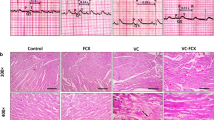
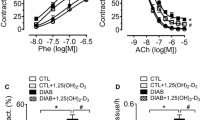
Abstract
Background
Type 2 Diabetes Mellitus is a chronic metabolic disease that causes endothelial damage and is an important risk factor for atherosclerosis. In the present study vitamin D3 supplementation in rats was used to determine the role of Osteoprotegerin (OPG)/Receptor activator kB ligand (RANKL) signalling in endothelial damage and changes in the expression levels of genes involved in this pathway. We hypothesized that vitamin D3 supplementation affects OPG and RANKL activity in the aorta.
Methods
Diabetes was induced in rats via injections of 40 mg/kg of streptozotocin followed by a high fructose (10%) diet. Group 2 (healthy) and 4 (diabetic) received 170 IU/kg of vitamin D3 weekly for 5 weeks, while Group 1 (healthy) and 2 (diabetic) received sterile saline. The aortas of each group were collected to analyse mRNA expression using the real-time PCR method and also to evaluate magnesium and calcium levels using inductively coupled plasma mass spectrometry.
Results
Opg and Il-1b expression levels were significantly associated with both diabetes and vitamin D3 supplementation in the aortas of the study groups (p ≤ 0.05). Opg mRNA expression was also found to correlate with both Icam-1 and Nos3 mRNA expression levels (r = 0.699, p = 0.001 and r = 0.622, p = 0.003, respectively). In addition, when mineral levels in the aortic tissues were compared among all groups, it was found that the interaction of diabetes and vitamin D3 supplementation significantly affected Mg levels and Mg/Ca ratios.
Conclusions
It is concluded that vitamin D3 supplementation has a modulatory effect on OPG/RANKL activity in the vessel wall by ameliorating endothelial damage in diabetes. This effect may contribute to the regulation of cytokine-mediated vascular homeostasis and mineral deposition in the aorta; therefore, further comprehensive studies are proposed to demonstrate this relationship.



Similar content being viewed by others
Abbreviations
- Bmp2:
-
Bone morphogenetic protein 2
- Ca:
-
Calcium
- CTL:
-
Healthy control
- CTL + VitD:
-
Control rats supplemented with Vitamin D3
- CVD:
-
Cardiovascular disease
- ECs:
-
Endothelial cells
- eNOS:
-
Endothelial nitric oxide synthase
- Gapdh:
-
Glyceraldehyde 3-phosphate dehydrogenase
- Icam-1:
-
Intercellular adhesion molecule-1
- ICP-MS:
-
Inductively coupled plasma mass spectrometry
- Il-1b:
-
Interleukin 1 β
- Mg:
-
Magnesium
- MMPs:
-
Metalloproteinases
- NF kB:
-
Nuclear factor кB
- Nos3:
-
Nitric oxide synthase 3
- OPG:
-
Osteoprotegerin
- Q-PCR:
-
Quantitative real-time PCR
- RANK:
-
Receptor activator кB
- RANKL:
-
Receptor activator кB Ligand
- Runx2:
-
RUNX family transcription factor 2
- STZ:
-
Streptozotocin
- T2DM:
-
Type 2 diabetes
- TNFa:
-
Tumor necrosis factor α
- VDR:
-
Vitamin D receptor
- VSMCs:
-
Vascular smooth muscle cells
References
Sami W, Ansari T, Butt NS, Hamid MRA. Effect of diet on type 2 diabetes mellitus: a review. Int J Health Sci (Qassim). 2017;11(2):65–71.
Leon BM, Maddox TM. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J Diabetes. 2015;6(13):1246–58.
Hadi HAR, Carr CS, Al Suwaidi J. Endothelial dysfunction: cardiovascular risk factors, therapy, and outcome. Vasc Health Risk Manag. 2005;1(3):183–98.
Hamilton SJ, Watts GF. Endothelial dysfunction in diabetes: pathogenesis, significance, and treatment. Rev Diabet Stud. 2013;10(2–3):133–56.
Widmer RJ, Lerman A. Endothelial dysfunction and cardiovascular disease. Glob Cardiol Sci Pract. 2014;3:43. https://doi.org/10.5339/gcsp.2014.43.
Rochette L, Meloux A, Rigal E, Zeller M, Cottin Y, Vergely C. The role of osteoprotegerin and its ligands in vascular function. Int J Mol Sci. 2019;20(3):705.
Alfaqih MA, Bashir N, Saadeh R, Khader Y, Barqawi M, Alqudah S. Dysregulation of the RANKL/RANK/OPG axis in thalassemia intermedia patients. BMC Res Notes. 2018;11(1):534.
Barchetta I, Ceccarelli V, Cimini FA, Bertoccini L, Fraioli A, Alessandri C, et al. Impaired bone matrix glycoprotein pattern is associated with increased cardio-metabolic risk profile in patients with type 2 diabetes mellitus. J Endocrinol Invest. 2018;42(5):513–20.
de Castro LF, Burke AB, Wang HD, Tsai J, Florenzano P, Pan KS, et al. Activation of RANK/RANKL/OPG pathway is involved in the pathophysiology of fibrous dysplasia and associated with disease burden. J Bone Miner Res. 2018;34(2):290–4.
Kosmopoulos M, Paschou SA, Grapsa J, Anagnostis P, Vryonidou A, Goulis DG, et al. The emerging role of bone markers in diagnosis and risk stratification of patients with coronary artery disease. Angiology. 2019;70:690–700. https://doi.org/10.1177/0003319718822625.
Pérez de Ciriza C, Lawrie A, Varo N. Osteoprotegerin in Cardiometabolic disorders. Int J Endocrinol. 2015;2015(3):1–15.
Tai N, Inoue D. Association between osteoporosis and atherosclerosis in dyslipidemia. Clin Calcium. 2019;29(2):237–43.
Bennett BJ, Scatena M, Kirk EA, Rattazzi M, Varon RM, Averill M, et al. Osteoprotegerin inactivation accelerates advanced atherosclerotic lesion progression and calcification in older ApoE −/− Mice. Arterioscler Thromb Vasc Biol. 2006;26(9):2117–24.
McGonigle JS, Giachelli CM, Scatena M. Osteoprotegerin and RANKL differentially regulate angiogenesis and endothelial cell function. Angiogenesis. 2009;12(1):35–46. https://doi.org/10.1007/s10456-008-9127-z.
Schoppet M, Schaefer JR, Hofbauer LC. Low serum levels of soluble RANK ligand are associated with the presence of coronary artery disease in men. Circulation. 2003;107(11):76e–76. https://doi.org/10.1161/01.CIR.0000060815.25798.02.
Boisson-Vidal C, Benslimane-Ahmim Z, Lokajczyk A, Heymann D, Smadja DM. Osteoprotegerin induces CD34+ differentiation in endothelial progenitor cells. Front Med. 2018;5:331.
Harper E, Forde H, Davenport C, Rochfort KD, Smith D, Cummins PM. Vascular calcification in type-2 diabetes and cardiovascular disease: Integrative roles for OPG RANKL and TRAIL. Vascul Pharmacol. 2016;82:30–40.
Mangan SH, Van Campenhout A, Rush C, Golledge J. Osteoprotegerin upregulates endothelial cell adhesion molecule response to tumor necrosis factor-alpha associated with induction of angiopoietin-2. Cardiovasc Res. 2007;76(3):494–505.
Heymann MF, Herisson F, Davaine JM, Charrier C, Battaglia S, Passuti N, et al. Role of the OPG/RANK/RANKL triad in calcifications of the atheromatous plaques: comparison between carotid and femoral beds. Cytokine. 2012;58(2):300–6.
Secchiero P, Corallini F, Pandolfi A, Consoli A, Candido R, Fabris B, et al. An increased osteoprotegerin serum release characterizes the early onset of diabetes mellitus and may contribute to endothelial cell dysfunction. Am J Pathol. 2006;169(6):2236–44.
Melamed ML, Thadhani RI. Mini-review vitamin D therapy in chronic kidney disease and end stage renal disease. Clin J Am Soc Nephrol. 2012;7:358–65.
Kim DH, Meza CA, Clarke H, Kim JS, Hickner RC. Vitamin D and endothelial function. Nutrients. 2020;12(2):1–17.
Anandabaskar N, Selvarajan S, Dkhar SA, Kamalanathan SK, Tamilarasu K, Bobby Z. Effect of Vitamin D supplementation on vascular functions and oxidative stress in type 2 diabetic patients with Vitamin D deficiency. Indian J Endocrinol Metab. 2017;21(4):555–63.
De Vita F, Lauretani F, Bauer J, Bautmans I, Shardell M, Cherubini A, et al. Relationship between vitamin D and inflammatory markers in older individuals. Age (Omaha). 2014;36(4):9694.
Takiishi T, Gysemans C, Bouillon R, Mathieu C. Vitamin D and diabetes. Endocrinol Metab Clin North Am. 2010;39(2):419–46.
Razzaque MS. The dualistic role of vitamin D in vascular calcifications. Kidney Int. 2011;79(7):708–14.
Hardwick LL, Jones MR, Brautbar N, Lee DBN. Magnesium absorption: mechanisms and the influence of vitamin D, calcium and phosphate. J Nutr. 1991;121:13–23.
Villa-Bellosta R. Impact of magnesium:calcium ratio on calcification of the aortic wall. PLoS ONE. 2017;12(6):e0178872.
Wilson RD, Islam MS. Fructose-fed streptozotocin-injected rat: an alternative model for type 2 diabetes. Pharmacol Rep. 2012;64(1):129–39.
Aydemir D, Salman N, Karimzadehkhouei M, Alaca BE, Turan B, Ulusu NN. Evaluation of the effects of aging on the aorta stiffness in relation with mineral and trace element levels: an optimized method via custom-built stretcher device. Biol Trace Elem Res. 2021;199:2644–52. https://doi.org/10.1007/s12011-020-02380-9.
Aydemir D, Karabulut G, Gok M, Barlas N, Ulusu NN. Data the DEHP induced changes on the trace element and mineral levels in the brain and testis tissues of rats. Data Br. 2019;26:104526.
Aydemir D, Simsek G, Ulusu NN. Dataset of the analyzing trace elements and minerals via ICP-MS: Method validation for the mammalian tissue and serum samples. Data Br. 2020;29:105218.
Glushakova O, Kosugi T, Roncal C, Mu W, Heinig M, Cirillo P, et al. Fructose induces the inflammatory molecule ICAM-1 in endothelial cells. J Am Soc Nephrol. 2008;19(9):1712–20.
Haruna Y, Morita Y, Komai N, Yada T, Sakuta T, Tomita N, et al. Endothelial dysfunction in rat adjuvant-induced arthritis: Vascular superoxide production by NAD(P)H oxidase and uncoupled endothelial nitric oxide synthase. Arthritis Rheum. 2006;54(6):1847–55.
Kinlay MB, Ganz MDP. Role of endothelial dysfunction in coronary artery disease and implications for therapy. Am J Cardiol. 1997;80:11I-16I.
Radenković M, Stojanović M, Prostran M. Experimental diabetes induced by alloxan and streptozotocin: the current state of the art. J Pharmacol Toxicol Methods. 2016;78:13–31.
Hou XD, Guan XQ, Cao YF, Weng ZM, Hu Q, Bin LH, et al. Inhibition of pancreatic lipase by the constituents in St. John’s wort: in vitro and in silico investigations. Int J Biol Macromol. 2020;145:620–33.
He Y, Yang T, Du Y, Qin L, Ma F, Wu Z, et al. High fat diet significantly changed the global gene expression profile involved in hepatic drug metabolism and pharmacokinetic system in mice. Nutr Metab (Lond). 2020. https://doi.org/10.1186/s12986-020-00456-w.
Song YQ, Guan XQ, Weng ZM, Liu JL, Chen J, Wang L, et al. Discovery of hCES2A inhibitors from: Glycyrrhiza inflata via combination of docking-based virtual screening and fluorescence-based inhibition assays. Food Funct. 2021;12(1):162–76.
Ma R, Deng XL, Du GL, Li C, Xiao S, Aibibai Y, Zhu J. Active vitamin D3, 1,25-(OH) 2 D 3, protects against macrovasculopathy in a rat model of type 2 diabetes mellitus. Genet Mol Res. 2016. https://doi.org/10.4238/gmr.15028113.
Valentini A, Cianfarani MA, Federici M, Tarantino U, Bertoli A. Osteoprotegerin in diabetic osteopathy. Nutr Metab Cardiovasc Dis. 2020;30:49–55.
Valderrábano RJ, Linares MI. Diabetes mellitus and bone health: epidemiology, etiology and implications for fracture risk stratification. Clin Diabetes Endocrinol. 2018;4(1):9.
Zannettino ACW, Holding CA, Diamond P, Atkins GJ, Kostakis P, Farrugia A, et al. Osteoprotegerin (OPG) is localized to the weibel-palade bodies of human vascular endothelial cells and is physically associated with von Willebrand factor. J Cell Physiol. 2005;204(2):714–23.
Benslimane-Ahmim Z, Poirier F, Delomenie C, Lokajczyk A, Grelac F, Galy-Fauroux I, et al. Mechanistic study of the proangiogenic effect of osteoprotegerin. Angiogenesis. 2013;16(3):575–93.
Zauli G, Corallini F, Bossi F, Fischetti F, Durigutto P, Celeghini C, et al. Osteoprotegerin increases leukocyte adhesion to endothelial cells both in vitro and in vivo. Blood. 2007;110(2):536–43.
García-Gómez MC, Vilahur G. Osteoporosis and vascular calcification: a shared scenario. Clin Investig Arterioscler. 2020;32:33–42.
Graves DT, Ding Z, Yang Y. The impact of diabetes on periodontal diseases. Periodontol 2000. 2020;82(1):214–24.
Riegel A, Maurer T, Prior B, Stegmaier S, Heppert V, Wagner C, et al. Human polymorphonuclear neutrophils express RANK and are activated by its ligand RANKL. Eur J Immunol. 2012;42(4):975–81.
Quercioli A, Mach F, Bertolotto M, Lenglet S, Vuilleumier N, Galan K, et al. Receptor activator of NF-κB ligand (RANKL) increases the release of neutrophil products associated with coronary vulnerability. Thromb Haemost. 2012;107(1):124–39.
Venuraju SM, Yerramasu A, Corder R, Lahiri A. Osteoprotegerin as a predictor of coronary artery disease and cardiovascular mortality and morbidity. J Am Coll Cardiol. 2010;55(19):2049–61. https://doi.org/10.1016/j.jacc.2010.03.013.
O’Brien CA. Control of RANKL gene expression. Bone. 2010;46(4):911–9.
Collin-Osdoby P. Regulation of vascular calcification by osteoclast regulatory factors RANKL and osteoprotegerin. Circ Res. 2004;95(11):1046–57.
Martinesi M, Bruni S, Stio M, Treves C. 1,25-Dihydroxyvitamin D3 inhibits tumor necrosis factor-α-induced adhesion molecule expression in endothelial cells. Cell Biol Int. 2006;30(4):365–75.
Charoenngam N, Holick MF. Immunologic effects of vitamin D on human health and disease. Nutrients. 2020;12(7):2097. https://doi.org/10.3390/nu12072097.
Martínez-Miguel P, Valdivielso JM, Medrano-Andrés D, Román-García P, Cano-Peñalver JL, Rodríguez-Puyol M, et al. The active form of vitamin D, calcitriol, induces a complex dual upregulation of endothelin and nitric oxide in cultured endothelial cells. Am J Physiol Endocrinol Metab. 2014;307(12):E1085–96.
Andrukhova O, Slavic S, Zeitz U, Riesen SC, Heppelmann MS, Ambrisko TD, Markovic M, Kuebler WM, Erben RG. Vitamin D is a regulator of endothelial nitric oxide synthase and arterial stiffness in mice. Mol Endocrinol. 2014;28(1):53–64. https://doi.org/10.1210/me.2013-1252.
Martinez-Moreno JM, Herencia C, Montes de Oca A, Muñoz-Castañeda JR, Rodríguez-Ortiz ME, Díaz-Tocados JM, Peralbo-Santaella E, Camargo A, Canalejo A, Rodriguez M, Velasco-Gimena F, Almaden Y. Vitamin D modulates tissue factor and protease-activated receptor 2 expression in vascular smooth muscle cells. FASEB J. 2016;30(3):1367–76. https://doi.org/10.1096/fj.15-272872 (Epub 2015 Dec 23. PMID: 26700731).
Zhang JY, Wu P, Chen D, Ning F, Lu Q, Qiu X, Hewison M, Tamblyn JA, Kilby MD, Lash GE. Vitamin D promotes trophoblast cell induced separation of vascular smooth muscle cells in vascular remodeling via induction of G-CSF. Front Cell Dev Biol. 2020;22(8): 601043. https://doi.org/10.3389/fcell.2020.601043.
White KA, Olabisi RM. Spatiotemporal control strategies for bone formation through tissue engineering and regenerative medicine approaches. Adv Healthc Mater. 2019;8(2):1801044.
Lee M-H, Kim Y-J, Kim H-J, Park H-D, Kang A-R, Kyung H-M, et al. BMP-2-induced Runx2 expression is mediated by Dlx5, and TGF-β1 opposes the BMP-2-induced osteoblast differentiation by suppression of Dlx5 expression. J Biol Chem. 2003;278(36):34387–94.
Byon CH, Sun Y, Chen J, Yuan K, Mao X, Heath JM, et al. Runx2-upregulated receptor activator of nuclear factor κB ligand in calcifying smooth muscle cells promotes migration and osteoclastic differentiation of macrophages. Arterioscler Thromb Vasc Biol. 2011;31(6):1387–96.
Sun M, Chang Q, Xin M, Wang Q, Li H, Qian J. Endogenous bone morphogenetic protein 2 plays a role in vascular smooth muscle cell calcification induced by interleukin 6 in vitro. Int J Immunopathol Pharmacol. 2017;30:227–37. https://doi.org/10.1177/0394632016689571.
Tintut Y, Patel J, Parhami F, Demer LL. Tumor necrosis factor-α promotes in vitro calcification of vascular cells via the cAMP pathway. Circulation. 2000;102(21):2636–42.
Aydemir D, Karabulut G, Şimşek G, Gok M, Barlas N, Ulusu NN. Impact of the Di(2-ethylhexyl) phthalate administration on trace element and mineral levels in relation of kidney and liver damage in rats. Biol Trace Elem Res. 2018;186(2):474–88. https://doi.org/10.1007/s12011-018-1331-0.
Liao F, Folsom AR, Brancati FL. Is low magnesium concentration a risk factor for coronary heart disease? The atherosclerosis risk in communities (ARIC) study. Am Heart J. 1998;136(3):480–90.
Proudfoot D. Calcium signaling and tissue calcification. Cold Spring Harb Perspect Biol. 2019;11(10):a035303.
Zeper LW, De Baaij JHF. Magnesium and calciprotein particles in vascular calcification: the good cop and the bad cop. Curr Opin Nephrol Hypertens. 2019;28:368–74.
Hénaut L, Massy ZA. Magnesium as a calcification inhibitor. Adv Chronic Kidney Dis. 2018;25:281–90.
Tebben PJ, Singh RJ, Kumar R. Vitamin D-mediated hypercalcemia: mechanisms, diagnosis, and treatment. Endocr Rev. 2016;37:521–47.
Aoshima Y, Mizobuchi M, Ogata H, Kumata C, Nakazawa A, Kondo F, et al. Vitamin D receptor activators inhibit vascular smooth muscle cell mineralization induced by phosphate and TNF-α. Nephrol Dial Transplant. 2012;27(5):1800–6.
Majeed F. Low levels of Vitamin D an emerging risk for cardiovascular diseases: a review. Int J Health Sci (Qassim). 2017;11(5):71–6.
Wang J, Zhou J, Robertson G, Lee V. Vitamin D in vascular calcification: a double-edged sword? Nutrients. 2018;10(5):652.
Bozic M, Álvarez Á, de Pablo C, Sanchez-Niño M-D, Ortiz A, Dolcet X, et al. Impaired vitamin D signaling in endothelial cell leads to an enhanced leukocyte-endothelium interplay: implications for atherosclerosis development. PLoS ONE. 2015;10(8):e0136863 (Bader M, editor).
Funding
This study was supported by the Scientific Research Projects Coordination Unit of Istanbul University (Project numbers: TYL-2019-32715 and TYL-2017-26139).
Author information
Authors and Affiliations
Contributions
GC conducted the experiments, performed the statistical analysis, wrote, and edited the original draft. MA, ASA, DA participated in experiments. MA, FKD, ASA, DA, NNU, TU, and EKB conceived and participated in the study design. EKB reviewed the manuscript. All authors contributed to and have approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Celebi, G., Anapali, M., Dagistanli, F.K. et al. Effect of vitamin D supplementation on OPG/RANKL signalling activities in endothelial tissue damage in diet-induced diabetic rat model. Pharmacol. Rep 74, 124–134 (2022). https://doi.org/10.1007/s43440-021-00332-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-021-00332-1