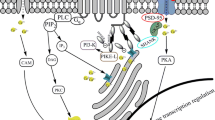
Abstract
Background
Cocaine use disorder (CUD) remains a severe health problem with no effective pharmacological therapy. One of the potential pharmacological strategies for CUD pharmacotherapy includes manipulations of the brain glutamatergic (Glu) system which is particularly involved in drug withdrawal and relapse. Previous research indicated a pivotal role of ionotropic N-methyl-d-aspartate (NMDA) receptors or metabotropic receptors’ type 5 (mGlu5) receptors in controlling the reinstatement of cocaine. Stimulation of the above molecules results in the activation of the downstream signaling targets such as neuronal nitric oxide synthase (nNOS) and the release of nitric oxide.
Methods
In this paper, we investigated the molecular changes in nNOS in the prefrontal cortex and nucleus accumbens following 3 and 10 days of cocaine abstinence as well as the effectiveness of nNOS blockade with the selective enzyme inhibitor N-ω-propyl-l-arginine hydrochloride (L-NPA) on cocaine seeking in male rats. The effect of L-NPA on locomotor activity in drug-naïve animals was investigated.
Results
Ten-day (but not 3-day) cocaine abstinence from cocaine self-administration increased nNOS gene and protein expression in the nucleus accumbens, but not in the prefrontal cortex. L-NPA (0.5–5 mg/kg) administered peripherally did not change locomotor activity but attenuated the reinstatement induced with cocaine priming or the drug-associated conditioned cue.
Conclusions
Our findings support accumbal nNOS as an important molecular player for cocaine seeking while its inhibitors could be considered as anti-cocaine pharmacological tools in male rats.
Graphical abstract





Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon request.
References
Global report on cocaine 2023. Glob Rep Cocaine. 2023.
Czeisler MÉ, Lane RI, Petrosky E, Wiley JF, Christensen A, Njai R, et al. Mental health, substance use, and suicidal ideation during the COVID-19 pandemic—United States, June 24–30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(32):1049–57.
Kampman KM. The treatment of cocaine use disorder. Sci Adv. 2019;5(10):1–8.
Cleva RM, Olive MF. mGlu receptors and drug addiction. Wiley Interdiscip Rev Membr Transp Signal. 2012;1:281–95.
Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, et al. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6(7):743–9.
Pomierny-Chamiolo L, Miszkiel J, Frankowska M, Pomierny B, Niedzielska E, Smaga I, et al. Withdrawal from cocaine self-administration and yoked cocaine delivery dysregulates glutamatergic mGlu5 and NMDA receptors in the rat brain. Neurotox Res. 2015;27(3):246–58.
Smaga I, Gawlińska K, Frankowska M, Wydra K, Sadakierska-Chudy A, Suder A, et al. Extinction training after cocaine self-administration influences the epigenetic and genetic machinery responsible for glutamatergic transporter gene expression in male rat brain. Neuroscience. 2020;451:99–110.
Martin G, Nie Z, Siggins GR. Metabotropic glutamate receptors regulate N-methyl-d-aspartate-mediated synaptic transmission in nucleus accumbens. J Neurophysiol. 1997;78(6):3028–38.
Attucci S, Albani-Torregrossa S, Moroni F, Pellegrini-Giampietro DE. Metabotropic glutamate receptors stimulate phospholipase D via different pathways in the adult and neonate rat hippocampus. Neurochem Res. 2001;26(10):1151–5.
Pisani A, Gubellini P, Bonsi P, Conquet F, Picconi B, Centonze D, et al. Metabotropic glutamate receptor 5 mediates the potentiation of N-methyl-d-aspartate responses in medium spiny striatal neurons. Neuroscience. 2001;106(3):579–87.
Ferraguti F, Shigemoto R. Metabotropic glutamate receptors. Cell Tissue Res. 2006;326(2):483–504.
Stefani MR, Moghaddam B. Activation of type 5 metabotropic glutamate receptors attenuates deficits in cognitive flexibility induced by NMDA receptor blockade. Eur J Pharmacol. 2010;639(1–3):26–32.
Spencer S, Kalivas PW. Glutamate transport: a new bench to bedside mechanism for treating drug abuse. Int J Neuropsychopharmacol. 2017;20(10):797–812.
Tzschentke TM, Schmidt WJ. Glutamatergic mechanisms in addiction. Mol Psychiatry. 2003;8(4):373–82.
Kew JNC, Kemp JA. Ionotropic and metabotropic glutamate receptor structure and pharmacology. Psychopharmacology. 2005;179(1):4–29.
Smaga I, Sanak M, Filip M. Cocaine-induced changes in the expression of NMDA receptor subunits. Curr Neuropharmacol. 2019;17(11):1039–55.
Smaga I, Wydra K, Suder A, Frankowska M, Sanak M, Caffino L, et al. The NMDA receptor subunit (GluN1 and GluN2A) modulation following different conditions of cocaine abstinence in rat brain structures. Neurotox Res. 2021;39(3):556–65.
Smaga I, Wydra K, Piechota M, Caffino L, Fumagalli F, Sanak M, et al. Cocaine abstinence modulates NMDA receptor subunit expression: an analysis of the GluN2B subunit in cocaine-seeking behavior. Prog Neuro-Psychopharmacol Biol Psychiatry. 2021;109: 110248.
DePoy LM, Zimmermann KS, Marvar PJ, Gourley SL. Induction and blockade of adolescent cocaine-induced habits. Biol Psychiatry. 2017;81(7):595–605.
Smaga I, Wydra K, Witek K, Surówka P, Suder A, Pieniążek R, et al. Intravenous administration of Tat-NR2B9c peptide, a PSD95 inhibitor, attenuates reinstatement of cocaine-seeking behavior in rats. Behav Brain Res. 2022;416: 113537.
Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357(3):593–615.
Garthwaite J, Garthwaite G, Palmer RMJ, Moncada S. NMDA receptor activation induces nitric oxide synthesis from arginine in rat brain slices. Eur J Pharmacol Mol Pharmacol. 1989;172(4–5):413–6.
Harada K, Aota M, Inoue T, Matsuda R, Mihara T, Yamaji T, et al. Anxiolytic activity of a novel potent serotonin 5-HT2C receptor antagonist FR260010: a comparison with diazepam and buspirone. Eur J Pharmacol. 2006;553(1–3):171–84.
Kim HS, Park WK. Nitric oxide mediation of cocaine-induced dopaminergic behaviors: ambulation-accelerating activity, reverse tolerance and conditioned place preference in mice. J Pharmacol Exp Ther. 1995;275:551–7.
Orsini C, Izzo E, Koob GF, Pulvirenti L. Blockade of nitric oxide synthesis reduces responding for cocaine self-administration during extinction and reinstatement. Brain Res. 2002;925(2):133–40.
Balda MA, Anderson KL, Itzhak Y. Adolescent and adult responsiveness to the incentive value of cocaine reward in mice: role of neuronal nitric oxide synthase (nNOS) gene. Neuropharmacology. 2006;51(2):341–9.
Smith ACW, Scofield MD, Heinsbroek JA, Gipson CD, Neuhofer D, Roberts-Wolfe DJ, et al. Accumbens nNOS interneurons regulate cocaine relapse. J Neurosci. 2017;37(4):742–56.
Siemsen BM, McFaddin JA, Haigh K, Brock AG, Nan Leath M, Hooker KN, et al. Amperometric measurements of cocaine cue and novel context-evoked glutamate and nitric oxide release in the nucleus accumbens core. J Neurochem. 2020;153(5):599–616.
Ghasemi M, Sadeghipour H, Mosleh A, Sadeghipour HR, Mani AR, Dehpour AR. Nitric oxide involvement in the antidepressant-like effects of acute lithium administration in the mouse forced swimming test. Eur Neuropsychopharmacol. 2008;18(5):323–32.
Beamer E, Otahal J, Sills GJ, Thippeswamy T. Nw-Propyl-l-arginine (L-NPA) reduces status epilepticus and early epileptogenic events in a mouse model of epilepsy: behavioural, EEG and immunohistochemical analyses. Eur J Neurosci. 2012;36(9):3194–203.
Srebro DP, Vučković S, Milovanović A, Vujović KS, Vučetić Č, Prostran M. Preventive treatment with dizocilpine attenuates oedema in a carrageenan model of inflammation: the interaction of glutamatergic and nitrergic signaling. Inflammopharmacology. 2019;27(1):121–8.
Frankowska M, Filip M, Przegaliński E. Effects of GABAB receptor ligands in animal tests of depression and anxiety. Pharmacol Rep. 2007;59(6):645–55.
Gawlińska K, Frankowska M, Gawliński D, Piechota M, Korostyński M, Filip M. Cocaine administration and its abstinence conditions modulate neuroglia. Int J Mol Sci. 2020;21(21):7970.
Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th ed. San Diego: Elsevier Academic Press; 2007.
Bagetta G, Rodinò P, Arabia A, Massoud R, Paoletti AM, Nisticò R, et al. Systemic administration of cocaine, given alone or in combination with sensory stimuli, differentially affects l-arginine-nitric oxide metabolism in discrete regions of the brain of rat. Neurosci Lett. 1999;266(3):153–6.
Loftis JM, Janowsky A. Regulation of NMDA receptor subunits and nitric oxide synthase expression during cocaine withdrawal. J Neurochem. 2000;75(5):2040–50.
Itzhak Y, Ali SF, Martin JL, Black MD, Huang PL. Resistance of neuronal nitric oxide synthase-deficient mice to cocaine-induced locomotor sensitization. Psychopharmacology. 1998;140(3):378–86.
Ohno M, Arai I, Watanabe S. N-Methyl-d-aspartate stimulates dopamine release through nitric oxide formation in the nucleus accumbens of rats. Brain Res. 1995;699(2):332–5.
Mungrue IN, Bredt DS. nNOS at a glance: implications for brain and brawn. J Cell Sci. 2004;117(13):2627–9.
Christopherson KS, Hillier BJ, Lim WA, Bredt DS. PSD-95 assembles a ternary complex with the N-methyl-d-aspartic acid receptor and a bivalent neuronal NO synthase PDZ domain. J Biol Chem. 1999;274(39):27467–73.
Hillier W, Wydrzynski T. The affinities for the two substrate water binding sites in the O2 evolving complex of photosystem II vary independently during S-state turnover. Biochemistry. 2000;39(15):4399–405.
Wydra K, Gołembiowska K, Suder A, Kamińska K, Fuxe K, Filip M. On the role of adenosine (A)2A receptors in cocaine-induced reward: a pharmacological and neurochemical analysis in rats. Psychopharmacology. 2015;232(2):421–35.
Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Rev. 1991;16(3):223–44.
Smaga I, Zaniewska M, Gawliński D, Faron-Górecka A, Szafrański P, Cegła M, et al. Changes in the cannabinoids receptors in rats following treatment with antidepressants. Neurotoxicology. 2017;63:13–20.
Wydra K, Witek K, Suder A, Filip M. Esketamine inhibits cocaine-seeking behaviour subsequent to various abstinence conditions in rats. Biomolecules. 2023;13(9):1411.
Frankowska M, Miszkiel J, Pomierny-Chamioło L, Pomierny B, Giannotti G, Suder A, et al. Alternation in dopamine D2-like and metabotropic glutamate type 5 receptor density caused by differing housing conditions during abstinence from cocaine self-administration in rats. J Psychopharmacol. 2019;33(3):372–82.
Frankowska M, Miszkiel J, Pomierny-Chamioło L, Pomierny B, Borelli AC, Suder A, et al. Extinction training following cocaine or MDMA self-administration produces discrete changes in D(2)-like and mGlu(5) receptor density in the rat brain. Pharmacol Rep. 2019;71(5):870–8.
Knackstedt LA, Schwendt M. mGlu5 receptors and relapse to cocaine-seeking: the role of receptor trafficking in postrelapse extinction learning deficits. Neural Plast. 2016;2016:9312508.
Knackstedt LA, Moussawi K, Lalumiere R, Schwendt M, Klugmann M, Kalivas PW. Extinction training after cocaine self-administration induces glutamatergic plasticity to inhibit cocaine seeking. J Neurosci. 2010;30(23):7984–92.
Neuhofer D, Spencer SM, Chioma VC, Beloate LN, Schwartz D, Kalivas PW. The loss of NMDAR-dependent LTD following cannabinoid self-administration is restored by positive allosteric modulation of CB1 receptors. Addict Biol. 2020;25(6):1–21.
McLeod AA. Later management of documented ischaemic heart disease: secondary prevention and rehabilitation. Br Med Bull. 2001;59:113–33.
Sales AJ, Hiroaki-Sato VA, Joca SRL. Participation of hippocampal nitric oxide synthase and soluble guanylate cyclase in the modulation of behavioral responses elicited by the rat forced swimming test. Behav Pharmacol. 2017;28(1):19–29.
Jefferys D, Funder J. Nitric oxide modulates retention of immobility in the forced swimming test in rats. Eur J Pharmacol. 1996;295(2–3):131–5.
Morato GS, Ortiga RM, Ferreira VMM. Involvement of nitric oxide-dependent pathways of dorsolateral periaqueductal gray in the effects of ethanol in rats submitted to the elevated plus-maze test. Behav Brain Res. 2004;153(2):341–9.
Vila-Verde C, Marinho ALZ, Lisboa SF, Guimarães FS. Nitric oxide in the prelimbic medial prefrontal cortex is involved in the anxiogenic-like effect induced by acute restraint stress in rats. Neuroscience. 2016;320:30–42.
Frankowska M, Gołda A, Wydra K, Gruca P, Papp M, Filip M. Effects of imipramine or GABAB receptor ligands on the immobility, swimming and climbing in the forced swim test in rats following discontinuation of cocaine self-administration. Eur J Pharmacol. 2010;627(1–3):142–9.
Hámor PU, Gobin CM, Schwendt M. The role of glutamate mGlu5 and adenosine A2a receptor interactions in regulating working memory performance and persistent cocaine seeking in rats. Prog Neuro-Psychopharmacol Biol Psychiatry. 2020;103: 109979.
Gobin C, Schwendt M. The cognitive cost of reducing relapse to cocaine-seeking with mGlu5 allosteric modulators. Psychopharmacology. 2020;237(1):115–25.
Lipaus IFS, Gomes EF, Martins CW, e Silva CM, Pires RGW, Malgarin F, et al. Impairment of spatial working memory and oxidative stress induced by repeated crack cocaine inhalation in rats. Behav Brain Res. 2019;359:910–7.
Fijał K, Nowak E, Leśkiewicz M, Budziszewska B, Filip M. Working memory deficits and alterations of ERK and CREB phosphorylation following withdrawal from cocaine self-administration. Pharmacol Rep. 2015;67(5):881–9.
Utkan T, Gocmez SS, Ozer C, Gacar N, Aricioglu F. Selective and nonselective neuronal NOS inhibitors impair cognitive function in the three panel runway and passive avoidance tasks in rats. Pharmacol Biochem Behav. 2012;101(4):515–9.
Cieślik P, Borska M, Wierońska JM. A comparative study of the impact of NO-related agents on MK-801- or scopolamine-induced cognitive impairments in the Morris water maze. Brain Sci. 2023;13(3):410.
Filip M, Przegaliński E. The role of the nitric oxide (NO) pathway in the discriminative stimuli of amphetamine and cocaine. Pharmacol Biochem Behav. 1998;59(3):703–8.
Brown GC, Bal-Price A. Inflammatory neurodegeneration mediated by nitric oxide, glutamate, and mitochondria. Mol Neurobiol. 2003;27(3):325–55.
Hartung H, Threlfell S, Cragg SJ. Nitric oxide donors enhance the frequency dependence of dopamine release in nucleus accumbens. Neuropsychopharmacology. 2011;36(9):1811–22.
Wiesinger H. Arginine metabolism and the synthesis of nitric oxide in the nervous system. Prog Neurobiol. 2001;64(4):365–91.
Acknowledgements
The authors would like to thank Karolina Wydra-Kolarska, Ph.D., Agata Suder, and Dawid Gawliński, Ph.D. for technical support.
Funding
This study was supported by statutory funds from the Maj Institute of Pharmacology Polish Academy of Sciences, Kraków, Poland.
Author information
Authors and Affiliations
Contributions
MF contributed to conceptualization, supervision, and funding acquisition; MFr, IS, and KG were involved in methodology and validation; MFr, IS, KG, and RP were involved in data curation; MFr, IS, and MF performed visualization; MF, MFr, IS, and KG were responsible for writing—original draft preparation; MF and MFr were responsible for writing—review and editing. All authors contributed to and proofread the manuscript and approved the final version.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Frankowska, M., Smaga, I., Gawlińska, K. et al. Further proof on the role of accumbal nNOS in cocaine-seeking behavior in rats. Pharmacol. Rep 76, 338–347 (2024). https://doi.org/10.1007/s43440-024-00571-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-024-00571-y