Abstract
Citrus fruits are among the most important economical crops, because of their nutritional value, medicinal importance, and unique flavor. Gas liquid chromatography-mass spectrometry of the hydro-distilled oil from the root resulted in the identification of 110 compounds with germacrene B (22%), aromadendrene (21.6%), α-santalene (7.1%), geijerene (4,81%), germacrene D (4.3%), and limonene (3.4%) as major constituents. In addition, chemical profiling the dichloromethane fraction of the root analyzed by high-performance liquid chromatography-photo diode array detector-electrospray tandem mass spectrometry afforded the identification of 43 compounds belonging to acridone alkaloids, coumarins, and flavonoids. Moreover, xanthyletin, citracridone I and II, clausarin, O-methylcitrusinine-I, and grandisinine were isolated as major metabolites using column chromatography and characterized depending on different spectroscopic techniques. Xanthyletin and citracridone I were investigated for their in vitro cytotoxicity against hepatocellular carcinoma and breast adenocarcinoma cell lines, and in vivo protective effect against cisplatin-induced nephrotoxicity and cardiotoxicity in different dose levels in a rat model. Xanthyletin and citracridone I showed protective activity against cisplatin-induced nephrotoxicity. It attenuated cisplatin-induced elevation of both serum urea and creatinine in a dose-dependent manner. Moreover, xanthyletin attenuated cisplatin-induced elevation of malondialdehyde and glutathione in both renal and cardiac tissues.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rutaceae, commonly known as Citrus family, consists of about 160 genera of flowering plants (Tamokou et al. 2017). The most economically important genera of the family include Citrus, Zanthoxylum, and Agathosma. The genus Citrus produces the most widely used fruits for human consumption, with an annual production of over 120 million tons of which oranges (C. sinensis) exceed 60% of the total production (Scordino and Sabatino 2014). The medicinal value of citrus fruits has been reported and extensively studied. However, a limited number of studies has been conducted to investigate the secondary metabolites and biological activities of citrus roots. Citrus sinensis (L.) Osbeck (Syn: Citrus × aurantium L.), Rutaceae (navel orange), fruits are rich in volatile oils, coumarins, carotenoids, flavonoids. They revealed the presence of volatile oil (Ogunjinmi et al. 2019) and acridone alkaloids (Yang et al. 1987).
In our investigation related to the chemical profile and biological activities of Citrus species growing in Egypt (El-Readi et al. 2010; Hamdan et al. 2010, 2011a, 2011b, 2013a, 2013b, 2014), we aimed to investigate the root of Citrus sinensis (L.) Osbeck, Rutaceae, for its chemical constituents and biological activities. A review of the current available literature indicates that few studies have been conducted on the phytochemical and biological investigations of the leaves, flowers, and fruits peels of this cultivated plant in Egypt (Hamdan et al. 2013b; Eldahshan and Halim 2016). However, to the best of our knowledge, no studies have been reported about the biological value and constituents of its root. Hence, we reported here the chemical constituents of C. sinensis hydro-distilled oil, and chemical profiling and the major compounds of the dichloromethane fraction. The major isolated compounds were investigated for the possible protective effects against cisplatin-induced nephrotoxicity and cardiotoxicity.
Materials and Methods
Plant Material
The root of Citrus sinensis (L.) Osbeck (Citrus × aurantium L.), Rutaceae, was collected from the vicinity of Benha (Tant Al Jazirah), Qalubiya province, Egypt (location: 30.358408 N, 31.0849537E), in May 2017 and a voucher specimen (R-CSN-1) was kept in the herbarium of the Pharmacognosy Department at Faculty of Pharmacy, Zagazig University, Egypt. The plant was identified by Dr. B. Holyel, Professor of Pomology, Benha University.
Volatile Oil Preparation and Analysis
Dried root (100 g) was subjected to hydro-distillation using a Clevenger-type apparatus for 6 h and the oil was extracted with hexane-diethyl ether (1:1 v/v). The solvent was removed under nitrogen stream to yield 0.2% of yellowish orange residue. One microliter of the volatile oil was dissolved in hexane (1 ml) prior to analysis. The gas liquid chromatography-mass spectrometry (GLC-MS) analysis was carried out as previously described by Hamdan et al. (2013a) (see Supplementary Material Text S1). Kovat’s retention indices (RI) were calculated with respect to a set of co-injected standard hydrocarbons (C8-C28).
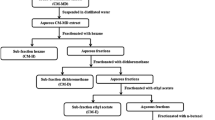
Extraction, Isolation, and HPLC-MS/MS Profiling
Dried root powder (2.5 kg) was exhaustively extracted with 80% aqueous ethanol (3 × 10 l). The extract was concentrated to yield 500 g of dried brownish-green residue, which was partitioned against light petroleum, dichloromethane, and ethyl acetate. The yields of different fractions were 65, 45, and 11 g, respectively. The dichloromethane fraction (30 g) was further chromatographed on a silica gel column eluted with hexane and mixtures of hexane and dichloromethane with increasing polarity, followed by methanol as summarized in Scheme S1. Further purification by column chromatography, repeated crystallization, and/or preparative thin-layer chromatography (TLC) afforded xanthyletin (1) (0.027% of plant dry weight), citracridone II (2) (0.0012%), clausarin (3) (0.0006%), O-methylcitrusinine I (4) (0.002%), grandisinine (5) (0.0016%), and citracridone I (6) (0.01%). The compounds were identified by UV, EI-MS, and 1D and 2D NMR. Chemical profiling the dichloromethane fraction by HPLC-PDA-ESI-MS/MS was performed as described previously (Hamdan et al. 2020) (see Supplementary Material Text S2).

Acute Toxicity Study
Animals
Male Wistar rats (180–200 g) were maintained under constant environmental conditions (3 animals per cage were placed at a temperature of 22 ± 2 °C with 12-h light and 12-h dark cycle). Rats were kept on bed of wood shaving and fed on standard rodent diet and water ad libitum. To investigate the possible toxic effect of the administered drugs on renal and cardiac tissues, xanthyletin (1) and citracridone I (6) at three dose levels (5, 50, 100 mg/kg) were injected to normal rats. Male Wistar rats were divided into seven groups, each group containing three rats. One week after acclimatization, both drugs were injected intraperitoneally (i.p.) in 3 doses (5, 50, and 100 mg/kg) into normal rats for 72 h (n = 3 each). Normal (N) control rats (n = 3) received 0.5% w/v carboxymethyl cellulose (CMC) (i.p.). At the end of the experiment, blood samples were collected for biochemical study, followed by sacrificing the animal. Kidneys and hearts were dissected and kept frozen at −80 °C for biochemical assays.
In Vivo Study
For the investigation of the possible protective effect of isolated compounds in three selected doses on cisplatin-induced nephrotoxicity and cardiotoxicity, twenty-four male rats were randomly divided into eight experimental groups (3 rats in each). The first group represent the normal control group which received 0.5% CMC for 3 days and normal saline at the last day. The second group (cisplatin, CP group) received 0.5% CMC (1 ml/kg) 30 min immediately before CP (Oncotec Pharma Produktion GmbH, Germany) administration i.p. once (7.5 mg/kg). The third to fifth groups (xanthyletin + CP groups) received both xanthyletin (1: 5, 50, and 100 mg/kg; i.p. once daily) and CP (7.5 mg/kg, once), respectively, at 30-min interval. The sixth to eighth groups (citracridone I + CP groups) received both citracridone I (6: 5, 50, and 100 mg/kg; i.p. once daily) and CP (7.5 mg/kg, once), respectively, at 30-min interval. Cisplatin was prepared in normal saline (0.9% NaCl) and i.p. administered. Xanthyletin (1) and citracridone I (6) were suspended in 0.5% CMC and were i.p. administered in 3 doses (5, 50, and 100 mg/kg) to either normal or cisplatin-injected rats.
Serum Collection and Tissue Homogenate Preparation
Blood was collected from animals by puncturing the retro-orbital venous sinus under anesthesia (thiopental sodium, 50 mg/kg, i.p.) and allowed to clot for 30 min and centrifuged at 1008 × g for 20 min. The serum samples were kept at −20 °C until further use. For preparation of tissue homogenate, all rats were weighed and sacrificed for isolation of kidney and heart. After the isolation, samples were immediately frozen in liquid nitrogen and stored at −80 °C until further analysis. The kidney and heart were minced into small pieces and homogenized in ice-cold phosphate buffer saline (0.05 M, pH 7) to obtain a 10% homogenate. The homogenate was centrifuged at 17,000 × g for 60 min at 4 °C and the supernatant was used for assay of reduced glutathione (GSH) and malondialdehyde (MDA) levels.
Nephro- and Cardio-protection
Renal function was determined by assaying serum urea and creatinine using commercially available kits (Bioclin, Santa Coloma, Spain). Cardiac injury was assessed by measuring serum creatinine kinase-MB (CK-MB) activity which was measured kinetically using spectrum diagnostics kit supplied by Egyptian Company for Biotechnology (Obour City Industrial Area, Cairo, Egypt). The concentration of MDA in kidney and heart tissues was determined as an index of lipid peroxidation, by using commercially available kits (Biodiagnostics Co, Giza, Egypt) and expressed as nanomoles of MDA/g of tissue. Reduced glutathione (GSH) content in tissue homogenate was determined by commercially available kits (Biodiagnostics Co, Giza, Egypt) and expressed as mg of GSH/g of tissue.
In Vitro Studies
Cytotoxicity
The cytotoxic effect of xanthyletin (1), citracridone I (6), and cisplatin was evaluated using hepatocellular carcinoma (HepG2) and breast adenocarcinoma (MCF-7) cells. Both cell lines were obtained from Nawah Scientific Inc. (Mokatam, Cairo, Egypt) as previously reported (Allam et al. 2018).
Molecular Docking
The protein structural file of the human cyclin-dependent kinase 9 (CDK9)/cyclinT1 complex with ATP (PDB accession code: 3BLQ) was downloaded from the protein data bank repository (www.rcsb.org). The protein structure was prepared for docking by adjusting the bond orders, adding missing hydrogen atoms, filling in missing side chains and loops with prime, and deleting artificial water molecules. Although it does not affect the docking simulation, we kept cyclinT1. The hydrogen bond network was assigned by sampling water orientations and adjusting the protonation states of the amino acids. Restrained energy minimization was carried out using OPLS3e to remove atomic clashes.
ATP atomic coordinates were selected to define the receptor grid for docking. To impart some degree of flexibility during the docking process, we modified the van der Waals radius scaling factor to 0.85 with a partial charge cutoff of 0.25. This allowed the ligand to use more space for docking. We allowed the rotation of receptor hydroxyl and thiol groups to simulate the actual ligand fitting. The 3D structures of the compounds were downloaded from PubChem (pubchem.ncbi.nlm.nih.gov). All acceptable protonation and tautomerization states were generated at pH 7.4 using LigPrep. After ligand preparation, we run the docking simulation using Glide SP (Standard precision). Ligand was treated as flexible, and the receptor was considered rigid with softened potentials.
Statistical Analysis
All the data were analyzed by one-way analysis of variance (ANOVA) using GraphPad Prism version 5 software. p < 0.05 values were considered as statistically significant. Values were represented as mean ± SEM, for three rats in each group.
Results and Discussion
Volatile Oil Composition
The root volatile oil prepared by hydro-distillation was subjected to gas chromatography coupled to mass spectrometry (GC/MS). A total of 110 compounds representing 97% were unambiguously identified (Table S1). Compound identification was based on comparison of the mass spectral data and RI with those reported in the literature (Adams 2007). The main components detected in the root were germacrene B (22%), aromadendrene (21.5%), α-santalene (7.1%), geijerene (4.8%), germacrene D (4.3%), and limonene (3.4%).
HPLC-PDA-ESI-MS/MS Profiling
Profiling of the dichloromethane fraction using HPLC-PDA-ESI-MS/MS in negative and positive ion modes (Fig. S1) afforded the identification of 43 secondary metabolites belonging to coumarins, flavonoids, and acridone alkaloids. The identification of the compounds was based on comparison of their MS and MS2 fragmentation data (Fig. S2) with the previously reported data. The compounds were ordered according to their relative retention time (Rt) to grandisine I as summarized in Table S2.
Isolated Compounds
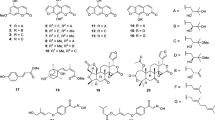
Since most of the identified compounds from HPLC-PDA-ESI-MS/MS are not commercially available, we attempted to isolate the major compounds. Six compounds were isolated from dichloromethane-soluble fraction of total root extract using column chromatography. The chemical structures of the isolated compounds were identified by interpretation of their spectral data including UV, EI-MS, 1H-NMR, 13C-NMR, COSY, HSQC, APT, and HMBC (see Supplementary Material Text S3–S8 and Figs. S3–S8) and comparison of the NMR data with those in the literature. The isolated compounds were xanthyletin (1), citracridone II (2), clausarin (3), O-methylcitrusinine I (4), grandisinine (5), and citracridone I (6).
Effect of Xanthyletin and Citracridone I
Normal Rats
In order to investigate whether the major compounds xanthyletin (1) and citracridone I (6) have any acute toxic effects on rats, their effects at three dose levels on animal behaviors, mortality, renal function, and heart injury of normal rats were first investigated. Compounds 1 and 6 did not produce any hazardous symptoms or death in normal rats. There were no significant changes in both serum creatinine and blood urea nitrogen (BUN) between vehicle and groups treated with varying doses of compounds 1 and 6. However, serum urea was significantly increased in 6 treated groups at 50 and 100 mg/kg dose levels (p < 0.05) when compared to the normal control group (Table 1). Treatment with both 1 and 6 at different dose levels did not affect creatine kinase-MB (CK-MB) when compared to the control group.
Cisplatin-Induced Toxicity
We further investigated whether 1 and 6 exerted nephro- and cardioprotective effects against cisplatin-induced toxicity. As shown in Fig. 1, cisplatin injection in a dose of 7.5 mg/kg induced a significant increase in both serum urea and creatinine levels as compared to the control group (p < 0.05). Injection of 1 for 3 days protected against cisplatin-induced nephrotoxicity as it attenuated cisplatin-induced elevation of both serum urea and creatinine (p < 0.05). On the other hand, pre-treatment with 6, at 5 mg/kg and 50 mg/kg, significantly (p < 0.05) prevented the elevation of serum urea levels. Only 1 and 6 in a dose of 5 mg/kg prevented the elevation of serum creatinine levels when compared to the cisplatin-treated group. Additionally, cisplatin injection in a dose of 7.5 mg/kg induced significant increase in creatine kinase (CK)-MB activity, a marker of cardiac injury, as compared to the control group (p < 0.05). Injection of 1 for 3 days protected against cisplatin-induced cardiotoxicity where it prevented cisplatin-induced elevation of CK-MB in a dose-dependent manner (p < 0.05). However, pre-treatment with 6 at 50 mg/kg and 100 mg/kg significantly (p < 0.05) prevented the elevation of serum CK-MB activity when compared to the cisplatin-treated group (Fig. 1).
Effect of xanthyletin (1) and citracridone I (6) at 5, 50, and 100 mg/kg (i.p.) on serum urea, creatinine, and creatine kinase-MB (CK-MB) levels in cisplatin-treated rats. Values are expressed as the mean ± SE of the mean; N = 3 animals; *p < 0.05, compared with the corresponding control group. @p < 0.05, compared with the corresponding cisplatin-treated group by one-way ANOVA and Tukey post hoc test
The toxicity of cisplatin is mainly associated with increased oxidative stress. For this reason, we measure the level of MDA, an oxidative stress marker. As shown in Fig. 2, cisplatin treatment significantly increased (p < 0.05) the renal and cardiac MDA levels as compared to the control group. Xanthyletin attenuated cisplatin-induced elevation of MDA in both renal and cardiac tissues in a dose-dependent manner compared to the cisplatin-treated group (p < 0.05). Citracridone I, in the highest dose level (100 mg/kg), exerted a pro-oxidant effect in renal tissues as it elevated renal MDA compared with the cisplatin-treated group (p < 0.05) while lower doses had no significant effect on renal MDA levels (p ˃0.05). On the other hand, citracridone I in all dose levels decreased cardiac MDA compared to cisplatin. Moreover, cisplatin decreased both renal and cardiac GSH compared with the normal control group (Fig. 2). Xanthyletin injection in all dose levels protected against cisplatin-induced GSH depletion in both renal and cardiac tissues compared with the cisplatin-treated group (P < 0.05). On the other hand, only the lowest dose of citracridone I was able to elevate GSH in both renal and cardiac tissues as compared to the cisplatin-treated group (p < 0.05, Fig. 2).
Effect of xanthyletin (1) and citracridone I (6) at 5, 50, and 100 mg/kg (i.p.) on renal and cardiac malondialdehyde (MDA) and reduced glutathione content (GSH) in cisplatin-treated rats. Values are expressed as the mean ± SE of the mean; N = 3 animals; *p < 0.05, compared with the corresponding control group. @p < 0.05, compared with the corresponding cisplatin-treated group by one-way ANOVA and Tukey post hoc test
Molecular Docking
The tested compounds fitted well within the binding site of CDK9 (Fig. 3 and Fig. S10). Most of the structures showed hydrogen bond to the hinge amino acid residue Met107 and electrostatic interactions to Mg+2 ion, which are conserved for the native ligands. Adding alkyl substituent improved the interaction in the gate keeper region; however, these affect other interactions with hinge regions. The conserved Lys49 residue showed strong ion-cation interactions with the aromatic rings. In general, the oxygen-containing functionalities orient themselves in the proper position to face the hinge region allowing for forming the required hydrogen bonds.
The interaction profile of xanthyletin (a) and citracridone I (b) with the binding site of cyclin-dependent kinase 9. Left, the 2D interaction map showing the interacting amino acids and the type of interaction. Right, the 3D figure of the protein-ligand complex showing protein as cartoon and ligand as sticks. The Mg+2 is shown as sphere
In Vitro Cytotoxicity Assays
The effects of xanthyletin (1), citracridone I (6), and cisplatin on the growth of HepG2 and MCF-7 cells were studied using the sulforhodamine B assay (SRB). As shown in Fig. 4, xanthyletin (1) possessed an IC50 values of 44.0 μM (HepG2) and 35.7 μM (MCF-7), citracridone I (6) IC50 values of 43.1 μM (HepG2) and 11.4 μM (MCF-7), and cisplatin possessed IC50 values of 1.06 (HepG2) and 1.6 μM (HepG2). On the other hand, a combination of both xanthyletin (1) and citracridone I (6) with cisplatin resulted in a significant increase in the cytotoxic effect of cisplatin on HepG2 cells compared with cisplatin alone, as shown in Fig. 5. In vitro study showed that combination of xanthyletin and cisplatin (IC50 concentration) resulted in a decrease in the percentage of cell viability to 42% of control. Similarly, combination of citracridone I and cisplatin (IC50 concentration) resulted in a decrease in the percentage of cell viability percentage to 38% of control, as shown in Fig. 5a, while a combination of 1 and 6, at an IC50 concentration, with cisplatin using a 30-min lag time did not produce any significant effect on cell viability of MCF-7 cells as compared with cisplatin alone (Fig. 5b).
Cytotoxic effects of different treatments in the combination with cisplatin against HepG2 cells (a) and MCF-7 cells (b) using SRB assay (n = 3), data expressed as the mean value of cell viability (% of control) ± SD, * significantly different from the control group at p < 0.05, @ significantly different from cisplatin alone at p < 0.05
Previous studies showed that xanthyletin derived from Zanthoxylum species has in vitro antioxidant activity (Zhang et al. 2014). It also possesses anti-inflammatory effects and scavenges superoxide radical (Chung et al. 2013). Citracridone I exerts antispasmodic, antioxidant, anti-diabetic, and anti-inflammatory effects (Yang et al. 1987; Bissim et al. 2020). The protective effect of xanthyletin and citracridone I against cisplatin-induced toxicity has been tested in the current study. Although cisplatin is an effective and potent anticancer drug against a wide range of solid cancers (Kart et al. 2010), its effect is complicated with cytotoxicity. It exerted dose-dependent nephrotoxicity and cardiotoxicity (Kang et al. 2009; Soliman et al. 2018).We reported previously that cisplatin impaired renal function and exerted renal injury following single-dose injection (Mahmoud and El Shazly 2013). Searching for new drugs that prevent cisplatin cytotoxicity on normal tissues without affecting their anticancer effects is a must. In the present study, we showed a significant protective effect of xanthyletin and citracridone I in different dose levels on cisplatin-induced nephrotoxicity and cardiotoxicity. It was reported previously that cisplatin causes lipid peroxidation in cardiac tissues (Soliman et al. 2018). This increased lipid peroxidation leads to impairment of membrane permeability and subsequent leakage of intracellular enzymes such as CK-MB.
Several previous studies also related the nephrotoxic and cardiotoxic effects of cisplatin to the generation of reactive oxygen species (ROS) in these tissues (Greggi Antunes et al. 2000; Wozniak et al. 2004). Increased generation of ROS causes lipid peroxidation and generation of lipid peroxidation products such as MDA (Garth and Kenneth 2008). Increased generation of ROS also consumed endogenous antioxidants in the cell such as GSH. Moreover, previous studies showed cisplatin inside cells can be changed into a highly reactive form, that reacts with thiol-containing molecules, such as GSH forming a conjugate leading to GSH depletion (Arany and Safirstein 2003; Hanigan and Devarajan 2003). Reduced glutathione is a potent endogenous non-enzymatic antioxidant. It plays an important role in protection of cellular and subcellular contents against oxidative stress. The tested compounds resorted these parameters to their normal values. Xanthyletin and citracridone I have been reported to have cytotoxic activity against human cancer cell lines (Ndendoung et al. 2014; Segun et al. 2018). Our molecular docking studies, which were performed using the Glide program, supported these previous investigations and revealed that xanthyletin and citracridone I are possibly good targets for CDK9. CDK9 inhibitors have recently emerged as a possible target for the development of anticancer drugs (Sonawane et al. 2016). CDK9 promotes RNA synthesis in genetic programs for cell growth, differentiation, and viral pathogenesis but does not regulate the cell cycle. Its inhibition contributes to the anticancer activity (Wang and Fischer 2008). Previous studies showed that siRNA knockdown of CDK9, instead of CDK1 or CDK2, reduced the levels of cyclin B1 and protooncogene protein, MYC in triple negative breast cancer (TNBC) cell lines (Rajput et al. 2016).
Finally, to investigate the possible cytotoxic effects of xanthyletin and citracridone I on cell viability of cancer cell lines and whether they affect the anticancer potential of cisplatin, xanthyletin and citracridone I alone or in combination with cisplatin was incubated with HepG2 and MCF-7 cancer cell lines. Both drugs alone decreased the cell viability of both cell lines indicating that both drugs possess cytotoxic effects against these cancer cell lines. Furthermore, both drugs potentiated the cytotoxic effect of cisplatin against HepG2 cells. But they did not affect the cytotoxic effect of cisplatin against MCF-7 cells. The cytotoxic effects of the tested drugs may be explained by our docking study results that these compounds can interact with CDK 9. We found also that both xanthyletin and citracridone either potentiate or did not affect the cytotoxic effects of cisplatin on cancer cells. These findings indicate that they have potential for further development for drugs as chemoprotective agents against cisplatin-induced nephro- and cardiotoxicity. These results deserve future investigation of their in vivo cytotoxic activity to unravel the molecular mechanisms for their antitumor potential.
References
Adams RP (2007) Identification of essential oil components by gas chromatography/mass spectrometry. Allured Publishing Corporation, Carol Stream, Illinois
Allam RM, Al-Abd AM, Khedr A, Sharaf OA, Nofal SM, Khalifa AE, Mosli HA, Abdel-Naim AB (2018) Fingolimod interrupts the cross talk between estrogen metabolism and sphingolipid metabolism within prostate cancer cells. Toxicol Lett 291:77–85. https://doi.org/10.1016/j.toxlet.2018.04.008
Arany I, Safirstein RL (2003) Cisplatin nephrotoxicity. Semin Nephrol 23:460–464. https://doi.org/10.1016/S0270-9295(03)00089-5
Bissim SM, Kenmogne SB, Lobe JS, Atangana AF, Bissoue AN, Langat MK, Isyaka SM, Lateef M, Emmanuel NH, Wansi JD, Ali MS, Waffo AF (2020) The chemistry and biological activities of Citrus clementina Hort. ex Tanaka (Rutaceae), a vegetatively propagated species. Nat Prod Res 35:4839–4842. https://doi.org/10.1080/14786419.2020.1731740
Chung CY, Hwang TL, Kuo LM, Kuo WL, Cheng MJ, Wu YH, Sung PJ, Chung MI, Chen JJ (2013) New benzo[c]phenanthridine and benzenoid derivatives, and other constituents from Zanthoxylum ailanthoides: effects on neutrophil pro-inflammatory responses. Int J Mol Sci 14:22395–22408. https://doi.org/10.3390/ijms141122395
Eldahshan OA, Halim AF (2016) Comparison of the composition and antimicrobial activities of the essential oils of green branches and leaves of Egyptian navel orange (Citrus sinensis (L.) Osbeck var. malesy). Chem Biodivers 13:681–685. https://doi.org/10.1002/cbdv.201500139
El-Readi MZ, Hamdan D, Farrag N, El-Shazly A, Wink M (2010) Inhibition of P-glycoprotein activity by limonin and other secondary metabolites from Citrus species in human colon and leukaemia cell lines. Eur J Pharmacol 626:139–145. https://doi.org/10.1016/j.ejphar.2009.09.040
Garth LN, Kenneth AC (2008) Molecular replacement in cancer therapy: reversing cancer metabolic and mitochondrial dysfunction, fatigue and the adverse effects of cancer. Therapy Curr Cancer Ther Rev 4(66):76. https://doi.org/10.2174/157339408783565484
Greggi Antunes LM, D'Arc CD, Bianchi MD (2000) Protective effects of vitamin C against cisplatin-induced nephrotoxicity and lipid peroxidation in adult rats: a dose-dependent study. Pharmacol Res 41:405–411. https://doi.org/10.1006/phrs.1999.0600
Hamdan D, Hamdan D, El-Readi MZ, Nibret E, Sporer F, Farrag N, El-Shazly A, Wink M (2010) Chemical composition of the essential oils of two Citrus species and their biological activities. Die Pharmazie 65:141–147. https://doi.org/10.1691/ph.2010.9731
Hamdan D, El-Readi Mahmoud Z, Tahrani A, Herrmann F, Kaufmann D, Farrag N, El-Shazly A, Wink M (2011a) Secondary metabolites of ponderosa lemon (Citrus pyriformis) and their antioxidant, anti-inflammatory, and cytotoxic activities. Z Naturforsch C 66:385–393. https://doi.org/10.1515/znc-2011-7-810
Hamdan D, El-Readi MZ, Tahrani A, Herrmann F, Kaufmann D, Farrag N, El-Shazly A, Wink M (2011b) Chemical composition and biological activity of Citrus jambhiri Lush. Food Chem 127:394–403. https://doi.org/10.1016/j.foodchem.2010.12.129
Hamdan D, Ashour M, Mulyaningsih S, El Sahzly A, Wink M (2013a) Chemical composition of the essential oils of variegated pink-fleshed lemon (Citrus x limon L. Burm. f.) and their anti-inflammatory and antimicrobial activities. Z Naturforsch C 68:275–284. https://doi.org/10.1515/znc-2013-7-804
Hamdan D, Mohamed M, Abdulla R, Ahmed S, El Sahzly A (2013b) Anti-inflammatory, insecticidal and antimicrobial activities and chemical composition of the essential oils of different plant organs from navel orange (Citrus sinensis (L.) Osbeck var. malesy) grown in Egypt. J Med Plant Res 7:1204–1215.
Hamdan DI, Mahmoud MF, Wink M, El-Shazly AM (2014) Effect of hesperidin and neohesperidin from bittersweet orange (Citrus aurantium var. bigaradia) peel on indomethacin-induced peptic ulcers in rats. Environ Toxicol Phar 37:907–915. https://doi.org/10.1016/j.etap.2014.03.006
Hamdan DI, El-Shiekh RA, El-Sayed MA, Khalil HM, Mousa MR, Al-Gendy AA, El-Shazly AM (2020) Phytochemical characterization and anti-inflammatory potential of Egyptian Murcott mandarin cultivar waste (stem, leaves and peel). Food Funct 211:8214–8236. https://doi.org/10.1039/D0FO01796E
Hanigan MH, Devarajan P (2003) Cisplatin nephrotoxicity: molecular mechanisms. Cancer Ther 1:47–61
Kang KP, Kim DH, Jung YJ, Lee AS, Lee S, Lee SY, Jang KY, Sung MJ, Park SK, Kim W (2009) Alpha-lipoic acid attenuates cisplatin-induced acute kidney injury in mice by suppressing renal inflammation. Nephrol Dial Transplant 24:3012–3020. https://doi.org/10.1093/ndt/gfp242
Kart A, Cigremis Y, Karaman M, Ozen H (2010) Caffeic acid phenethyl ester (CAPE) ameliorates cisplatin-induced hepatotoxicity in rabbit. Exp Toxicol Pathol 62:45–52. https://doi.org/10.1016/j.etp.2009.02.066
Mahmoud MF, El Shazly SM (2013) Pioglitazone protects against cisplatin induced nephrotoxicity in rats and potentiates its anticancer activity against human renal adenocarcinoma cell lines. Food Chem Toxicol 51:114–122. https://doi.org/10.1016/j.fct.2012.09.006
Ndendoung S, Tamokou JD, Lamshöft M, Talontsi F, Meli Lannang A, Sarkar P, Bag PK, Spiteller M (2014) LC-MS guided isolation of antibacterial and cytotoxic constituents from Clausena anisata. Med Chem Res 23:3929–4300. https://doi.org/10.1007/s00044-014-1233-4
Ogunjinmi OE, Olawore NO, Maharaj VJ (2019) Chemical examination of essential oil from stem, roots and fruit peels of Nigerian Citrus jambhiri. J Essent Oil-Bear Plants 22:1614–1621. https://doi.org/10.1080/0972060X.2018.1490208
Rajput S, Khera N, Guo Z, Hoog J, Li S, Ma CX (2016) Inhibition of cyclin dependent kinase 9 by dinaciclib suppresses cyclin B1 expression and tumor growth in triple negative breast cancer. Oncotarget 7:56864–56875. https://doi.org/10.18632/oncotarget.10870
Scordino M, Sabatino L (2014) Characterization of polyphenolic profile of Citrus fruit by HPLC/PDA/ESI/MS-MS. In: Watson RR (ed) Polyphenols in Plants. Academic Press, San Diego, pp 187–199. https://doi.org/10.1016/B978-0-12-397934-6.00009-7
Segun PA, Ismail FM, Ogbole OO, Nahar L, Evans AR, Ajaiyeoba EO, Sarker SD (2018) Acridone alkaloids from the stem bark of Citrus aurantium display selective cytotoxicity against breast, liver, lung and prostate human carcinoma cells. J Ethnopharmacol 227:131–138. https://doi.org/10.1016/j.jep.2018.08.039
Soliman AF, Anees LM, Ibrahim DM (2018) Cardioprotective effect of zingerone against oxidative stress, inflammation, and apoptosis induced by cisplatin or gamma radiation in rats. Naunyn-Schmiedeb Arch Phar 391:819–832. https://doi.org/10.1007/s00210-018-1506-4
Sonawane YA, Taylor MA, Napoleon JV, Rana S, Contreras JI, Natarajan A (2016) Cyclin dependent kinase 9 inhibitors for cancer therapy. J Med Chem 59:8667–8684. https://doi.org/10.1021/acs.jmedchem.6b00150
Tamokou JD, Mbaveng AT, Kuete V (2017) Antimicrobial activities of African medicinal spices and vegetables. In: Kuete V (ed) Medicinal spices and vegetables from Africa. Academic Press, pp 207–237. https://doi.org/10.1016/B978-0-12-809286-6.00008-X
Wang S, Fischer PM (2008) Cyclin-dependent kinase 9: a key transcriptional regulator and potential drug target in oncology, virology and cardiology. Trends Pharmacol Sci 29:302–313. https://doi.org/10.1016/j.tips.2008.03.003
Wozniak K, Czechowska A, Blasiak J (2004) Cisplatin-evoked DNA fragmentation in normal and cancer cells and its modulation by free radical scavengers and the tyrosine kinase inhibitor STI571. Chem Biol Interact 147:309–318. https://doi.org/10.1016/j.cbi.2004.03.001
Yang MS, Wu TS, Wang CH (1987) Citracridone-I: a new antispasmodic from root barks of Citrus depressa. Planta Med 53:143–147. https://doi.org/10.1055/s-2006-962657
Zhang Y, Luo Z, Wang D, He F, Li D (2014) Phytochemical profiles and antioxidant and antimicrobial activities of the leaves of Zanthoxylum bungeanum. Sci World J 2014:181072. https://doi.org/10.1155/2014/181072
Acknowledgements
The authors are grateful to Prof. M. Wink and Dr. M. Sobeh, Institute of Pharmacy and Molecular Biotechnology, Heidelberg University, Germany, for performing LC-MS analysis. The authors are also beholden to Ass. Prof. M. L. Ashour (Faculty of Pharmacy, Ain Shams University) and Ass. Prof. M. E. Mohamed (School of Clinical Pharmacy, King Faisal University, Saudi Arabia) for carrying out EIMS and NMR analysis for some isolated bioactive metabolites.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
DIH extracted the plant and elucidated the isolated compounds. GC-MS and HPLC-MS analyses were performed by DIH, RAA, and AME. Pharmacological experiments were carried out by MFM. MAN carried out molecular docking study. DIH, RAA, and MFM wrote the draft. AME supervised laboratory work. All authors revised and approved the manuscript before submission.
Corresponding author
Ethics declarations
Protection of Human and Animal Subjects
The authors declare that all animal experiments were performed after the approval of institutional animal ethical committee of the Faculty of Pharmacy, Zagazig University (P7-6-2016), and animals were handled following the International Animal Ethics Guidelines, ensuring minimum animal suffering.
Supplementary Information
ESM 1
(PDF 2114 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hamdan, D.I., Attia, R.A., Nael, M.A. et al. Chemical Profiling of Citrus sinensis Root and the Effects of Its Secondary Metabolites on Cisplatin-Induced Renal and Cardiac Toxicities. Rev. Bras. Farmacogn. 33, 106–115 (2023). https://doi.org/10.1007/s43450-022-00294-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43450-022-00294-2