Abstract
Doxorubicin, sold under the brand name Adriamycin among others, is an important drug for cancer therapy; however, its use is limited by its cardiotoxicity. Ginsenoside Rg2 is extracted from Panax ginseng C.A.Mey., Araliaceae, which is believed to have cardioprotective properties. However, to date, there have been no reports on whether ginsenoside Rg2 could protect cardiomyocytes against doxorubicin. In this study, we investigated the action and the underlying mechanisms of cardioprotection of ginsenoside Rg2 upon doxorubicin treatment. Cell counting kit-8 was used to determine cell viability; in addition, terminal deoxynucleotidyl transferase–mediated dUTP nick-end labeling staining was used to detect apoptotic cells. Western blotting was used to investigate the relevant pathways. LY294002, a phosphatidylinositol 3-kinase inhibitor, was also used in this study. Ginsenoside Rg2 significantly (p < 0.01) neutralized cardiomyocyte apoptosis induced by doxorubicin in a dose-dependent manner, but this effect was blocked by LY294002. Furthermore, ginsenoside Rg2 upregulated protein kinase B phosphorylation through the phosphatidylinositol 3-kinase/protein kinase B pathway and inhibited p53 expression. These results suggest that ginsenoside Rg2 could attenuate doxorubicin-induced cardiomyocyte apoptosis via the phosphatidylinositol 3-kinase/protein kinase B pathway.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Doxorubicin (DOX) is an effective drug for the treatment of malignant neoplasia. However, the application of DOX is limited by its cardiotoxicity. Previous studies have shown that long-term DOX application leads to the high morbidity of irreversible and late-onset dilated cardiomyopathy (Gupta et al. 2022). Therefore, new strategies are needed to control DOX cardiotoxicity and protect cardiac myocytes from damage.
Ginseng, the root of Panax ginseng C.A.Mey., Araliaceae, is a Chinese herbal medicine widely used for its health benefits and has been used to treat cardiovascular diseases in the USA (Kaufman et al. 2002), Canada (Pharand et al. 2003), and Europe (Nilsson et al. 2001). To date, over 100 ginsenosides have been identified and many of them have been studied for the treatment of various diseases (Huang et al. 2017). As a famous traditional Chinese medicine, ginseng is now widely used to reinforce human endurance and resistance to fatigue and physical stress, owing to its role in improving energy and longevity (Liu and Xiao 1992). Many previous studies have demonstrated that ginseng plays a role in immunomodulation, cancer suppression, cardiac protection, and neuroprotection (Xiang et al. 2008).
Ginsenoside Rg2 (1) is one of the major component of the ginsenoside extracted from P. ginseng (Jeon et al. 2021). The formula of Rg2 is C42H72O13 and its molecular weight is 785.03. This metabolite is a dammarane which is substituted by hydroxy groups at the 3-β, 6-α, 12-β, and 20 pro-S positions, in which the hydroxy group at position 6 has been converted to the corresponding α-l-rhamnopyranosyl-(1 → 2)-β-d-glucopyranoside, and in which a double bond has been introduced at the C24-C25 position. It has various pharmacological functions, such as anti-myocardial fibrotic (Li et al. 2020), vascular dementia-ameliorating (Cui et al. 2017), glucose- and fat metabolism–improving (Cheng et al. 2020), antioxidant (Jeon et al. 2021), and antidepressant (Ren et al. 2017) effects. However, the effect of ginsenoside Rg2 on myocardial injury protection and its underlying mechanism remain unclear. It has been shown that ginsenosides Rg1, Rg3, Rb1, and Rh2 (Wang et al. 2012; Li et al. 2017; Zhang et al. 2017; Xu et al. 2018) can alleviate cardiotoxicity induced by DOX, but there are no reports on ginsenoside Rg2.

In view of previous studies, we hypothesized that ginsenoside Rg2 could have the same effect of attenuating DOX-induced cardiomyocytes apoptosis as other ginsenosides. To test this hypothesis, we examined whether ginsenoside Rg2 is functional in H9c2 cells (Rat BDIX heart myoblast) treated with DOX. The expression of Akt and p53 was determined to demonstrate the possible mechanism of action. We aimed to determine whether Rg2 could protect cells from apoptosis through the phosphatidylinositol 3-kinase (PI3K)/Akt (protein kinase B)/p53 signaling pathway.
Materials and Methods
Cell Culture
H9c2 cells were cultured in 10% DMEM, of which the Dulbecco’s Modified Eagle’s Medium (DMEM) contained 10% fetal bovine serum (FBS) and 1% P-S (100 U/ml penicillin and 100 μg/ml streptomycin), at 37 °C in a 5% CO2 incubator. H9c2 cells were treated with 0.25% trypsin at 80% confluency and passaged at a 1:4 ratio approximately every 3 days. The cells used in subsequent experiments were in the logarithmic growth phase. Each subsequent experiment was performed at least in triplicate.
Cell Cytotoxicity Assay
DOX and ginsenoside Rg2 were respectively first dissolved in dimethyl sulfoxide (DMSO) to 100 mM and then diluted by 0.5% DMEM (DMEM contained 0.5% FBS and 1% P-S) to different experiment concentrations. H9c2 cells were seeded in 96-well plates at a density of 1 × 104 cells/well and incubated at 37 °C for 24 h in 0.5% DMEM. The cells were then treated with different concentrations of DOX (2.5, 5, 10, 15, and 20 μM) or ginsenoside Rg2 (purity of 98.18%; Chengdu Must Bio-Technology Co., Ltd., Chengdu, China, lot number: MUST-20040501) at 200, 250, 300, 350, and 400 μM for another 24 h. After that, the cell supernatant was replaced with 10% cell counting kit-8 (CCK-8) diluent and incubated at 37 °C for 1 h before the CCK-8 assay was performed. The absorbance value was measured at 450 nm using a multifunctional enzyme standard instrument (Synergy H1, BioTek). Cell viability was calculated according to the cell viability (%) = (OD treatment/OD Control) × 100 (Tong et al. 2021).
To investigate the effect of ginsenoside Rg2 on DOX-induced cardiomyocyte apoptosis, H9c2 cells were first treated with ginsenoside Rg2 for 24 h followed by the addition of DOX (5 μM) for another 24 h. Thereafter, the CCK-8 assay was performed. To determine the underlying mechanism, H9c2 cells were pretreated with LY294002 (LY294) (1 μM), a PI3K inhibitor, for 30 min before ginsenoside Rg2 intervention.
Apoptosis Assay
Terminal deoxynucleotidyl transferase–mediated dUTP nick-end labeling (TUNEL) staining was used to detect morphological features of apoptosis. After treatment with different concentrations (100, 200, and 250 μM) of ginsenoside Rg2 for 24 h, H9c2 cells were treated with DOX (5 μM) for another 24 h. Next, the cell supernatant was replaced with immune staining fix solution for 30 min. After washing with phosphate-buffered saline, the cells were mixed with enhanced immunostaining permeabilization solution for 5 min. The TUNEL solution was then added to the cells and incubated for 60 min at 37 °C, followed by detection using a 200-fold fluorescence microscope (Olympus BX50, Tokyo, Japan). Cell apoptosis ratio was calculated as the percentage of apoptotic cells number per total cells number per field (Cui et al. 2021).
ROS Assay
The reactive oxygen species assay kit was used to analyze reactive oxygen species (ROS) levels in cardiomyocytes. H9c2 cells were seeded in 96-well plates at a density of 1 × 104 cells/well. After intervention with ginsenoside Rg2 (250 μM), DOX (5 μM), and LY294 (1 μM), DCFH-DA (10 μM) was added to the cells, followed by incubation for 20 min at 37 °C. The cells were then washed three times with DMEM, and ROS absorbance was measured by a multifunctional enzyme standard instrument at the excitation wavelength of 488 nm and emission wavelength of 525 nm (Liu et al. 2020).
Western Blot Analysis
Briefly, the total proteins of H9c2 cells were extracted according to the manufacturer’s instructions. The protein concentration was determined using the bicinchoninic acid protein concentration determination kit. After denaturation at 95 °C for 5 min, proteins were separated via SDS-PAGE (10% separating gel and 5% stacking gel, 60 V for 30 min, 100 V for 1 h). The separated proteins were transferred to polyvinylidene difluoride membranes at 350 mA for 1 h. Protein ladder markers were used to observe the molecular weight positions. The membranes were pre-incubated in blocking buffer-TBST containing 5% skim milk (w/v) for 1 h at room temperature (nearly 20 °C) and then incubated overnight at 4 °C with primary antibodies at a dilution of 1:1000. After washing with TBST, the membranes were incubated with a secondary antibody (1:2000 dilution) for 1 h at room temperature. Protein bands were detected using the ECL chemiluminescence system and a gel imaging system (Chemiscope 6300). ImageJ software was used for the gray value statistics. GAPDH was used as the loading control (Xi et al. 2020).
Statistical Analysis
All data are presented as mean ± standard deviation and were analyzed using SPSS Statistics 25.0. An independent sample t-test was used to compare the two groups. Comparison among three groups (above) was performed via one-way analysis of variance, and the least significant difference test was performed between groups. Non-normal distribution was represented by the median, and the rank sum test was adopted. Statistical significance was assumed if p reached a value < 0.05. Graphs were created using GraphPad Prism 7.0.
Results and Discussion
Cancer is one of the main causes of death worldwide, and chemotherapy is an important treatment method. Although DOX is an important chemotherapeutic, it exhibits dose-dependent cardiotoxicity. Although the detailed mechanism of DOX-induced cardiac toxicity has not been fully elucidated, numerous studies have indicated that DOX-related myocardial damage is possibly correlated with apoptosis, inflammation, oxidative stress, and autophagy (Mantawy et al. 2014). It has been reported that after the administration of DOX, excessive ROS are produced in cardiomyocytes, accompanied by the upregulation of p53 and caspase-3, thereby activating apoptotic pathways (Khafaga et al. 2018), which eventually lead to cardiac dysfunction.
Inhibition of Apoptosis
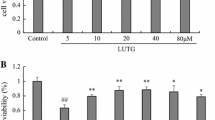
First, a DOX-induced apoptosis model in cardiomyocytes was established. The cytotoxicity of DOX in H9c2 cells was analyzed using the CCK-8 assay, using the appropriate concentration of DOX (5 μM) at 24 h (Fig. 1A). The cytotoxicity of ginsenoside Rg2 was also measured using the CCK-8 assay, which demonstrated that the maximum safe concentration was 300 μM after 24 h of treatment (Fig. 1B). Then, the effect of simultaneous ginsenoside Rg2 and DOX treatment in H9c2 cells were tested, showing that ginsenoside Rg2 significantly increased the cell viability in a dose-dependent manner (Fig. 1C). To further investigate the effect of simultaneous administration of ginsenoside Rg2 and DOX, H9c2 cells were pretreated with ginsenoside Rg2 (100, 200, and 250 μM) for 24 h before DOX (5 μM) treatment followed by TUNEL staining (Fig. 1D). These results showed that ginsenoside Rg2 notably reduced the apoptotic rate compared with that of the DOX-treated cells alone, and the apoptotic rate was even lower at 200 μM and 250 μM than at 100 μM ginsenoside Rg2 (p < 0.01) (Fig. 1E). These data suggested that ginsenoside Rg2 inhibited cardiomyocyte apoptosis against DOX.
Ginsenoside Rg2 neutralized DOX-induced cardiomyocyte apoptosis. CCK-8 was used to measure H9c2 cells viability under the influence of DOX (A), Rg2 (B), and DOX with different concentrations of Rg2 (C). Morphological features of cell apoptosis in H9c2 cells were detected via TUNEL staining, in green (D), and cell apoptosis ratio was calculated using ImageJ (E). The scale is 100 μm in picture D, and the white arrow points to the bright apoptotic cells. Data were obtained from at least three independent experiments. ▲p < 0.01 vs control group; ★p < 0.01 vs DOX group; □p < 0.05; and ■p < 0.01 vs Rg2 (200 μM) group. DOX doxorubicin; CCK-8 cell counting kit-8
Inhibition of ROS Generation
As excessive ROS are generated by DOX, ROS content in H9c2 cells was tested after treatment with ginsenoside Rg2 and DOX using the ROS assay kit. We found a higher level of ROS in the DOX (p < 0.01) and DOX + Rg2 + LY294 (p < 0.05) groups than in the control group. Meanwhile, ROS was markedly reduced in the DOX + Rg2 group (p < 0.01) (Fig. 2A). These results suggest that ginsenoside Rg2 could reduce ROS generation caused by DOX.
Ginsenoside Rg2 decreased ROS generation and apoptosis through PI3K/Akt pathway. A ROS assay was used to test the expression of ROS absorption. B CCK-8 assay was used to detect the cell viability. Data were obtained from at least three independent experiments. ▲p < 0.01 and △p < 0.05 vs control group; ★p < 0.01 vs DOX group; ■p < 0.01 vs Rg2 (250 μM) group. ROS reactive oxygen species; PI3K phosphatidylinositol 3-kinase; Akt protein kinase B; CCK-8 cell counting kit-8
Upregulation of PI3K/Akt Pathway
To further clarify the mechanism by which ginsenoside Rg2 inhibits apoptosis and ROS generation in cardiomyocytes, LY294, an inhibitor of PI3K, was used for treatment. ROS content was dramatically upregulated in H9c2 cells after DOX treatment, which was distinctly inhibited by ginsenoside Rg2. LY294 notably counteracted the inhibitory effect of ginsenoside Rg2 (Fig. 2A). We found that ginsenoside Rg2 significantly inhibited cardiomyocyte apoptosis induced by DOX, which was suppressed by LY294 (Fig. 2B). These data suggest that the effect of ginsenoside Rg2 on cardiomyocyte apoptosis and ROS generation occurs through the PI3K/Akt pathway.
To further reveal the mechanism of action of ginsenoside Rg2 against DOX, the PI3K/Akt/p53 pathway was studied. It was found that Akt did not significantly differ between the DOX and DOX + Rg2 groups, but p-Akt was both upregulated in the DOX group and DOX + Rg2 group, and the value of p-Akt/Akt was notably increased in H9c2 cells when treated with ginsenoside Rg2, which was inhibited by LY294 (Fig. 3A and B). Meanwhile, DOX dramatically increased both p53 and p-p53 expression in H9c2 cells. Simultaneous ginsenoside Rg2 treatment significantly reduced p53 expression (p < 0.01), and although it did not significantly inhibit p-p53, a trend could be observed, although this observation is not within the function of LY294 (Fig. 3A and B). Taken together, these results indicate that ginsenoside Rg2 upregulates Akt phosphorylation through the PI3K/Akt pathway and inhibits p53 expression.
Ginsenoside Rg2 upregulated Akt phosphorylation and inhibited DOX upregulation of p53 expression. A The level of Akt, p-Akt, p53, and p-p53 proteins in H9c2 cells was detected via Western blotting. B ImageJ was used to measure the gray value of strip. Data were obtained from at least three independent experiments. ▲p < 0.01 and △p < 0.05 vs control group; ★p < 0.01 and ☆p < 0.05 vs DOX group; □p < 0.05 and ■p <0.01 vs DOX + Rg2 group. Akt protein kinase B; DOX doxorubicin
In our study, it was found that DOX led to cardiomyocyte apoptosis and induced ROS generation at certain concentrations, which was consistent with previous studies. Also, ginsenoside Rg2 significantly reduced cardiomyocyte apoptosis and ROS generation caused by DOX. Ginsenoside Rg2 is one of the active principles found in ginseng. The ginsenosides have displayed multiple functions. For instance, ginsenoside Rg3 can inhibit proliferation, metastasis, and angiogenesis, as well as activate apoptosis and promote immunoreaction in cancer (Sun et al. 2017). In terms of anti-apoptosis, ginsenoside Rb3 exerted anti-apoptotic effects on cardiomyocytes via the PPARα pathway (Chen et al. 2019), whereas ginsenoside Rg1 attenuated dopamine-induced apoptosis in PC12 cells by suppressing oxidative stress (Chen et al. 2003). Ginsenoside Rd was also reported to attenuate the mitochondria-dependent apoptotic pathway via Akt/GSK-3β signaling in myocardial ischemia/reperfusion injury (Wang et al. 2013). Ginsenoside Rg2 (1) was previously reported to be capable of relieving cardiotoxicity induced by trastuzumab (Liu et al. 2022), but no report on DOX. In our study, the anti-apoptotic function of ginsenoside Rg2 in DOX-treated cells was confirmed using TUNEL staining, of which the effect was dose-dependent.
To further identify the mechanism of action of ginsenoside Rg2 against DOX, the PI3K/Akt pathway was investigated. The PI3K/Akt pathway is thought to be closely related to differentiation, proliferation, apoptosis, and oxidative stress. Its activation is known to reduce cardiomyocyte apoptosis (Shu et al. 2019). p53, a downstream factor of the PI3K/Akt pathway, is a classic tumor suppressor and its activation has been reported to accelerate cardiomyocyte apoptosis (Sun et al. 2014). Previous studies have also demonstrated that Akt phosphorylation and p53 inhibition could inhibit DOX-induced cardiomyocyte apoptosis and improve myocardial systolic function (Chen et al. 2015). In this study, our data illustrated that the ginsenoside Rg2–mediated inhibition of cardiomyocyte apoptosis induced by DOX was repressed by LY294. And results of our ROS assay also showed that there’s a trend that ginsenoside Rg2–induced suppression of DOX-induced ROS could be inhibited by LY294, though of no obvious significance. In addition, Western blot analysis indicated that ginsenoside Rg2 notably upregulated p-Akt and p-Akt/t-Akt, which was also suppressed by LY294. These results demonstrated that the effect of ginsenoside Rg2 against DOX was mediated through the PI3K/Akt pathway. Consistently, in a mouse model of myocardial infarction, ginsenoside Rg2 was also shown to improve cardiac function by promoting Akt phosphorylation (Li et al. 2020). Our results also showed that DOX significantly upregulated p53 expression, which was remarkably inhibited by ginsenoside Rg2. Although no significant difference was observed in the levels of p-p53 upon ginsenoside Rg2 treatment, there was a downregulation trend. However, it is unclear whether LY294 represses the expression of p53 and p-p53 and elevates p-p53/p53.
Conclusion
This study is the first to report that ginsenoside Rg2 can potentially suppress DOX-triggered cardiotoxicity. The protective effect of ginsenoside Rg2 against DOX-induced toxicity was shown to be associated with decreased production of ROS, downregulation of p53, and increased Akt phosphorylation. The attenuation of DOX-induced cardiomyocyte apoptosis triggered by ginsenoside Rg2 was mediated by the PI3K/Akt pathway. However, the underlying mechanism involving the PI3K/Akt pathway remains to be further explored. It is proposed that upregulation and downregulation of Akt with plasmid transfection and RNA interference in the following research of ginsenoside Rg2 on DOX could be useful to clarify the precise mechanism. And additionally, given the anti-tumor effects of ginsenosides, it is hoped that the application of ginsenoside Rg2 in combination with DOX will improve cardiotoxicity and inhibit tumor cell activity at the same time.
References
Chen RC, Xu XD, Zhi Liu X, Sun GB, Zhu YD, Dong X, Wang J, Zhang HJ, Zhang Q, Sun XB (2015) Total flavonoids from Clinopodium chinense (Benth.) O. Ktze protect against doxorubicin-induced cardiotoxicity in vitro and in vivo. Evid Based Complement Alternat Med 2015:472565. https://doi.org/10.1155/2015/472565
Chen X, Wang Q, Shao M, Ma L, Guo D, Wu Y, Gao P, Wang X, Li W, Li C, Wang Y (2019) Ginsenoside Rb3 regulates energy metabolism and apoptosis in cardiomyocytes via activating PPARα pathway. Biomed Pharmacother 120:109487. https://doi.org/10.1016/j.biopha.2019.109487
Chen XC, Zhu YG, Zhu LA, Huang C, Chen Y, Chen LM, Fang F, Zhou YC, Zhao CH (2003) Ginsenoside Rg1 attenuates dopamine-induced apoptosis in PC12 cells by suppressing oxidative stress. Eur J Pharmacol 473:1–7. https://doi.org/10.1016/s0014-2999(03)01945-9
Cheng B, Gao W, Wu X, Zheng M, Yu Y, Song C, Miao W, Yang Z, He Y, Liu C, Yang W, Yang X, Li Y, Zhang F, Gao Y (2020) Ginsenoside Rg2 ameliorates high-fat diet-induced metabolic disease through SIRT1. J Agric Food Chem 68:4215–4226. https://doi.org/10.1021/acs.jafc.0c00833
Cui J, Wang J, Zheng M, Gou D, Liu C, Zhou Y (2017) Ginsenoside Rg2 protects PC12 cells against β-amyloid(25-35)-induced apoptosis via the phosphoinositide 3-kinase/Akt pathway. Chem Biol Interact 275:152–161. https://doi.org/10.1016/j.cbi.2017.07.021
Cui Y, Pu R, Ye J, Huang H, Liao D, Yang Y, Chen W, Yao Y, He Y (2021) LncRNA FAM230B promotes gastric cancer growth and metastasis by regulating the miR-27a-5p/TOP2A axis. Dig Dis Sci 66:2637–2650. https://doi.org/10.1007/s10620-020-06581-z
Gupta MK, Sun Y, Stenson KT, Naga Prasad SV (2022) Anthracycline cardiotoxicity is associated with elevated beta1-adrenergic receptor density. J Am Heart Assoc 11:e023457. https://doi.org/10.1161/JAHA.121.023457
Huang GX, Khan I, Li XA, Chen L, Leong WK, Ho LT, Hsiao WLW (2017) Ginsenosides Rb3 and Rd reduce polyps formation while reinstate the dysbiotic gut microbiota and the intestinal microenvironment in Apc(Min/+) mice. Sci Rep-UK 7:14. https://doi.org/10.1038/s41598-017-12644-5
Jeon H, Huynh DTN, Baek N, Nguyen TLL, Heo KS (2021) Ginsenoside-Rg2 affects cell growth via regulating ROS-mediated AMPK activation and cell cycle in MCF-7 cells. Phytomedicine 85:153549. https://doi.org/10.1016/j.phymed.2021.153549
Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA (2002) Recent patterns of medication use in the ambulatory adult population of the United States – the Slone survey. JAMA 287:337–344. https://doi.org/10.1001/jama.287.3.337
Khafaga AF, El-Sayed YS (2018) All-trans-retinoic acid ameliorates doxorubicin-induced cardiotoxicity: in vivo potential involvement of oxidative stress, inflammation, and apoptosis via caspase-3 and p53 down-expression. Naunyn Schmiedeberg's Arch Pharmacol 391:59–70. https://doi.org/10.1007/s00210-017-1437-5
Li L, Ni J, Li M, Chen J, Han L, Zhu Y, Kong D, Mao J, Wang Y, Zhang B, Zhu M, Gao X, Fan G (2017) Ginsenoside Rg3 micelles mitigate doxorubicin-induced cardiotoxicity and enhance its anticancer efficacy. Drug Deliv 24:1617–1630. https://doi.org/10.1080/10717544.2017.1391893
Li X, Xiang N, Wang Z (2020) Ginsenoside Rg2 attenuates myocardial fibrosis and improves cardiac function after myocardial infarction via AKT signaling pathway. Biosci Biotechnol Biochem 84:2199–2206. https://doi.org/10.1080/09168451.2020.1793292
Liu CX, Xiao PG (1992) Recent advances on ginseng research in China. J Ethnopharmacol 36:27–38. https://doi.org/10.1016/0378-8741(92)90057-x
Liu G, Zhang J, Sun F, Ma J, Qi X (2022) Ginsenoside Rg2 attenuated trastuzumab-induced cardiotoxicity in rats. Biomed Res Int 2022:8866660. https://doi.org/10.1155/2022/8866660
Liu H, Weng XJ, Yao JY, Zheng J, Lv X, Zhou XH, Jiang H, Li ST (2020) Neuregulin-1beta protects the rat diaphragm during sepsis against oxidative stress and inflammation by activating the PI3K/Akt pathway. Oxidative Med Cell Longev 2020:1720961. https://doi.org/10.1155/2020/1720961
Mantawy EM, El-Bakly WM, Esmat A, Badr AM, El-Demerdash E (2014) Chrysin alleviates acute doxorubicin cardiotoxicity in rats via suppression of oxidative stress, inflammation and apoptosis. Eur J Pharmacol 728:107–118. https://doi.org/10.1016/j.ejphar.2014.01.065
Nilsson M, Trehn G, Asplund K (2001) Use of complementary and alternative medicine remedies in Sweden. A population-based longitudinal study within the northern Sweden MONICA Project. J Intern Med 250:225–233. https://doi.org/10.1046/j.1365-2796.2001.00882.x
Pharand C, Ackman ML, Jackevicius CA, Paradiso-Hardy FL, Pearson GJ, Canadian Cardiovasc Pharmacists N (2003) Use of OTC and herbal products in patients with cardiovascular disease. Ann Pharmacother 37:899–904. https://doi.org/10.1345/aph.1C163
Ren Y, Wang JL, Zhang X, Wang H, Ye Y, Song L, Wang YJ, Tu MJ, Wang WW, Yang L, Jiang B (2017) Antidepressant-like effects of ginsenoside Rg2 in a chronic mild stress model of depression. Brain Res Bull 134:211–219. https://doi.org/10.1016/j.brainresbull.2017.08.009
Shu Z, Yang Y, Yang L, Jiang H, Yu X, Wang Y (2019) Cardioprotective effects of dihydroquercetin against ischemia reperfusion injury by inhibiting oxidative stress and endoplasmic reticulum stress-induced apoptosis via the PI3K/Akt pathway. Food Funct 10:203–215. https://doi.org/10.1039/c8fo01256c
Sun A, Zou Y, Wang P, Xu D, Gong H, Wang S, Qin Y, Zhang P, Chen Y, Harada M, Isse T, Kawamoto T, Fan H, Yang P, Akazawa H, Nagai T, Takano H, Ping P, Komuro I, Ge J (2014) Mitochondrial aldehyde dehydrogenase 2 plays protective roles in heart failure after myocardial infarction via suppression of the cytosolic JNK/p53 pathway in mice. J Am Heart Assoc 3:e000779. https://doi.org/10.1161/jaha.113.000779
Sun M, Ye Y, Xiao L, Duan X, Zhang Y, Zhang H (2017) Anticancer effects of ginsenoside Rg3 (Review). Int J Mol Med 39:507–518. https://doi.org/10.3892/ijmm.2017.2857
Tong Y, Liu L, Wang R, Yang T, Wen J, Wei S, Jing M, Zou W, Zhao Y (2021) Berberine attenuates chronic atrophic gastritis induced by MNNG and its potential mechanism. Front Pharmacol 12:644638. https://doi.org/10.3389/fphar.2021.644638
Wang H, Yu P, Gou H, Zhang J, Zhu M, Wang ZH, Tian JW, Jiang YT, Fu FH (2012) Cardioprotective effects of 20(S)-ginsenoside Rh2 against doxorubicin-induced cardiotoxicity in vitro and in vivo. Evid Based Complement Alternat Med 2012:506214. https://doi.org/10.1155/2012/506214
Wang Y, Li X, Wang X, Lau W, Wang Y, Xing Y, Zhang X, Ma X, Gao F (2013) Ginsenoside Rd attenuates myocardial ischemia/reperfusion injury via Akt/GSK-3β signaling and inhibition of the mitochondria-dependent apoptotic pathway. PLoS One 8:e70956. https://doi.org/10.1371/journal.pone.0070956
Xi Z, Qiao Y, Wang J, Su H, Bao Z, Li H, Liao X, Zhong X (2020) Gastrodin relieves inflammation injury induced by lipopolysaccharides in MRC-5 cells by up-regulation of miR-103. J Cell Mol Med 24:1451–1459. https://doi.org/10.1111/jcmm.14826
Xiang YZ, Shang HC, Gao XM, Zhang BL (2008) A comparison of the ancient use of ginseng in traditional Chinese medicine with modern pharmacological experiments and clinical trials. Phytother Res 22:851–858. https://doi.org/10.1002/ptr.2384
Xu ZM, Li CB, Liu QL, Li P, Yang H (2018) Ginsenoside Rg1 prevents doxorubicin-induced cardiotoxicity through the inhibition of autophagy and endoplasmic reticulum stress in mice. Int J Mol Sci 19. https://doi.org/10.3390/ijms19113658
Zhang Y, Wang Y, Ma Z, Liang Q, Tang X, Tan H, Xiao C, Gao Y (2017) Ginsenoside Rb1 inhibits doxorubicin-triggered H9C2 cell apoptosis via aryl hydrocarbon receptor. Biomol Ther (Seoul) 25:202–212. https://doi.org/10.4062/biomolther.2016.066
Funding
This work was supported by the National Natural Science Foundation of China (No. 81804010) and Longyi Scholar Project of National Clinical Research Base of Traditional Chinese Medicine of Longhua Hospital (LYTD-83).
Author information
Authors and Affiliations
Contributions
BQ and MM conducted the experiments and drafted the manuscript. ZM and LSh collected and analyzed the data. BD and DZ reviewed and edited the manuscript. YW and WZ conceived and designed the study. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Qiu, B., Mao, M., Ma, Z. et al. Ginsenoside Rg2 Attenuates Doxorubicin-induced Cardiomyocyte Apoptosis via the PI3K/Akt Pathway. Rev. Bras. Farmacogn. 32, 433–439 (2022). https://doi.org/10.1007/s43450-022-00261-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43450-022-00261-x