https://doi.org/10.1016/j.rehab.2016.01.006
Pelletier JP, Martel-Pelletier J, Abramson SB (2001) Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum 44:1237–1247. https://doi.org/10.1002/1529-0131(200106)
Peng YJ, Lu JW, Lee CH, Lee HS, Chu YH, Ho YJ, Liu FC, Huang CJ, Wu CC, Wang CC (2021) Cardamonin attenuates inflammation and oxidative stress in interleukin-1beta-stimulated osteoarthritis chondrocyte through the Nrf2 pathway. Antioxidants 10:862. https://doi.org/10.3390/antiox10060862
Robinson WH, Lepus CM, Wang Q, Raghu H, Mao R, Lindstrom TM, Sokolove J (2016) Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol 12:580–592. https://doi.org/10.1038/nrrheum.2016.136
Scanzello CR (2017) Role of low-grade inflammation in osteoarthritis. Curr Opin Rheumatol 29:79–85. https://doi.org/10.1097/BOR.0000000000000353
Shen J, Yang D, Zhou X, Wang Y, Tang S, Yin H, Wang J, Chen R, Chen J (2019) Role of autophagy in zinc oxide nanoparticles-induced apoptosis of mouse LEYDIG cells. Int J Mol Sci 20. https://doi.org/10.3390/ijms20164042
Sun P, Xue Y (2022) Silence of TANK-binding kinase 1 (TBK1) regulates extracellular matrix degradation of chondrocyte in osteoarthritis by janus kinase (JAK)-signal transducer of activators of transcription (STAT) signaling. Bioengineered 13:1872–1879. https://doi.org/10.1080/21655979.2021.2018976
Thomas AC, Hubbard-Turner T, Wikstrom EA, Palmieri-Smith RM (2017) Epidemiology of post-traumatic osteoarthritis. J Athl Train 52:491–496. https://doi.org/10.4085/1062-6050-51.5.08
van Beuningen HM, de Vries-van Melle ML, Vitters EL, Schreurs W, van den Berg WB, van Osch GJ, van der Kraan PM (2014) Inhibition of TAK1 and/or JAK can rescue impaired chondrogenic differentiation of human mesenchymal stem cells in osteoarthritis-like conditions. Tissue Eng Part A 20:2243–2252. https://doi.org/10.1089/ten.TEA.2013.0553
Wang FS, Kuo CW, Ko JY, Chen YS, Wang SY, Ke HJ, Kuo PC, Lee CH, Wu JC, Lu WB, Tai MH, Jahr H, Lian WS (2020) Irisin mitigates oxidative stress, chondrocyte dysfunction and osteoarthritis development through regulating mitochondrial integrity and autophagy. Antioxidants 9:810. https://doi.org/10.3390/antiox9090810
Wang W, Li J, Li F, Peng J, Xu M, Shangguan Y, Li Y, Zhao Y, Qiu C, Qu R, Li W, Zhang C, Zhang T (2019) Scutellarin suppresses cartilage destruction in osteoarthritis mouse model by inhibiting the NF-kappaB and PI3K/AKT signaling pathways. Int Immunopharmacol 77:105928. https://doi.org/10.1016/j.intimp.2019.105928
Wang W, Niu Y, Jia Q (2022) Physical therapy as a promising treatment for osteoarthritis: a narrative review. Front Physiol 13:1011407. https://doi.org/10.3389/fphys.2022.1011407
Wang Y, Zhao W, Hao J, Xu W, Luo Y, Wu W, Yang Z, Liang Z, Huang K (2014) Changes in biosynthesis and metabolism of glutathione upon ochratoxin A stress in Arabidopsis thaliana. Plant Physiol Biochem 79:10–18. https://doi.org/10.1016/j.plaphy.2014.03.001
Woo SU, Sangai T, Akcakanat A, Chen H, Wei C, Meric-Bernstam F (2017) Vertical inhibition of the PI3K/Akt/mTOR pathway is synergistic in breast cancer. Oncogenesis 6:e385. https://doi.org/10.1038/oncsis.2017.86
Wu KJ, Hsieh MT, Wu CR, Wood WG, Chen YF (2012) Green tea extract ameliorates learning and memory deficits in ischemic rats via its active component polyphenol epigallocatechin-3-gallate by modulation of oxidative stress and neuroinflammation. Evid-Based Comp Alt Med 2012:163106. https://doi.org/10.1155/2012/163106
Xu C, Zhai Z, Ying H, Lu L, Zhang J, Zeng Y (2022) Curcumin primed ADMSCs derived small extracellular vesicle exert enhanced protective effects on osteoarthritis by inhibiting oxidative stress and chondrocyte apoptosis. J Nanobiotechnol 20:123. https://doi.org/10.1186/s12951-022-01339-3
Yang XW, Li YH, Zhang H, Zhao YF, Ding ZB, Yu JZ, Liu CY, Liu JC, Jiang WJ, Feng QJ, Xiao BG, Ma CG (2016) Safflower yellow regulates microglial polarization and inhibits inflammatory response in LPS-stimulated Bv2 cells. Int J Immunopathol Pharmacol 29:54–64. https://doi.org/10.1177/0394632015617065
Zheng W, Feng Z, You S, Zhang H, Tao Z, Wang Q, Chen H, Wu Y (2017) Fisetin inhibits IL-1beta-induced inflammatory response in human osteoarthritis chondrocytes through activating SIRT1 and attenuates the progression of osteoarthritis in mice. Int Immunopharmacol 45:135–147. https://doi.org/10.1016/j.intimp.2017.02.009
Zhou M (2013) The effect of hydroxy safflower yellow A on inflammatory reaction in myocardium of the rats after acute myocardial infarction. Afr J Pharm Pharmacol 7:643–649. https://doi.org/10.5897/AJPP12.1181
Zhou Q, Ren Q, Jiao L, Huang J, Yi J, Chen J, Lai J, Ji G, Zheng T (2022) The potential roles of JAK/STAT signaling in the progression of osteoarthritis. Front Endocrinol 13:1069057. https://doi.org/10.3389/fendo.2022.1069057
Funding
This work was financially supported by the Natural Science Foundation of Sichuan Province (no. 2023NSFSC1803), Innovative topics of Affiliated Sport Hospital of CDSU (no. LCCX22B01), and Innovation team of Chengdu Sport University (No. CXTD201805).
Author information
Authors and Affiliations
Contributions
The conception and design of the study: SJ and MW; acquisition of data: SJ, PL, LT, and YT; analysis and interpretation of data: XL, BH, and YX; drafting the article or revising it critically for important intellectual content: SJ. All authors gave a final approval of the version to be submitted.
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ju, S., Liu, P., Tan, L. et al. Hydroxysafflor Yellow A Regulates Inflammation and Oxidative Stress by Suppressing the HIF-1α/JAK/STAT3 Signaling Pathway to Attenuate Osteoarthritis. Rev. Bras. Farmacogn. 33, 1022–1030 (2023). https://doi.org/10.1007/s43450-023-00429-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43450-023-00429-z