Abstract
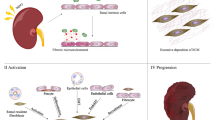
Renal tubulointerstitial fibrosis (RTIF) is a common feature and inevitable consequence of all progressive chronic kidney diseases, leading to end-stage renal failure regardless of the initial cause. Although research over the past few decades has greatly improved our understanding of the pathophysiology of RTIF, until now there has been no specific treatment available that can halt the progression of RTIF. Norcantharidin (NCTD) is a demethylated analogue of cantharidin, a natural compound isolated from 1500 species of medicinal insect, the blister beetle (Mylabris phalerata Pallas), traditionally used for medicinal purposes. Many studies have found that NCTD can attenuate RTIF and has the potential to be an anti-RTIF drug. This article reviews the recent progress of NCTD in the treatment of RTIF, with emphasis on the pharmacological mechanism of NCTD against RTIF.


Similar content being viewed by others
Availability of data and materials
This article has no additional data.
Abbreviations
- α-SMA:
-
α-Smooth muscle actin
- BMP:
-
Bone morphogenetic protein
- BSA:
-
Bovine serum albumin
- CaN:
-
Calcineurin
- CDKN1A:
-
Cyclin-dependent kinase inhibitor 1A
- CKDs:
-
Chronic kidney diseases
- Col-I:
-
Collagen I
- Col IV:
-
Type IV Collagen
- CRF:
-
Chronic renal failure
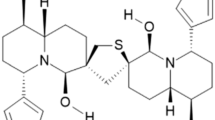
- CTD:
-
Cantharidin
- CTGF:
-
Connective tissue growth factor or CCN2
- DN:
-
Diabetic nephropathy
- ECM:
-
Extracellular matrix
- EGFR:
-
Epidermal growth factor receptor
- EMT:
-
Epithelial-mesenchymal transition
- EZH2:
-
Zeste homolog 2
- FN:
-
Fibronectin, a fibrosis marker
- Fn14:
-
Fibroblast growth factor-inducible 14
- FoxO:
-
Forkhead box O
- GAS5:
-
Growth arrest-specific transcript 5
- H3 K79:
-
Histone H3 lysine79
- HEA:
-
N6-(2-Hydroxyethyl) adenosine
- HK-2 cells:
-
Human renal proximal tubular epithelial cell line
- ITGB1:
-
Integrin beta-1
- JNK:
-
C-Jun amino terminal kinase
- KLF5:
-
Krüppel- like factor 5
- lncRNAs:
-
Long noncoding RNA molecules
- miRNAs:
-
MicroRNAs
- MMP9:
-
Matrix metalloproteinase 9
- ncRNAs:
-
Non-coding RNAs
- NCTD:
-
Norcantharidin
- NF-κB:
-
Nuclear factor-kappaB
- NFATc:
-
Nuclear factor of activated T cells cytosolic component
- Nrf2:
-
Nuclear factor erythroid2-related factor2
- OA:
-
Okadaic acid
- PAI-1:
-
Plasminogen activator inhibitor-1 or SERPINE1
- Pink1:
-
PTEN-induced kinase 1
- PON:
-
Protein overload nephropathy
- PP2A:
-
Protein phosphatase 2A
- PP2Aa:
-
A structural subunit A of PP2A
- PP2Ab:
-
A highly variable regulatory subunit B of PP2A
- PP2Ac:
-
A catalytic subunit C of PP2A
- p-Smad3:
-
C-terminal-phosphorylated Smad3
- RTIF:
-
Renal tubulointerstitial fibrosis
- SHH:
-
Sonic Hedgehog
- SIS3:
-
A specific inhibitor of Smad3
- Smad:
-
Mothers against decapentaplegic homolog
- Snail1:
-
A transcription factor of E-cadherin
- SncRNAs:
-
Short ncRNAs
- Sp1:
-
Special protein1
- STAT3:
-
Signal transducer and activator of transcription 3
- TGF-β1:
-
Transforming growth factor-β1
- TWEAK:
-
Tumor necrosis factor-like weak inducer of apoptosis
- UUO:
-
Unilateral ureteral obstruction
- XIST:
-
X-inactive specific transcript
References
Honkanen RE. Cantharidin, another natural toxin that inhibits the activity of serine/threonine protein phosphatases types 1 and 2A. FEBS Lett. 1993;330(3):283–6. https://doi.org/10.1016/0014-5793(93)80889-3.
Zheng J, Wang JJ, Ma HM, Shen MQ, Qian ZM, Bao YX. Norcantharidin down-regulates iron contents in the liver and spleen of lipopolysaccharide-treated mice. Redox Rep. 2022;27(1):119–27. https://doi.org/10.1080/13510002.2022.2088011.
Wang GS. Medical uses of mylabris in ancient China and recent studies. J Ethnopharmacol. 1989;26(2):147–62. https://doi.org/10.1016/0378-8741(89)90062-7.
Hsieh CH, Chao KS, Liao HF, Chen YJ. Norcantharidin, derivative of cantharidin, for cancer stem cells. Evid Based Complement Altern Med. 2013;2013: 838651. https://doi.org/10.1155/2013/838651.
Liu FY, Li Y, Peng YM, Ye K, Li J, Liu YH, et al. Norcantharidin ameliorates proteinuria, associated tubulointerstitial inflammation and fibrosis in protein overload nephropathy. Am J Nephrol. 2008;28(3):465–77. https://doi.org/10.1159/000112850.
Li Y, Ge Y, Liu FY, Peng YM, Sun L, Li J, et al. Norcantharidin, a protective therapeutic agent in renal tubulointerstitial fibrosis. Mol Cell Biochem. 2012;361(1–2):79–83. https://doi.org/10.1007/s11010-011-1091-z.
Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-β: the master regulator of fibrosis. Nat Rev Nephrol. 2016;12(6):325–38. https://doi.org/10.1038/nrneph.2016.48.
Humphreys BD. Mechanisms of renal fibrosis. Annu Rev Physiol. 2018;80:309–26. https://doi.org/10.1146/annurev-physiol-022516-034227.
Rauchman M, Griggs D. Emerging strategies to disrupt the central TGF-β axis in kidney fibrosis. Transl Res. 2019;209:90–104. https://doi.org/10.1016/j.trsl.2019.04.003.
Higgins CE, Tang J, Mian BM, Higgins SP, Gifford CC, Conti DJ, et al. TGF-β1-p53 cooperativity regulates a profibrotic genomic program in the kidney: molecular mechanisms and clinical implications. FASEB J. 2019;33(10):10596–606. https://doi.org/10.1096/fj.201900943R.
Higgins CE, Tang J, Higgins SP, Gifford CC, Mian BM, Jones DM, et al. The genomic response to TGF-β1 dictates failed repair and progression of fibrotic disease in the obstructed kidney. Front Cell Dev Biol. 2021;9: 678524. https://doi.org/10.3389/fcell.2021.678524.
Liang S, Wu YS, Li DY, Tang JX, Liu HF. Autophagy and renal fibrosis. Aging Dis. 2022;13(3):712–31. https://doi.org/10.14336/AD.2021.1027.
Afsar B, Afsar RE. Sodium-glucose cotransporter inhibitors and kidney fibrosis: review of the current evidence and related mechanisms. Pharmacol Rep. 2023;75(1):44–68. https://doi.org/10.1007/s43440-022-00442-4.
Genovese F, Manresa AA, Leeming DJ, Karsdal MA, Boor P. The extracellular matrix in the kidney: a source of novel non-invasive biomarkers of kidney fibrosis? Fibrogenesis Tissue Repair. 2014;7(1):4. https://doi.org/10.1186/1755-1536-7-4.
Grynberg K, Ma FY, Nikolic-Paterson DJ. The JNK signaling pathway in renal fibrosis. Front Physiol. 2017;8:829. https://doi.org/10.3389/fphys.2017.00829.
Qi R, Yang C. Renal tubular epithelial cells: the neglected mediator of tubulointerstitial fibrosis after injury. Cell Death Dis. 2018;9(11):1126. https://doi.org/10.1038/s41419-018-1157-x.
Klahr S, Morrissey J. Obstructive nephropathy and renal fibrosis. Am J Physiol Renal Physiol. 2002;283:F861–75. https://doi.org/10.1152/ajprenal.00362.2001.
Dendooven A, Ishola DA Jr, Nguyen TQ, Van der Giezen DM, Kok RJ, Goldschmeding R, Joles JA. Oxidative stress in obstructive nephropathy. Int J Exp Pathol. 2011;92:202–10. https://doi.org/10.1111/j.1365-2613.2010.00730.x.
Gewin LS. Renal fibrosis: primacy of the proximal tubule. Matrix Biol. 2018;68–69:248–62. https://doi.org/10.1016/j.matbio.2018.02.006.
Nørregaard R, Mutsaers HAM, Frøkiær J, Kwon TH. Obstructive nephropathy and molecular pathophysiology of renal interstitial fibrosis. Physiol Rev. 2023;103(4):2827–72. https://doi.org/10.1152/physrev.00027.2022.
Cockwell P, Fisher LA. The global burden of chronic kidney disease. Lancet. 2020;395:662–4. https://doi.org/10.1016/S0140-6736(19)32977-0.
Ruiz-Ortega M, Rayego-Mateos S, Lamas S, Ortiz A, Rodrigues-Diez RR. Targeting the progression of chronic kidney disease. Nat Rev Nephrol. 2020;16:269–88. https://doi.org/10.1038/s41581-019-0248-y.
Rende U, Guller A, Goldys EM, Pollock C, Saad S. Diagnostic and prognostic biomarkers for tubulointerstitial fibrosis. J Physiol. 2023;601(14):2801–26. https://doi.org/10.1113/JP284289.
Boor P, Ostendorf T, Floege J. Renal fibrosis: novel insights into mechanisms and therapeutic targets. Nat Rev Nephrol. 2010;6(11):643–56. https://doi.org/10.1038/nrneph.2010.
Bani-Hani AH, Campbell MT, Meldrum DR, Meldrum KK. Cytokines in epithelial-mesenchymal transition: a new insight into obstructive nephropathy. J Urol. 2008;180(2):461–8. https://doi.org/10.1016/j.juro.2008.04.001.
Duffield JS. Cellular and molecular mechanisms in kidney fibrosis. J Clin Investig. 2014;124(6):2299–306. https://doi.org/10.1172/JCI72267.
Falke LL, Gholizadeh S, Goldschmeding R, Kok Robbert RJ, Nguyen TQ. Diverse origins of the myofibroblast-implications for kidney fibrosis. Nat Rev Nephrol. 2015;11(4):233–44. https://doi.org/10.1038/nrneph.2014.246.
Trionfini P, Benigni A, Remuzzi G. MicroRNAs in kidney physiology and disease. Nat Rev Nephrol. 2015;11(1):23–33. https://doi.org/10.1038/nrneph.2014.202.
Chen L, Yang T, Lu DW, Zhao H, Feng YL, Chen H, et al. Central role of dysregulation of TGF-β/Smad in CKD progression and potential targets of its treatment. Biomed Pharmacother. 2018;101:670–81. https://doi.org/10.1016/j.biopha.2018.02.090.
Böttinger EP. TGF-beta in renal injury and disease. Semin Nephrol. 2007;27(3):309–20. https://doi.org/10.1016/j.semnephrol.2007.02.009.
Munoz-Felix JM, Gonzalez-Nunez M, Martinez-Salgado C, Lopez-Novoa JM. TGF-β/BMP proteins as therapeutic targets in renal fibrosis. Where have we arrived after 25 years of trials and tribulations? Pharmacol Ther. 2015;156:44–58. https://doi.org/10.1016/j.pharmthera.2015.10.003.
Cao J, Li J, Zhao H, Feng YL, Chen H, et al. Febuxostat prevents renal interstitial fibrosis by the activation of BMP-7 signaling and inhibition of USAG-1 expression in rats. Am J Nephrol. 2015;42(5):369–78. https://doi.org/10.1159/000443023.
Zhang ZH, Mao JR, Chen H, Zhao H, Feng YL, Chen H, et al. Removal of uremic retention products by hemodialysis is coupled with indiscriminate loss of vital metabolites. Clin Biochem. 2017;50(18):1078–86. https://doi.org/10.1016/j.clinbiochem.2017.09.012.
Syn WK, Jung Y, Omenetti A, Abdelmalek M, Guy CD, Yang L, et al. Hedgehog-mediated epithelial-to-mesenchymal transition and fibrogenic repair in nonalcoholic fatty liver disease. Gastroenterology. 2009;137(4):1478-1488.e8. https://doi.org/10.1053/j.gastro.2009.06.051.
Bai Y, Lu H, Lin C, Xu Y, Hu D, Liang Y, et al. Sonic hedgehog-mediated epithelial-mesenchymal transition in renal tubulointerstitial fibrosis. Int J Mol Med. 2016;37(5):1317–27. https://doi.org/10.3892/ijmm.2016.2546.
Guan Y, Quan D, Chen K, Kang L, Yang D, Wu H, et al. Kaempferol inhibits renal fibrosis by suppression of the sonic hedgehog signaling pathway. Phytomedicine. 2023;108:154246. https://doi.org/10.1016/j.phymed.2022.
Huffstater T, Merryman WD, Gewin LS. Wnt/beta-catenin in acute kidney injury and progression to chronic kidney disease. Semin Nephrol. 2020;40(2):126–37. https://doi.org/10.1016/j.semnephrol.2020.01.004.
Zhang Y, Jin D, Kang X, Xu Y, Hu D, Liang Y, et al. Signaling pathways involved in diabetic renal fibrosis. Front Cell Dev Biol. 2021;9: 696542. https://doi.org/10.3389/fcell.2021.696542.
Ghosh AK, Vaughan DE. PAI-1 in tissue fibrosis. J Cell Physiol. 2012;227:493–507. https://doi.org/10.1002/jcp.22783.
Flevaris P, Vaughan D. The role of plasminogen activator inhibitor type-1 in fibrosis. Semin Thromb Hemost. 2017;43:169–77. https://doi.org/10.1055/s-0036-1586228.
Kelly K, Plotkin J, Vulgamott ASL, Dagher PC. p53 mediates the apoptotic response to GTP depletion after renal ischemia-reperfusion: protective role of a p53 inhibitor. J Am Soc Nephrol. 2003;14:128–38. https://doi.org/10.1097/01.asn.0000040596.23073.01.
Huber MA, Azoitei N, Baumann B, Grunert S, Sommer A, Pehamberger H, et al. NF-kappaB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J Clin Investig. 2004;114(4):569–81. https://doi.org/10.1172/JCI21358.
Pan J, Jiang Y, Huang Y, Zhang H, Wang X, Luo C, et al. Liuwei Dihuang decoction drug-containing serum attenuates transforming growth factor-β1-induced epithelial-mesenchymal transition in HK-2 cells by inhibiting NF-κB/snail signaling pathway. Curr Pharm Biotechnol. 2023;24(12):1589–602. https://doi.org/10.2174/1389201024666230228100718.
Zhang L, Chen L, Gao C, Grunert S, Sommer A, Pehamberger H, et al. Loss of histone H3 K79 methyltransferase Dot1l facilitates kidney fibrosis by upregulating endothelin 1 through histone deacetylase 2. J Am Soc Nephrol. 2020;31:337–49. https://doi.org/10.1681/asn.2019070739.
Li ZL, Lv LL, Wang B, Tang TT, Feng Y, Cao JY, et al. The profibrotic effects of MK-8617 on tubulointerstitial fibrosis mediated by the KLF5 regulating pathway. FASEB J. 2019;33(11):12630–43. https://doi.org/10.1096/fj.201901087RR.
Li J, Liu L, Zhou WQ, Cai L, Xu ZG, Rane MJ. Roles of Krüppel-like factor 5 in kidney disease. J Cell Mol Med. 2021;25(5):2342–55. https://doi.org/10.1111/jcmm.16332.
Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med. 2010;16(5):535–43. https://doi.org/10.1038/nm.2144. (1p following 143).
Liu N, Guo JK, Pang M, Cai L, Xu ZG, Rane MJ, et al. Genetic or pharmacologic blockade of EGFR inhibits renal fibrosis. J Am Soc Nephrol. 2012;23(5):854–67. https://doi.org/10.1681/ASN.2011050493.
Liu N, Wang L, Yang T, Xiong C, Xu L, Shi Y, et al. EGF receptor inhibition alleviates hyperuricemic nephropathy. J Am Soc Nephrol. 2015;26(11):2716–29. https://doi.org/10.1681/ASN.2014080793.
Shi Y, Tao M, Chen H, Ma X, Wang Y, Hu Y, et al. Ubiquitin-specific protease 11 promotes partial epithelial-to-mesenchymal transition by deubiquitinating the epidermal growth factor receptor during kidney fibrosis. Kidney Int. 2023;103(3):544–64. https://doi.org/10.1016/j.kint.2022.11.027.
Zeng D, Xiao Z, Xu Q, Luo H, Wen L, Tang C, et al. Norcantharidin protects against renal interstitial fibrosis by suppressing TWEAK-mediated Smad3 phosphorylation. Life Sci. 2020;260: 118488. https://doi.org/10.1016/j.lfs.2020.118488.
Kriegel AJ, Liu Y, Cohen B, Usa K, Liu Y, Liang M. MiR-382 targeting of kallikrein 5 contributes to renal inner medullary interstitial fibrosis. Physiol Genom. 2012;44(4):259–67. https://doi.org/10.1152/physiolgenomics.00173.2011.
Chung ACK, Dong Y, Yang W, Zhong X, Li R, Lan HY. Smad7 suppresses renal fibrosis via altering expression of TGF-β/Smad3-regulated microRNAs. Mol Ther. 2013;21(2):388–98. https://doi.org/10.1038/mt.2012.251.
Wang B, Yao K, Wise AF, Lau R, Shen HH, Tesch GH, et al. miR-378 reduces mesangial hypertrophy and kidney tubular fibrosis via MAPK signaling. Clin Sci (Lond). 2017;131(5):411–23. https://doi.org/10.1042/CS20160571.
Mercer TR, Mattick JS. Structure and function of long noncoding RNAs in epigenetic regulation. Nat Struct Mol Biol. 2013;20(3):300–7. https://doi.org/10.1038/nsmb.2480.
He Y, Wu Y, Huang C, Meng X, Ma T, Wu B, et al. Inhibitory effects of long noncoding RNA MEG3 on hepatic stellate cells activation and liver fibrogenesis. Biochim Biophys Acta. 2014;1842(11):2204–15. https://doi.org/10.1016/j.bbadis.2014.08.015.
Zhou Q, Chung ACK, Huang XR, Dong Y, Yu X, Lan HY. Identification of novel long noncoding RNAs associated with TGF-β/Smad3-mediated renal inflammation and fibrosis by RNA sequencing. Am J Pathol. 2014;184(2):409–17. https://doi.org/10.1016/j.ajpath.2013.10.007.
Su H, Wan C, Song A, Qiu Y, Xiong W, Zhang C. Oxidative stress and renal fibrosis: mechanisms and therapies. Adv Exp Med Biol. 2019;1165:585–604. https://doi.org/10.1007/978-981-13-8871-2_29.
Yin Q, Liu H. Connective tissue growth factor and renal fibrosis. Adv Exp Med Biol. 2019;1165:365–80. https://doi.org/10.1007/978-981-13-8871-2_17.
Wang J, Zhu H, Huang L, Zhu X, Sha J, Li G, et al. Nrf2 signaling attenuates epithelial-to-mesenchymal transition and renal interstitial fibrosis via PI3K/Akt signaling pathways. Exp Mol Pathol. 2019;111: 104296. https://doi.org/10.1016/j.yexmp.2019.104296.
Sheng L, Zhuang S. New insights into the role and mechanism of partial epithelial-mesenchymal transition in kidney fibrosis. Front Physiol. 2020;11:569322. https://doi.org/10.3389/fphys.2020.569322.
Hou T, Xiao Z, Li Y, You YH, Li H, Liu YP, et al. Norcantharidin inhibits renal interstitial fibrosis by downregulating PP2Ac expression. Am J Transl Res. 2015;7(11):2199–211 (PMC4697700).
Xiao Z, Wen L, Zeng D, Yin D, Zhou X, Tang C, et al. Protein phosphatase 2A inhibiting β-catenin phosphorylation contributes critically to the anti-renal interstitial fibrotic effect of norcantharidin. Inflammation. 2020;43(3):878–91. https://doi.org/10.1007/s10753-019-01173-0.
Ruby M, Gifford CC, Pandey R, Raj VS, Sabbisetti VS, Ajay AK. Autophagy as a therapeutic target for chronic kidney disease and the roles of TGF-β1 in autophagy and kidney fibrosis. Cells. 2023;12(3):412. https://doi.org/10.3390/cells12030412.
Li Y, Liu FY, Peng YM, Yin D, Zhou X, Tang C, et al. Norcantharidin inhibits proliferation and fibronectin expression of HK-2 cells induced by albumin in vitro. Cell Biol Int. 2011;35(12):1239–41. https://doi.org/10.1042/CBI20100850.
Li Y, Chen Q, Liu FY, Peng YM, Wang S, Li J, et al. Norcantharidin inhibits the expression of extracellular matrix and TGF-β1 in HK-2 cells induced by high glucose independent of calcineurin signal pathway. Lab Investig. 2011;91(12):1706–16. https://doi.org/10.1038/labinvest.2011.119.
Li Y, Sun Y, Liu F, Sun L, Li J, Duan S, et al. Norcantharidin inhibits renal interstitial fibrosis by blocking the tubular epithelial-mesenchymal transition. PLoS ONE. 2013;8(6): e66356. https://doi.org/10.1371/journal.pone.0066356.
Li Y, Chen Q, Liu FY, Peng YM, Hou T, Duan SB, et al. Norcantharidin attenuates tubulointerstitial fibrosis in rat models with diabetic nephropathy. Ren Fail. 2011;33(2):233–41. https://doi.org/10.3109/0886022X.2011.553305.
Okada H, Kikuta T, Kobayashi T, Inoue T, Kanno Y, Takigawa M, et al. Connective tissue growth factor expressed in tubular epithelium plays a pivotal role in renal fibrogenesis. J Am Soc Nephrol. 2005;16(1):133–43. https://doi.org/10.1681/ASN.2004040339.
Phanish MK, Winn SK, Dockrell ME. Connective tissue growth factor-(CTGF, CCN2)—a marker, mediator and therapeutic target for renal fibrosis. Nephron Exp Nephrol. 2010;114(3):e83-92. https://doi.org/10.1159/000262316.
Janssens V, Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signaling. Biochem J. 2001;353(Pt 3):417–39. https://doi.org/10.1042/0264-6021:3530417.
Mumby M. The 3D structure of protein phosphatase 2A: new insights into a ubiquitous regulator of cell signaling. ACS Chem Biol. 2007;2(2):99–103. https://doi.org/10.1021/cb700021z.
Pais SM, Téllez-Iñón MT, Capiati DA. Serine/threonine protein phosphatases type 2A and their roles in stress signaling. Plant Signal Behav. 2009;4(11):1013–5. https://doi.org/10.4161/psb.4.11.9783.
Hill TA, Stewart SG, Gordon CP, Ackland SP, Gilbert J, Sauer B, et al. Norcantharidin analogues: synthesis, anticancer activity and protein phosphatase 1 and 2A inhibition. Chem Med Chem. 2008;3(12):1878–92. https://doi.org/10.1002/cmdc.200800192.
Deng L, Tang S. Norcantharidin analogues: a patent review (2006–2010). Expert Opin Ther Pat. 2011;21(11):1743–53. https://doi.org/10.1517/13543776.2011.629190.
Kadioglu O, Kermani NS, Kelter G, Schumacher U, Fiebig HH, Greten HJ, et al. Pharmacogenomics of cantharidin in tumor cells. Biochem Pharmacol. 2014;87(3):399–409. https://doi.org/10.1016/j.bcp.2013.10.025.
Luo HW, Yin DD, Xiao Z, Wen L, Liao YJ, Tang CY, et al. Anti-renal interstitial fibrosis effect of norcantharidin is exerted through inhibition of PP2Ac-mediated C-terminal phosphorylation of Smad3. Chem Biol Drug Des. 2021;97(2):293–304. https://doi.org/10.1111/cbdd.13781.
Wiley SR, Winkles JA. TWEAK, a member of the TNF superfamily, is a multifunctional cytokine that binds the TweakR/Fn14 receptor. Cytokine Growth Factor Rev. 2003;14(3–4):241–9. https://doi.org/10.1016/s1359-6101(03)00019-4.
Poveda J, Vázquez-Sánchez S, Sanz AB, Ortiz A, Ruilope LM, Ruiz-Hurtado G. TWEAK-Fn14 as a common pathway in the heart and the kidneys in cardiorenal syndrome. J Pathol. 2021;254(1):5–19. https://doi.org/10.1002/path.5631.
Zhang Y, Zeng W, Xia Y. TWEAK/Fn14 axis is an important player in fibrosis. J Cell Physiol. 81–2021;236(5):3304–16. https://doi.org/10.1002/jcp.30089.
Hu G, Liang L, Liu Y, Wen L, Liao YJ, Tang CY, et al. TWEAK/Fn14 interaction confers aggressive properties to cutaneous squamous cell carcinoma. J Investig Dermatol. 2019;139:796–806. https://doi.org/10.1016/j.jid.2018.09.035.
Doerner JL, Wen J, Xia Y, Wen L, Liao YJ, Tang CY, et al. TWEAK/Fn14 signaling involvement in the pathogenesis of cutaneous disease in the MRL/lpr model of spontaneous lupus. J Investig Dermatol. 2015;135(8):1986–95. https://doi.org/10.1038/jid.2015.124.
Poveda J, Sanz AB, Carrasco S, Ruiz-Ortega M, Cannata-Ortiz P, Sanchez-Nino MD, et al. Bcl3: a regulator of NF-κB inducible by TWEAK in acute kidney injury with anti-inflammatory and antiapoptotic properties in tubular cells. Exp Mol Med. 2017;49(7): e352. https://doi.org/10.1038/emm.2017.89.
Zhu C, Zhang L, Liu Z, Li C, Bai Y. TWEAK/Fn14 interaction induces proliferation and migration in human airway smooth muscle cells via activating the NF-κB pathway. J Cell Biochem. 2018;119(4):3528–36. https://doi.org/10.1002/jcb.26525.
Itoigawa Y, Harada N, Harada S, Ruiz-Ortega M, Cannata-Ortiz P, Sanchez-Nino MD, et al. TWEAK enhances TGF-beta-induced epithelial-mesenchymal transition in human bronchial epithelial cells. Respir Res. 2015;16(1):48. https://doi.org/10.1186/s12931-015-0207-5.
Son A, Oshio T, Kawamura YI, Hagiwara T, Yamazaki M, Inagaki-Ohara K, et al. TWEAK/Fn14 pathway promotes a T helper 2-type chronic colitis with fibrosis in mice. Mucosal Immunol. 2013;6(6):1131–42. https://doi.org/10.1038/mi.2013.10.
Liu Z, Xue L, Liu Z, Huang J, Wen J, Hu J, Bo L, et al. Tumor necrosis factor-like weak inducer of apoptosis accelerates the progression of renal fibrosis in lupus nephritis by activating SMAD and p38 MAPK in TGF-β1 signaling pathway. Mediat Inflamm. 2016;2016:8986451. https://doi.org/10.1155/2016/8986451.
Wang A, Zhang F, Xu H, Huang J, Wen J, Hu J, Bo L, et al. TWEAK/Fn14 promotes pro-inflammatory cytokine secretion in hepatic stellate cells via NF-κB/STAT3 pathways. Mol Immunol. 2017;87:67–75. https://doi.org/10.1016/j.molimm.2017.04.003.
Valino-Rivas L, Cuarental L, Grana O, Bucala R, Leng L, Sanz A, Gomez G, et al. TWEAK increases CD74 expression and sensitizes to DDT proinflammatory actions in tubular cells. PLoS ONE. 2018;13(6): e0199391. https://doi.org/10.1371/journal.pone.0199391.
Ucero AC, Benito-Martin A, Fuentes-Calvo I, Santamaria B, Blanco J, Lopez-Novoa JM, et al. TNF-related weakinducer of apoptosis (TWEAK) promotes kidney fibrosis and Ras-dependent proliferation of cultured renal fibroblast. Biochim Biophys Acta Gen Subj. 2013;1832(10):1744–55. https://doi.org/10.1016/j.bbadis.2013.05.032.
Xia Y, Campbell SR, Broder A, Santamaria B, Blanco J, Lopez-Novoa JM, et al. Inhibition of the TWEAK/Fn14 pathway attenuates renal disease in nephrotoxic serum nephritis. Clin Immunol. 2012;145(2):108–21. https://doi.org/10.1016/j.clim.2012.08.008.
Hotta K, Sho M, Yamato I, Shimada K, Harada H, Akahori T, et al. Direct targeting of fibroblast growth factor-inducible 14 protein protects against renal ischemia reperfusion injury. Kidney Int. 2011;79(2):179–88. https://doi.org/10.1038/ki.2010.379.
Xia W, He Y, Gan Y, Zhang B, Dai G, Ru F, et al. Long non-coding RNA: an emerging contributor and potential therapeutic target in renal fibrosis. Front Genet. 2021;12: 682904. https://doi.org/10.3389/fgene.2021.682904.
Xiao H, Liao Y, Tang C, Xiao Z, Luo H, Li J, et al. RNA-Seq analysis of potential lncRNAs and genes for the anti-renal fibrotic effect of norcantharidin. J Cell Biochem. 2019;120(10):17354–67. https://doi.org/10.1002/jcb.28999.
Ørom UA, Derrien T, Beringer M, Gumireddy K, Gardini A, Bussotti G, et al. Long noncoding RNAs with enhancer-like function in human cells. Cell. 2010;143(1):46–58. https://doi.org/10.1016/j.cell.2010.09.001.
Fatica A, Bozzoni I. Long non-coding RNAs: new players in cell differentiation and development. Nat Rev Genet. 2014;15(1):7–21. https://doi.org/10.1038/nrg3606.
Chen H, Fan Y, Jing H, Tang S, Zhou J. Emerging role of lncRNAs in renal fibrosis. Arch Biochem Biophys. 2020;692: 108530. https://doi.org/10.1016/j.abb.2020.108530.
Gu YY, Dou JJ, Huang XR, Liu XS, Lan HY. Transforming growth factor-β and long non-coding RNA in renal inflammation and fibrosis. Front Physiol. 2021;12: 684236. https://doi.org/10.3389/fphys.2021.684236.
Wang M, Wang S, Yao D, Yan Q, Lu W. A novel long non-coding RNA CYP4B1-PS1-001 regulates proliferation and fibrosis in diabetic nephropathy. Mol Cell Endocrinol. 2016;426:136–45. https://doi.org/10.1016/j.mce.2016.02.020.
Zhou Q, Huang XR, Yu X, Yu J, Lan HY. Long noncoding RNA Arid2-IR is a novel therapeutic target for renal inflammation. Mol Ther. 2015;23(6):1034–43. https://doi.org/10.1038/mt.2015.31.
Feng M, Tang PM, Huang XR, Sun SF, You YK, Xiao J, et al. TGF-β mediates renal fibrosis via the Smad3-Erbb4-IR long noncoding RNA axis. Mol Ther. 2018;26(1):148–61. https://doi.org/10.1016/j.ymthe.2017.09.024.
Chen W, Zhang L, Zhou ZQ, Sun SF, You YK, Xiao J, et al. Effects of long non-coding RNA LINC00963 on renal interstitial fibrosis and oxidative stress of rats with chronic renal failure via the foxo signaling pathway. Cell Physiol Biochem. 2018;46(2):815–28. https://doi.org/10.1159/000488739.
Yang J, Shen Y, Yang X, Long Y, Chen S, Lin X, et al. Silencing of long noncoding RNA XIST protects against renal interstitial fibrosis in diabetic nephropathy via microRNA-93-5p-mediated inhibition of CDKN1A. Am J Physiol Renal Physiol. 2019;317(5):F1350–8. https://doi.org/10.1152/ajprenal.00254.2019.
Zhang L, Zhao S, Zhu Y. Long noncoding RNA growth arrest-specific transcript 5 alleviates renal fibrosis in diabetic nephropathy by downregulating matrix metalloproteinase 9 through recruitment of enhancer of zeste homolog 2. FASEB J. 2020;34(2):2703–14. https://doi.org/10.1096/fj.201901380RR.
Moghaddas Sani H, Hejazian M, Hosseinian Khatibi SM, Ardalan M, Zununi VS. Long non-coding RNAs: an essential emerging field in kidney pathogenesis. Biomed Pharmacother. 2018;99:755–65. https://doi.org/10.1016/j.biopha.2018.01.122.
Martin K, Pritchett J, Liewellyn J, Mullan AF, Athwal VS, Dobie R, et al. PAK proteins and YAP-1 signalling downstream of integrin beta-1 in myofibroblasts promote liver fibrosis. Nat Commun. 2016;7:12502. https://doi.org/10.1038/ncomms12502.
Yeh Y, Wei W, Wang Y, Lin S, Sung J, Tang M. Transforming growth factor-{beta}1 induces Smad3-dependent {beta}1 integrin gene expression in epithelial-to-mesenchymal transition during chronic tubulointerstitial fibrosis. Am J Pathol. 2010;177(4):1743–54. https://doi.org/10.2353/ajpath.2010.091183.
Chuang PY, He JC. JAK/STAT signaling in renal diseases. Kidney Int. 2010;78:231–4. https://doi.org/10.1038/ki.2010.158.
Chakraborty D, Šumová B, Mallano T, Chen CW, Distler A, Bergmann C, et al. Activation of STAT3 integrates common profibrotic pathways to promote fibroblast activation and tissue fibrosis. Nat Commun. 2017;8(1):1130. https://doi.org/10.1038/s41467-017-01236-6.
Lu S, Fan H, Li K, Fan X. Suppression of Elp2 prevents renal fibrosis and inflammation induced by unilateral ureter obstruction (UUO) via inactivating Stat3-regulated TGF-β1 and NF-κB pathways. Biochem Biophys Res Commun. 2018;501(2):400–7. https://doi.org/10.1016/j.bbrc.2018.04.227.
Patel AS, Song JW, Chu SG, Mizumura K, Osorio JC, Shi Y, et al. Epithelial cell mitochondrial dysfunction and PINK1 are induced by transforming growth factor-beta1 in pulmonary fibrosis. PLoS ONE. 2015;10(3): e0121246. https://doi.org/10.1371/journal.pone.0121246.
Bueno M, Lai YC, Romero Y, Mizumura K, Osorio JC, Shi Y, et al. PINK1 deficiency impairs mitochondrial homeostasis and promotes lung fibrosis. J Clin Investig. 2015;125(2):521–38. https://doi.org/10.1172/JCI74942.
Tian J, Xiao Z, Wei J, Mizumura K, Osorio JC, Shi Y, et al. NCTD prevents renal interstitial fibrosis via targeting Sp1/lncRNA Gm26669 axis. Int J Biol Sci. 2021;17(12):3118–32. https://doi.org/10.7150/ijbs.59195.
Pereira SG, Oakley F. Nuclear factor-kappaB1: regulation and function. Int J Biochem Cell Biol. 2008;40(8):1425–30. https://doi.org/10.1016/j.biocel.2007.05.004.
Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007;8(1):49–62. https://doi.org/10.1038/nrm2083.
Huang Y, Liu Q, Liu K, Yagasaki K, Zhang G. Suppression of growth of highly-metastatic human breast cancer cells by norcantharidin and its mechanisms of action. Cytotechnology. 2009;59(3):201–8. https://doi.org/10.1007/s10616-009-9210-3.
Zheng R, Zhu R, Li X, Li X, Shen L, Chen Y, et al. N6-(2-Hydroxyethyl) adenosine from cordyceps cicadae ameliorates Renal interstitial fibrosis and prevents inflammation via TGF-β1/Smad and NF-κB signaling pathway. Front Physiol. 2018;9:1229. https://doi.org/10.3389/fphys.2018.01229.
Enwere EK, Holbrook J, Lejmi-Mrad R, Vineham J, Timusk K, Sivaraj B, et al. TWEAK and cIAP1 regulate myoblast fusion through the noncanonical NF-κB signaling pathway. Sci Signal. 2012;5(246):75. https://doi.org/10.1126/scisignal.2003086.
Enwere EK, Lacasse EC, Adam NJ, Korneluk RG. Role of the TWEAK-Fn14-cIAP1-NF-κB signaling axis in the regulation of myogenesis and muscle homeostasis. Front Immunol. 2014;5:34. https://doi.org/10.3389/fimmu.2014.00034.
Funding
This work was supported by National Natural Science Foundation of China (NSFC82003702) and Shanghai-Sailing Program (no. 21YF1438000).
Author information
Authors and Affiliations
Contributions
Writing—original draft preparation: QSY, JBJ and QG; writing—review and editing: QG; supervision, QG; funding acquisition: QG; project administration: QG and YXB. All authors read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no confict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yun, QS., Bao, YX., Jiang, JB. et al. Mechanisms of norcantharidin against renal tubulointerstitial fibrosis. Pharmacol. Rep 76, 263–272 (2024). https://doi.org/10.1007/s43440-024-00578-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-024-00578-5