Abstract
Over the last several decades, the percentage of patients suffering from different forms of arthritis has increased due to the ageing population and the increasing risk of civilization diseases, e.g. obesity, which contributes to arthritis development. Osteoarthritis and rheumatoid arthritis are estimated to affect 50–60% of people over 65 years old and cause serious health and economic problems. Currently, therapeutic strategies are limited and focus mainly on pain attenuation and maintaining joint functionality. First-line therapies are nonsteroidal anti-inflammatory drugs; in more advanced stages, stronger analgesics, such as opioids, are required, and in the most severe cases, joint arthroplasty is the only option to ensure joint mobility. Cannabinoids, both endocannabinoids and synthetic cannabinoid receptor (CB) agonists, are novel therapeutic options for the treatment of arthritis-associated pain. CB1 receptors are mainly located in the nervous system; thus, CB1 agonists induce many side effects, which limit their therapeutic efficacy. On the other hand, CB2 receptors are mainly located in the periphery on immune cells, and CB2 modulators exert analgesic and anti-inflammatory effects in vitro and in vivo. In the current review, novel research on the cannabinoid-mediated analgesic effect on arthritis is presented, with particular emphasis on the role of the CB2 receptor in arthritis-related pain and the suppression of inflammation.
Graphic abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The term “arthritis” refers to a widely understood joint disease that is accompanied by pain and movement limitations. However, arthritis is not an organ-specific disease. According to The Centers for Disease Control and Prevention, there are several types of arthritis, the most common of which are osteoarthritis (OA), rheumatoid arthritis (RA), juvenile idiopathic arthritis (JIA), gout and fibromyalgia. In recent years, the percentage of patients suffering from OA and RA has increased due to the ageing population and an increasing risk of diseases such as obesity and type II diabetes [1,2,3,4]. Obesity contributes to direct mechanical cartilage degeneration, because overload joint is more exposed to wear and tear damage. Arthritis, especially OA development progress as a “self-propelling wheel” with inflammation and cartilage degradation as main contributors [4]. In turn, increased risk of RA is directly related to insulin resistance and linked to systemic inflammation induced by several proinflammatory factors (e.g. TNFa, IL-6). Moreover, the prevalence of type II diabetes is also increased in patients with RA [3]. The other risk factor that may contribute to arthritis development are joint injuries [5, 6]. Musculoskeletal diseases have long-term consequences not only for patients but also for society as a whole, such as economic problems [7]. Arthritis is a leading cause of disability and one of the most common conditions among chronic users of opioids in the U.S. [8]. This condition may affect 30% of people aged 18–64 and approximately 50–60% of people over 65 years old [9]. The number of people suffering from arthritis is highly underestimated, especially among younger patients and is more common in women than in men [10]. By 2040, the number of patients reporting activity limitations due to arthritis is estimated to increase by 52% to 34.6 million people in the U.S. (11.4% of all adults) [11], which may cause vast healthcare costs.
Current therapeutic strategies focus mainly on pain attenuation and maintaining joint functionality. First-line treatments are nonsteroidal anti-inflammatory drugs (NSAIDs). However, in the advanced stages of disease, NSAIDs are not sufficient, and patients need to take stronger analgesics, such as opioids, which have undesirable side effects and may be addictive. In the most severe cases, joint arthroplasty is required to maintain patient mobility [12,13,14]. The abovementioned therapeutic strategies focus mainly on symptomatic treatment; therefore, the search for novel therapies is urgently needed. Cannabinoids are a promising option for pain alleviation in OA and RA not only due to their analgesic effect, but also anti-inflammatory and anti-apoptotic properties [15, 16].
Moreover, cannabinoids may help to reduce the doses of opioids used by patients to relieve pain [17]. There are two types of cannabinoid receptors (CB1 and CB2). Direct activation of CB1 receptors may lead to adverse psychotropic side effects [18, 19], which limits their use in clinical practice. In contrast, CB2 modulators seem to be safer and do not cause negative central nervous system side effects [20, 21]. Understanding of the role of the CB2 receptor in pain came from the analgesic effect of the fatty acid ethanolamide–palmitoylethanolamide (PEA). The effect was blocked by the CB2 receptor antagonist SR141716A [22], although PEA had no significant affinity for either CB1 or CB2 [23]. This finding indicates that PEA does not directly activate CB2 but functions via indirect mechanisms [24].
Both OA and RA are accompanied by prolonged inflammatory states with different intensities. CB2 receptors have been found on immune cells [25], which indicates that these receptors play a role in inflammatory state modulation. Chondrocytes from OA joints, even in degenerated tissue, have been indicated to express CB2 receptors [26]. Moreover, CB2 receptors play important roles in osteoblast migration and bone formation. Mice with inactivated CB2 receptors (CB2−/−) developed osteoporosis significantly more often than control animals, while an in vitro osteoblast culture study showed that a CB2 agonist promoted bone nodule formation in wild-type osteoblasts [27]. This finding demonstrates the ability of CB2 receptors in joint tissues to respond to cannabinoid treatment. Several preclinical studies have demonstrated the analgesic and anti-inflammatory effects of CB2 modulators in arthritis models in vivo and in vitro. The current review discusses novel research on cannabinoid-mediated analgesic effects in OA and RA, with particular emphasis on the role of the CB2 receptor. The influence of cannabinoid compounds on arthritis-associated inflammation, pain and joint homeostasis are described and discussed.
Pathogenesis of arthritis
There are several types of arthritis, and OA and RA are two the most common types. A schematic changes that take place during arthritis progression are presented in the Fig. 1. Both diseases develop within the joints; however, there are some important differences between them. OA progresses slowly, leading to movement limitations and, in more severe cases, to a complete disability [28, 29]. Cartilage and subchondral bone degeneration and osteophyte formation, followed by synovial membrane inflammation (synovitis), lead to movement limitations and chronic pain [30]. Until recently, OA had been considered a “wear and tear” disease, indicating that cartilage degeneration caused by age and/or obesity was the primary cause of the disease. However, a few years ago, The Osteoarthritis Research Society (OARSI) reformulated the definition of OA, adding an inflammatory component as crucial for OA diagnosis. Joint inflammation is closely associated with macrophage influx and cytokine production, which drives the production of aggrecanases and matrix metalloproteinases (MMPs). Elevated levels of several proinflammatory factors were found in serum samples from OA patients in comparison to those of healthy individuals, but the levels were lower than those in serum samples from RA patients [31]. Interleukin 1β (IL-1β) and tumour necrosis factor alpha (TNFα) are two main proinflammatory factors produced by macrophages that influence the production of other inflammatory factors [32]. Other cytokines (e.g. IL-6, IL-8, IL-15 and IL-18) and chemokines (e.g. CCL2/3/4/5/19/21 and CXCL12) also play crucial roles in synovitis and OA progression [33, 34]. Inflammatory changes can be observed not only in late OA but also in the early stages (in patients with a median age of 34 years). Favero et al. proved, that in both early- and late-stage OA an important role plays synovium-meniscus cross-talk. IL-6 and IL-8 protein levels were elevated in synovium-meniscus co-cultures from patients from both groups (with early or late-OA stage), however, the changes were higher in the late OA. In both OA stages, CCL2/MCP‐1 was produced at higher levels by synovium compared with meniscus culture, while CCL5/RANTES protein release was significantly increased in co-cultures from early OA patients, in comparison to late-OA samples. MMP‐3 and MMP-10 protein release was increased in both early and end‐stage OA co-cultures compared to meniscus monocultures, while in the late OA the increase was approximately ten times higher in comparison to early stage. Tissue inhibitors of metalloproteinases (TIMPs) differed between groups: TIMP-2 is probably involved in the early OA, while TIMP-4 in the late stage of the disease [35]. Sohn et al. showed that Gc-globulin, a1-microglobulin and a2-macroglobulin could act via Toll-like receptor 4 (TLR4) to induce macrophage-dependent production of proinflammatory factors [31]. Thus, low-grade, prolonged inflammation is thought to be a pivotal factor in OA progression; however, a direct factor that initiates OA development is currently still unknown. Cartilage breakdown triggers the synovial membrane to release inflammatory factors into the joint space. The inflamed joint exhibit disrupted degradation-repair balance, which leads to further proteolytic enzyme production and cartilage degradation. The disease progresses in a self-perpetuating cycle in which cartilage degeneration triggers synovitis, which leads to further degradation [36]. Additionally, subchondral bone degradation seems to play a very important role in OA progression. Osteoclast-chondrocyte crosstalk, described by Hu et al., plays a significant role in joint homeostasis and OA development [37]. Since cartilage has low regenerative properties (because of the lack of innervation and blood vessels), conventional OA treatment is limited to pain attenuation and maintaining joint mobility [38]. First-line treatment is oral administration of paracetamol or NSAIDs, whereas opioid use is necessary for severe OA pain. Intra-articular injections of corticosteroids, hyaluronic acid (viscosupplementation) or mesenchymal stem cells also provide beneficial therapeutic effects on OA patients. Additional options that support pain alleviation and maintain joint functionality are exercise and physical therapy [39]. In OA, physiotherapy is recommended as an integral part of treatment. Both aquatic and land-based exercise therapy has similar beneficial effects [40], also manual and exercise therapies similarly improve patient’s mobility and there is no added benefit from a combination of these two therapies [41]. According to Juhl et al., exercise program for knee OA treatment should focus on improving aerobic capacity, quadriceps muscle strength and lower extremity performance and should be carried out three times a week [42]. In comparison to standard treatment, physical exercise may be similarly effective to hyaluronic acid injections and provide significant functional improvement in patients with moderate OA [43, 44]. In elderly OA patients, exercise increased muscle myofibrillar and sarcoplasmic protein fractional synthesis rates, whereas NSAIDs treatment reduced the level of circulating prostaglandin F2α [45]. Neuromuscular exercise effectively improved the performance of everyday activities in OA patients up to 12 months and provided greater improvement in knee symptoms (such as swelling, stiffness, etc.) in comparison do NSAID-treated group [46].
Schematic summary of arthritis pathogenesis. Pathological changes, such as swelling, synovial membrane outgrowth and inflammation (synovitis), are observed in both OA- and RA-affected joints. Although the inflammatory state is an important factor in the development of OA, it is not as critical as in RA, which is a typical inflammatory disease. In the synovial fluid, several inflammatory factors, such as cytokines, chemokines, macrophages, neutrophils, fibroblasts, chondrocytes and osteoblasts, can be found. Gradual cartilage degeneration, which is characteristic of OA progression, causes bone exposure and pain, while released cartilage fragments potentiate synovitis within the joint capsule
RA is an inflammatory disease characterized by persistent synovitis, systemic inflammation and autoantibodies, which lead to joint damage and disability [47]. In 2007, 1.3 million adults in the U.S. were affected by RA [48]. Women suffer from RA more frequently than men [49], and it is estimated that 1 in 12 women and 1 in 20 men will develop RA during their lifetime [50]. The main symptoms are swelling and stiffness in multiple joints (most commonly the wrists, proximal interphalangeal joints and metacarpophalangeal joints), pain and systemic symptoms (e.g. fatigue, weight loss and low-grade fever) [51]. Genetic factors are important, and the heritability of RA seems to be ~ 40% [52]. Approximately 100 specific genetic loci have been identified as associated with an increased risk of RA [53]. Apart from genetic factors, environmental factors also play a role in RA development, among which the most important are exposure to tobacco smoke, air pollution and obesity [54]. There is also evidence that imbalance in the gut microbiota and infections can contribute to RA development [55, 56]. Septic arthritis affects 2–6 per 100,000 people per year [57]. Joint inflammation is caused by bacteria, mycobacterial or fungi and the diagnosis is based on the synovial fluid analysis. The most common cause of septic arthritis is Staphylococcus aureus, which is responsible for 37–56% of cases [58, 59]. The study of Konig et al. proved the presence of autoantigens that are primary immune targets in RA in gingival crevicular fluid of patients with periodontal disease. They also identified a periodontal pathogen Aggregatibacter actinomycetemcomitans as a possible bacteria which trigger autoimmunity in RA [56].
The main factors involved in RA progression are TNFα, IL-6, IL-1, IL-17, IL-23, IL-21, IL-12, granulocyte–macrophage colony-stimulating factor (GM-CSF), CXCL8, Th2 cytokines and type 1 interferons (IFNs) [60, 61]. Circulating levels of IFN-γ, TNFα, IL-17 and IL-12 are elevated in RA patients or are produced by mitogen-stimulated peripheral blood mononuclear cells; moreover, lower levels of the anti-inflammatory factor interleukin IL-10 have been detected [62]. RA is treated with disease-modifying anti-rheumatic drugs (DMARDs), such as methotrexate, leflunomide or sulfasalazine. Early diagnosis and treatment are crucial for RA progression, and a window of opportunity may be present within the first year, especially in the first 3 months of disease onset. It was proven that very early DMARD treatment (in the first 3 months after symptom onset) gives better results than if the treatment is started after 12 months of RA symptoms [63]. However, in some patients, DMARDs are not effective. Pain in RA may arise from joint pathology and peripheral, spinal and supraspinal processing of pain signals. Sensitization mechanisms may occur at both peripheral and central levels and contribute to hyperalgesia and allodynia [64].
The endocannabinoid system
The endocannabinoid system (ECS) plays an important role in several processes, including neurodegenerative and neurological disease development [65, 66], stress-induced responses [67], pain processing [68] and immune system modulation [69], and may also link the gut microbiota and depression [70]. There are two main endocannabinoids, anandamide (AEA) and 2-arachidonoylglycerol (2-AG), and two main types of cannabinoid receptors, CB1 and CB2. AEA is more selective for CB1, while 2-AG has almost the same affinity for both CB receptors [71]. In addition, endocannabinoids may act via other receptors, such as transient receptor potential channels (TRPVs) or GPR receptors. Chondrocytes from patients with OA express a wide range of receptors (CB1, CB2, GPR55, PPRα, PPRγ). Most of receptors are highly expressed even in cells from degenerated cartilage; however, the number of chondrocytes displaying immunopositivity for GPR18 and TRPV1 is significantly decreased in degenerate cartilage [26]. Richardson et al. proved the presence of CB1 and CB2 protein in the synovial membrane taken from OA or RA patients; moreover, the synovial fluid of these patients contained both AEA and 2-AG, which were not detectable in healthy controls [72]. Endocannabinoids are not stored in the cell but are synthesized on demand from membrane-bound phospholipids, and their release is vesicle-independent. In numerous brain regions, endocannabinoid signalling occur in a retrograde manner, and cannabinoid receptors are localized on presynaptic neurons [73]. At the periphery, an important role in endocannabinoid signalling plays immune system. Leukocytes and other immune cells are shown to express CB2 and to a less extent: CB1 receptors. Endocannabinoids in the immune system are mostly produced by macrophages, lymphocytes, astrocytes, dendritic cells, microglia and monocytes [74, 75]. Endocannabinoids play important role in the enteric nervous system of gastrointestinal tract, where CB receptors are localized on the enteric nerve terminals. In the enteric nervous system, endocannabinoids exert inhibitory actions on neurotransmission to reduce motility and secretion [76], however, this mechanism is mediated mostly via CB1 receptors [77]. In the nervous system, CB receptors are distributed on peripheral terminals of primary afferent neurons, where play role in pain modulation: upon activation, modulate transducer ion channels and regulate neuron excitability [78].
Although AEA and 2-AG share similarities in their chemical structures, they are synthesized and degraded via two distinct Ca2+-dependent enzymatic pathways. AEA is synthesized in two stages (Fig. 2). First, N-arachidonoyl phosphatidylethanolamine (NAPE) is synthetized from glycerophospholipid (GPL) and phosphatidylethanolamine (PtdEth) with the participation of calcium-dependent membrane-associated trans-N-acyltransferase (NAT) and calcium-independent NAT (RPL-1). Second, the enzymatic hydrolysis of NAPE is catalysed by NAPE-selective phospholipase D (NAPE-PLD) and leads to AEA and phosphatidic acid production. 2-AG is produced from diacylglycerols (DAGs). Phosphoinositol bis-phosphate (PIP2) is catalysed by PIP2-selective phospholipase C (PLC), while phosphatidic acid (PA) is catalysed by PA phosphohydrolase (PAP), causing the formation of DAGs. Then, DAGs are converted into 2-AG by sn-1 selective-DAG lipases (DAGLs). The degradation pathway involves fatty acid amide hydrolase (FAAH)-mediated degradation of AEA and monoglycerol lipase (MAGL)-mediated degradation of 2-AG. AEA is degraded to arachidonic acid (AA) and ethanolamine (EtA), while 2-AG is degraded to AA and glycerol (G) [79, 80]. These pathways are the major pathways for the synthesis and degradation of endocannabinoids. In parallel, endocannabinoids can be synthesized and degraded via alternative pathways, and particular products may be responsible for several endocannabinoid effects. For example, AA is further metabolized on the cyclooxygenase pathway (by COX-1, COX-2) to prostaglandins, or on lipoxygenase pathway (by LOX-5, LOX-8, LOX-12, LOX-15) to leukotrienes, lipoxins and 8-, 12-, 15- hydroperoxyeicosatetraenoic acid. These metabolites might act as proinflammatory agents and generate nociceptive effects. This paradoxical effect proves ECS complexity and impede its modulation to achieve analgesic effect. It is attractive to suggest that the anti-inflammatory actions of NSAIDs are due to the COX inhibition, interestingly some NSAIDs also have the ability to inhibit FAAH (e.g. ibuprofen [81]). Moreover, FAAH inhibition might fail to induce analgesic effect due to the binding of endocannabinoids to other receptors, e.g. pro-nociceptive TRPV1. Novel dual-acting drugs, targeting endocannabinoid and endovanilloid system via interaction with FAAH enzyme together with TRPV1 receptor or COX-2 might lead to development of more efficient strategy for pain treatment [82, 83]. Finally, there is an evidence that AA might serve as a substrate to AEA production on the FAAH reversed pathway [84]. It was proven, that FAAH in the liver might work in reverse: in mice after partial hepatectomy, when the AA and EtA increase dramatically, FAAH activity might be switched for AEA generation form AA and EtA [85, 86]. Piscitelli and Di Marzo demonstrated that the ECS shows high redundancy, e.g. both AEA and 2-AG—besides classic route—are also inactivated through alternative biochemical routes, including hydrolysis and oxidation with several enzymes involved in this process. Moreover, endocannabinoids interact also with other than CB receptors, while the products of endocannabinoid catabolism may act on their own targets. This multidimensionality of the ECS system impedes its use in the treatment of several pathological conditions. However, knowledge of ECS redundancy could contribute to the development of novel analgesics, such as “dirty drugs” (compounds with more than one mechanism of action), and the use of certain natural products that are not currently used in the clinic [87]. Modulating the activity of the ECS seems to be a promising therapeutic strategy for several diseases, including arthritis, and is described in more detail in this review.
The main AEA and 2-AG synthesis and degradation pathways. Abbreviations: NAT: calcium-dependent membrane-associated trans-N-acyltransferase; RPL-1: calcium-independent NAT; GPL: glycerophospholipid; PtdEth: phosphatidylethanolamine; NAPE: N-arachidonoyl phosphatidylethanolamine; NAPE-PLD: NAPE-selective phospholipase D; AEA: anandamide; FAAH: fatty acid amide hydrolase; EtA: ethanolamine; AA: arachidonic acid; PIP2: phosphoinositol bis-phosphate; PLC: PIP2-selective phospholipase C; PA: phosphatidic acid; PAP: phosphatidic acid phosphohydrolase; DAGs: diacylglycerols; DAGL: sn-1 selective-DAG lipases; 2-AG: 2-arachidonylglycerol; MAGL: monoglycerol lipase; G: glycerol
Cannabinoid receptors
There are two types of cannabinoid receptors: CB1 and CB2. Both are sevenfold membrane-bound receptors associated with Gi/o proteins (GPCRs), and have more than one endogenous ligand (in contrast to most GPCRs) [88]. After activation, CB receptors inhibit adenylyl cyclase (AC) activity, leading to decreases in cAMP levels and stimulating mitogen-activated protein kinase (MAPK) and Akt cascades [89, 90]. Moreover, cannabinoids also act through other receptors, such as transient receptor potential channels (TRPV1, TRPV4, TRPM8 and TRPA1) and orphan receptors (GPR18 and GPR55) [91, 92]. CB1 receptors are most highly expressed in the central and peripheral nervous systems, mainly in presynaptic terminals. Due to the central location of these receptors, CB1 agonists induce a number of adverse effects, such as sleepiness, anxiety, euphoria and cognitive impairment, which limit their use in clinical practice. CB2 receptors are located mainly in the periphery. CB2 receptors were first discovered on immune cells [25, 93]; however, there is evidence of CB2 expression in the central nervous system [94] such as in the cerebellum [95] or brainstem [96] and particularly on microglial cells [97]. In comparison to CB1 receptors, CB2 receptor expression is much less abundant; hence, it was initially thought that CB2 receptors were absent from the nervous system. CB2 receptors located on neurons and microglia in the spinal cord contribute to central sensitization in OA [98]. CB2 receptors might play a role in the several neurological diseases, such as Alzheimer disease [99, 100], depression [101, 102], Parkinson disease [103] or memory formation [104, 105]. However, since CB2 in the other than peripheral location has been investigated recently, more studies are needed to clarify its role in various conditions.
Chondrocytes from OA joints and degenerated tissues have been proven to express a wide range of cannabinoid receptors [26]. Pajak et al. proved that the protein level of the CB2 receptor increased significantly during OA progression in rat joint tissues [106]. This finding demonstrates the potential role of cannabinoids in OA treatment. The CB2 receptor gene polymorphism Q63R is associated with increased arthritis risk [107, 108]. CB2 is also important for the regulation of osteoblast differentiation and bone formation. Mice with inactivated CB2 receptors developed osteoporosis with relative uncoupling of bone resorption from bone formation, while in primary osteoblasts from CB2−/− mice, a reduced capacity to form bone nodules in vitro was observed (in wild-type osteoblast cell culture, the CB2 agonist HU-308 promoted bone node formation) [27]. Mice lacking the CB2 receptor suffered from more severe OA induced by surgical destabilization of the medial meniscus and spontaneous OA than WT mice [109]. In an in vitro study of human RA synovial fibroblasts (FLSs), the CB2 receptor was shown to play a role in IL-1β-induced inflammation. CB2 knockdown resulted in reductions in IL-1β-induced IL-6, IL-8, ENA-78, RANTES, cyclooxygenase-2 (COX-2), MMP-2 and MMP-9 production, while CB2 overexpression increased IL-6, IL-8 and ENA-78 expression [110]. Additionally, CB2 knockout resulted in decreased production of proteoglycans in cultures of murine articular chondrocytes in vitro compared to chondrocytes from WT animals [109]. Both synthetic [111] and plant-derived cannabinoids [112] appear to be promising candidates for pain treatment and inhibiting arthritis development.
The role of endocannabinoids in arthritis-associated pain and inflammation
Alternations in AEA have been proven to play role during neuropathic pain development [113] and OA [114]. During OA progression, AEA synthesis and degradation enzymes were elevated in the spinal cord, synovial membrane and cartilage several days in animals after OA induction [114]. AEA and 2-AG levels were augmented in the synovial fluid of dogs with OA [115]. AEA was also elevated in the joint in a posttraumatic OA mouse model [116]. In contrast to those in healthy volunteers, AEA and 2-AG have been detected in the synovial fluid of OA and RA patients [72]. Increased plasmatic levels of 2-AG and the upregulation of CB1 and CB2 receptor gene expression in peripheral blood lymphocytes were also detected in OA patients compared with healthy subjects [117]. Moreover, in patients with OA, 2-AG levels are negatively correlated with leptin levels in cerebrospinal fluid, suggesting a role of 2-AG in food intake [118]. Intrathecal injection of 2-AG or AEA dose-dependently decreased carrageenan-induced mechanical allodynia in rats [119]. In in vitro bovine cartilage explants, AEA dose-dependently inhibited the release of sulphated glycosaminoglycans (GAGs) [120]. Many studies indicate the anti-inflammatory and analgesic effects of endocannabinoids. Blocking FAAH, which results in an increase in AEA, attenuates the development of arthritis and hyperalgesia in mice [121, 122]. Blocking MAGL also reduces mechanical hypersensitivity in OA rats [123]. These examples demonstrate the role of endocannabinoids and the entire ECS in arthritis pathogenesis and treatment possibilities.
CB2 receptor-dependent modulation of arthritis-associated pain and progression
Mechanism of action of CB2 modulators
The literature provides strong evidence for pain reduction by CB2 receptor modulators in arthritis, however, the exact mechanism of action is not clear. The role of synthetic cannabinoids with higher affinities for CB2 than CB1 [124] in arthritis is described in detail in this review and summarized in Table 1.
A major problem of cannabis-based drugs is their nonspecificity, interactions with receptors other than CB receptors and differences in effects in preclinical studies compared to clinical trials. Soethoudt et al. proved that most CB2 agonists exhibit reduced selectivity regarding binding affinity and functional efficacy on mouse CB2 versus CB1 compared to human orthologues. Antagonists exhibited the opposite effect [125]. Moreover, the same study revealed that HU-308 induced differences in signalling effects between the human and mouse CB2 receptors. This finding may indicate that HU-308 is a well-balanced agonist in all tested signal transduction pathways (GTPgS, cAMP, b-AR, pERK and GIRK) via human CB2 receptors but is significantly biased towards G-protein activation via murine CB2 receptors [125]. JWH-015 and HU-210 are agonists of GPR55 [126, 127]. JWH-015 also decreases the chemotaxis of monocytes [128]; however, it was proven to induce anti-inflammatory effects in the absence of CB2 receptors, which suggests a role of noncanonical or off-target receptors. Using molecular docking and molecular dynamics analyses, Fechtner et al. showed that JWH-015 favourably bound to glucocorticoid receptor (GR), and GR knockdown reduced the anti-inflammatory effect of JWH-015 [129]. JWH-015 and JWH-133 modulate interferon α (IFNα) and TNFα responses in primary human plasmacytoid dendritic cells [130]. In an in vitro study of IL-1β-stimulated synoviocytes, JWH-133 mediated the association of CB2 with TAK1 kinase to increase nuclear translocation of the transcription factors NF-κB p65 and AP-1 [110]. Most CB2 modulators exert anti-inflammatory effects on arthritis animal and in vitro models [131,132,133]. CB2 agonists can also exert paradoxical effects, which may be explained by their involvement via other receptors. For example, GW405833 reduced the mechanosensitivity of afferent nerve fibres in control joints but caused nociceptive responses in OA joints. This effect may be triggered by TRPV1 receptors, which can induce a nociceptive effect [134]. Additionally, the inhibition of enzymes responsible for endocannabinoid degradation (FAAH and MAGL) is effective in pain reduction in arthritis. URB595 (FAAH inhibitor) induces an increase in AEA, while MJN110 (MAGL inhibitor) enhances 2-AG levels. AEA and 2-AG are endogenous ligands of CB receptors, and these compounds are nonselective and show moderate binding affinities towards both receptors [125]. However, their mechanism of action depends on both CB receptors and can be blocked by specific CB1 or CB2 antagonists.
Analgesic and anti-inflammatory effects of CB2 modulators
Several studies have revealed the analgesic potential of CB2 receptor modulators, whereas in vitro models have confirmed the anti-inflammatory and anti-degenerative effects of CB2-targeting compounds. In collagen-induced arthritis (CIA) in mice, CB2 receptor expression was markedly higher in arthritic animals than in the control group [135]. JWH-133 suppressed CIA, synovial hyperplasia, inflammatory responses, cartilage damage and bone destruction. Moreover, JWH-133 repolarized the macrophage phenotype from M1 to M2, promoted anti-inflammatory IL-10 expression and diminished TNFα, IL-1β and IL-6 levels [135]. Fukuda et al. proved that JWH-133 reduced arthritis scores, inflammatory cell infiltration, bone destruction and anti-CII IgG1 production in CIA mice [136]. In sodium monoiodoacetate (MIA)-induced OA, systemic administration of JWH-133 reduced pain, inflammation, spinal astrogliosis and MMP-2 and MMP-9 activity in treated rats. In turn, spinal JWH-133 administration diminished noxious-induced responses in spinal neurons [98]. OA mice with CB1 knockout show effective manifestations that are not observed in CB2-KO mice. JWH-133 and ACEA (CB1 agonist) ameliorated nociceptive and affective alterations [117]. In healthy rats, JWH-133 increased synovial blood flow, and the effect was blocked by the CB2 receptor antagonist AM630 and the TRPV1 receptor antagonist SB366791. This finding indicates the role of the TRPV1 receptor in the mechanism of action of JWH-133. In arthritic animals, the vasodilatory effect of JWH-133 was diminished [137]. JWH-133 also exerts anti-inflammatory effects in vitro. JWH-133 reduces IL-6, MMP-3 and CCL2 production in TNFα-stimulated RA/OA FLSs [136]; however, the pretreatment of RA FLSs with JWH-133 before inflammatory stimulation did not reduce the IL-1β-induced increase in IL-6 and IL-8 expression and augmented COX-2 expression [110]. JWH-133 also increased osteoclast formation in osteoblast-bone marrow co-cultures in vitro [138].
The receptor activator of nuclear factor κB ligand/osteoprotegerin ratio (RANKL/OPG) is an important indicator of bone homeostasis and remodelling. This ratio determines osteoclast proliferation and activity and, therefore, controls bone formation and resorption [139]. In RA patients, the RANKL/OPG ratio was significantly decreased [140]. JWH-015, another CB2 agonist, ameliorated pain in arthritic rats, inhibited bone destruction, increased RANKL and decreased OPG levels [129]. JWH-015 also increased synovial blood flow in healthy animals [137]. In human RA FLSs in vitro, JWH-015 inhibited the ability of IL-1β to induce the production of IL-6, IL-8 and COX-2; however, this effect was partially mediated by the GR receptor, since the effect of JWH-015 persisted after CB2 knockdown [129].
Similar to JWH-133, HU-308, a selective CB2 agonist, reduced osteoclast formation in osteoblast-bone marrow co-cultures [138]. In IL-1β-, TNFα- or LPS-stimulated OA/RA FLSs, HU-308 diminished FLS proliferation but also inhibited MMP-3, MMP-13, IL-6 production and IL-1β-induced activation of extracellular ERK1/2 and p38 MAPK [141]. In LPS-stimulated mouse peritoneal macrophages from WT mice, HU-308 decreased the levels of IL-6 and TNFα but had no effect on macrophages from CB2-KO mice, suggesting the participation of the CB2 receptor in the mechanism of action of HU-308 [132]. The same study revealed that in the CIA mouse model, HU-308 did not inhibit the incidence of CIA development but alleviated the severity of CIA and decreased joint swelling, synovial inflammation, joint destruction and serum levels of anti-collagen II antibodies [132]. In surgically induced OA in CB2-KO and WT mice, HU-308 reduced OA severity. More severe OA was observed in CB2-KO mice than in WT mice [109].
WIN55,212-2, a nonselective CB agonist, has been widely studied in vitro, and its anti-inflammatory and anti-degradative properties have been verified. In TNFα-stimulated RA FLSs, WIN55,212-2 showed dose-dependent anti-inflammatory effects. At low concentrations, WIN55,212-2 decreased IL-6, IL-8 and MMP-3 production (an effect independent of CB1 or CB2 activation, but attenuated by TRPV1 or TRPA1 inhibition) and increased FLS adhesion. While higher concentrations of WIN55,212-2 reduced IL-6, IL-8 levels, adhesion and proliferation of FLS, it increased extracellular MMP-3 level [133]. In primary human OA articular chondrocyte cultures, WIN55,212-2 mesylate inhibited the activity of a disintegrin and metalloproteinase with thrombospondin motif 4 (ADAMTS-4, a major contributor to the pathogenesis of OA) and syndecan-1 expression, which suggests an antiarthritic effect [142]. WIN55,212-2 also regulates the gene and protein levels of MMPs and tissue inhibitors of metalloproteinases (TIMPs). MMP-3, MMP-13, TIMP-1 and TIMP-2 gene expression and MMP-3 and MMP-13 protein secretion were decreased after WIN55,212-2 treatment in IL-1β-stimulated chondrocyte cultures [143]. WIN55-212-2 administration in both IL-1α-stimulated bovine chondrocytes and explants, reduced proteoglycan, collagen degradation, iNOS, COX-2 expression and NF-κB activation. But the reduction in NO production was observed only in IL-1α-stimulated bovine articular chondrocytes which potentiated by AM281 and AMM630. Also WIN55,212-2-induced decrease in sulphated glycosaminoglycans was reported [120, 144]. WIN55-212,2 was also proven to decrease IL-6 and IL-8 expression; however, this effect was not inhibited by CB1 or CB2 antagonists, which indicates a CB receptor-independent mechanism of action. A similar effect occurred with the nonselective CB agonist CP55,940 [145], which was also proven to stimulate osteoclast formation in vitro [146].
Another selective CB2 agonist, A-796260, increased grip force in a rat OA model [147]. 4-Quinolone-3-carboxamide (4Q3C) was proven to reduce bone erosion, joint destruction, osteoclast formation and the general severity of arthritis in mice. Moreover, the same study revealed that 4Q3C diminished the proinflammatory factors TNFα, IL-1β and COX-2 and the RANKL/OPG ratio [131]. GW405833 reduced the joint afferent firing rate in control animals but caused nociceptive responses in OA joints and increased hindlimb incapacitance in rats. This effect was diminished by the CB2 receptor antagonist AM630 and the TRPV1 receptor antagonist SB366791, which proves that lack of selectivity of GW405833 and the involvement of TRPV1 receptors in this mechanism of action [134]. The plant-derived CB2 agonist β-caryophyllene (BCP) alleviated the severity of collagen antibody-induced arthritis, deceased MMP-3 and MMP-9 expression in joints, reduced proinflammatory cytokine expression and increased anti-inflammatory cytokine expression [112]. The studies described in this paragraph are summarized in Table 2.
Attenuation of OA pain and inflammation by inhibiting endocannabinoid degradation
Apart from the administration of CB2 agonists, ECS modulation may also be achieved via the inhibition of AEA or 2-AG degradation. Blocking the enzyme FAAH results in prolonged local AEA accumulation. One of the best studied FAAH inhibitors is URB597. Kinsey et al. proved that prolonged FAAH inhibition (in genetically modified FAAH−/− mice or after repeated URB597 administration) reduces the severity of CIA. Decreases in hyperalgesia and the severity of CIA in FAAH−/− mice were reversed by chronic administration of the CB2 receptor antagonist SR144528, while the effect of URB597 was prevented by the acute administration of the CB1 receptor antagonist rimonabant. This finding suggests that prolonged CB2 receptor activation diminishes CIA severity, whereas acute CB1 receptor activation reduces hyperalgesia in a CIA model [121]. In the kaolin/carrageenan-induced joint inflammation model, low doses of URB597 reduced hyperaemia and leukocyte rolling and adhesion. Moreover, improvements in hindlimb weight bearing and withdrawal thresholds were observed, which were mediated by CB1 receptors. Importantly, the effects on leukocyte rolling and hyperaemia were blocked by both CB1 and CB2 antagonists, while the reduction in leukocyte adhesion was independent of CB receptor activation [148]. In two rodent OA models, Schuelert et al. proved that peripheral administration of URB597 significantly reduced the afferent firing rate and hindlimb incapacitance (the effect was blocked by the CB1 receptor antagonist AM251), while local URB597 injection reduced mechanonociception and pain (this effect was mediated by CB1 receptors) [149]. Because FAAH inhibitors did not exhibit sufficient efficacy in clinical trials (despite their analgesic effects in animal studies), the efficacy of dual-acting FAAH and TRPV1 inhibitor (OMDM-198) in pain reduction was tested. OMDM-198 showed anti-hyperalgesia effects in an OA animal model. The effectiveness was comparable to that of a selective TRPV1 antagonist (SB-366,791) and a selective FAAH inhibitor (URB597) and was blocked by AM251 and olvanil (TRPV1 inhibitor) [83].
In turn, MAGL blockade results in the inhibition of 2-AG degradation. In the MIA-induced OA rat model, acute MJN110 administration was proven to significantly elevate brain 2-AG levels. A single injection of MJN110 restored weight-bearing asymmetry and lowered the withdrawal threshold. Both effects were blocked by SR144528, a CB2 receptor antagonist, while CB1 blockade by SR141716A inhibited only the restoration of weight-bearing asymmetry. In the same study, repeated MJN110 administration resulted in antinociceptive tolerance at a high dose (5 mg/kg); however, a low (1 mg/kg) dose reduced pain but did not alter joint histology. MJN100 inhibited the expression of membrane-associated PGE synthase-1 in the spinal cords of OA rats [150]. Another MAGL inhibitor, KLM29, reduced pain in the MIA-induced OA rat model, and this effect was blocked by CB1 and CB2 antagonists. The COX-2 inhibitor celecoxib was also administered, a significant reduction in joint pain and inflammation was noticed and mechanical allodynia development was prevented in the later OA stages [123]. Studies on the analgesic effect of selected inhibitors of endocannabinoid-degrading enzymes in arthritis are summarized in Table 3.
Future prospects
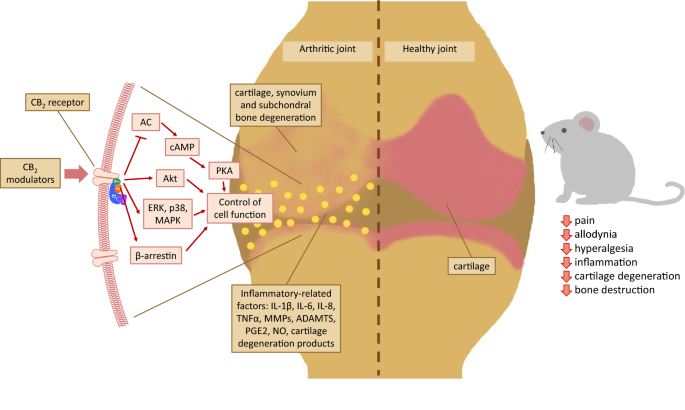
The studies presented in this review confirm the role of the ECS in arthritis pathogenesis and the efficacy of both endocannabinoids and novel synthetic CB2 modulators in pain management. Chronic pain of various origins is currently a global problem. In more severe cases, patients are forced to use stronger analgesics such as opioids, which are effective in alleviating pain but have a high potential for addiction. Opioid use disorder symptoms are increasingly diagnosed in chronically ill patients. Cannabinoid-based opioid replacement therapy may be an analgesic alternative that can help to ease opioid withdrawal symptoms and decrease the likelihood of addiction relapse [151]. In chronic and inflammatory pain models in rodents, combined mu opioid receptor (MOR) and CB2 receptor agonism resulted in significant synergistic pain inhibition and reduced opioid-induced side effects [152]. In turn, La Porta et al. proved that after OA induction, CB1KO and CB2KO animals developed allodynia at similar levels to wild-type mice, whereas in CB2xP transgenic animals (overexpressing CB2 receptors), allodynia was significantly attenuated. Moreover, the role of functional interactions between the ECS and the opioid system in the control of joint pain has been described [153]. These data suggest that cannabinoids can be an efficient alternative for opioids and reduce the opioid doses used by patients with chronic pain.
Apart from the abovementioned advantages of cannabinoids in chronic pain, ECS modulation itself might be a useful strategy for treating arthritis and the accompanying pain and inflammation. Although endocannabinoids are not selective for the CB2 receptor, they have been proven to diminish hyperalgesia in various arthritis animal models and prevent joint damage. AEA or 2-AG degradation inhibitors in combination with other currently available treatment strategies, such as the coadministration of COX-2 inhibitors, may give improved results in both pain alleviation and anti-inflammatory effects [82].
Cannabinoids not only alleviate joint hyperalgesia but also may help to prevent joint damage, chronic pain development and disease progression [121]. In addition to endocannabinoids, synthetic CB2 modulators exert analgesic and anti-inflammatory effects in various in vitro and in vivo arthritis models [133, 154, 155]. However, cannabinoid therapy is still not widely used. One of the main drawbacks of cannabinoids is their psychoactive component, which is mainly associated with CB1 receptors, which are localized mostly in the central nervous system. The peripheral location of the CB2 receptor, especially on immune cells, makes it a better therapeutic target, with very limited side effects. CB2 modulators, which have been proven to have analgesic and anti-inflammatory properties with no or mild negative side effects might be an interesting alternative to NSAID therapy, especially in the early, non-severe arthritis stages. Although there are indications for effective arthritis therapy, further preclinical studies need. Despite the fact that arthritis is a common disease that affects an increasing number of patients every year, the endocannabinoid approach to treatment is still not very popular in therapeutic practice. Cannabinoid-based drugs (especially those targeting CB1 receptors) possess dose-limiting side effects and may have modest clinical efficacy. However, increasing interest in products containing cannabinoid extracts has been observed in recent years, which may lead to the use of cannabinoids on a larger scale but might also carry the risk of overdose and the treatment of cannabinoids not as drugs but as harmless dietary supplements. As of today, clinical trials with synthetic CB2 modulators in arthritis are still limited, nevertheless, in vivo studies offer hope for an effective clinical therapy in the future.
Abbreviations
- 2-AG:
-
2-Arachidonoylglycerol
- AEA:
-
Anandamide
- ADAMTS:
-
Disintegrin and metalloproteinase with thrombospondin motifs
- BCP:
-
β-Caryophyllene
- CB:
-
Cannabinoid receptor
- CIA:
-
Collagen-induced arthritis
- CCL2:
-
Chemokine (C–C motif) ligand 2
- COX-2:
-
Cyclooxygenase-2
- DMARDs:
-
Disease-modifying anti-rheumatic drugs
- ECS:
-
Endocannabinoid system
- FAAH:
-
Fatty acid amide hydrolase
- FLS:
-
Fibroblast-like synoviocytes
- GM-CSF:
-
Granulocyte–macrophage colony-stimulating factor
- GPCR:
-
G protein-coupled receptor
- GR:
-
Glucocorticoid receptor
- IL:
-
Interleukin
- JIA:
-
Juvenile idiopathic arthritis
- MAGL:
-
Monoglycerol lipase
- MMP:
-
Matrix metalloproteinase
- NSAIDs:
-
Nonsteroidal anti-inflammatory drugs
- OA:
-
Osteoarthritis
- OPG:
-
Osteoprotegerin
- PEA:
-
Palmitoylethanolamide
- RA:
-
Rheumatoid arthritis
- RANKL:
-
Receptor activator of nuclear factor κB ligand
- RASFs:
-
Rheumatoid arthritis synovial fibroblasts
- TLR4:
-
Toll-like receptor 4
- TNFα:
-
Tumour necrosis factor α
- TRPV1:
-
Transient receptor potential cation channel subfamily V member 1
References
Kloppenburg M, Berenbaum F. Osteoarthritis year in review 2019: epidemiology and therapy. Osteoarthr Cartil. 2020;28:242–8. https://doi.org/10.1016/j.joca.2020.01.002.
Koonce RC, Bravman JT. Obesity and osteoarthritis: more than just wear and tear. J Am Acad Orthop Surg. 2013;21:161–9. https://doi.org/10.5435/JAAOS-21-03-161.
Nicolau J, Lequerré T, Bacquet H, Vittecoq O. Rheumatoid arthritis, insulin resistance, and diabetes. Jt Bone Spine. 2017;84:411–6. https://doi.org/10.1016/j.jbspin.2016.09.001.
Rehling T, Bjørkman ASD, Andersen MB, Ekholm O, Molsted S. Diabetes is associated with musculoskeletal pain, osteoarthritis, osteoporosis, and rheumatoid arthritis. J Diabetes Res. 2019;2019:1–6.
Roos EM. Joint injury causes knee osteoarthritis in young adults. Curr Opin Rheumatol. 2005;17:195–200. https://doi.org/10.1097/01.bor.0000151406.64393.00.
Anderson DD, Chubinskaya S, Guilak F, Martin JA, Oegema TR, Olson SA, et al. Post-traumatic osteoarthritis: improved understanding and opportunities for early intervention. J Orthop Res. 2011;29:802–9. https://doi.org/10.1002/jor.21359.
Murphy NJ, Eyles JP, Hunter DJ. Hip Osteoarthritis: Etiopathogenesis and Implications for Management. Adv Ther. 2016;33(11):1921-1946. https://doi.org/10.1007/s12325-016-0409-3
Hudson TJ, Edlund MJ, Steffick DE, Tripathi SP, Sullivan MD. Epidemiology of regular prescribed opioid use: results from a National Population-Based Survey. J Pain Symptom Manage. 2008;36:280–8. https://doi.org/10.1016/j.jpainsymman.2007.10.003.
Jafarzadeh SR, Felson DT. Updated estimates suggest a much higher prevalence of arthritis in United States adults than previous ones. Arthritis Rheumatol. 2018;70:185–92. https://doi.org/10.1002/art.40355.
Barbour KE, Helmick CG, Boring M, Brady TJ. Vital signs: prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation—United States, 2013–2015. Morb Mortal Wkly Rep. 2017;66:246–53.
Hootman JM, Helmick CG, Barbour KE, Theis KA, Boring MA. Updated projected prevalence of self-reported doctor-diagnosed arthritis and arthritis-attributable activity limitation among US adults, 2015–2040. Arthritis Rheumatol. 2016;68:1582–7. https://doi.org/10.1002/art.39692.
Köhler BM, Günther J, Kaudewitz D, Lorenz HM. Current therapeutic options in the treatment of rheumatoid arthritis. J Clin Med. 2019;8:938.
Hermann W, Lambova S, Muller-Ladner U. Current treatment options for osteoarthritis. Curr Rheumatol Rev. 2018;14:108–16. https://doi.org/10.2174/1573397113666170829155149.
Anandacoomarasamy A, March L. Current evidence for osteoarthritis treatments. Ther Adv Musculoskelet Dis. 2010;2:17–28. https://doi.org/10.1177/1759720X09359889.
Blake DR, Robson P, Ho M, Jubb RW, McCabe CS. Preliminary assessment of the efficacy, tolerability and safety of a cannabis-based medicine (Sativex) in the treatment of pain caused by rheumatoid arthritis. Rheumatology. 2006;45:50–2. https://doi.org/10.1093/rheumatology/kei183.
Philpott HT, O’Brien M, McDougall JJ. Attenuation of early phase inflammation by cannabidiol prevents pain and nerve damage in rat osteoarthritis. Pain. 2017;158:2442–51. https://doi.org/10.1097/j.pain.0000000000001052.
Takakuwa KM, Hergenrather JY, Shofer FS, Schears RM. The impact of medical cannabis on intermittent and chronic opioid users with back pain: how cannabis diminished prescription opioid usage. Cannabis Cannabinoid Res. 2020;5:263–70. https://doi.org/10.1089/can.2019.0039.
Moreira FA, Grieb M, Lutz B. Central side-effects of therapies based on CB1 cannabinoid receptor agonists and antagonists: focus on anxiety and depression. Best Pract Res Clin Endocrinol Metab. 2009;23:133–44. https://doi.org/10.1016/j.beem.2008.09.003.
Volkow ND, Baler RD, Compton WM, Weiss SRB. Adverse health effects of marijuana use. N Engl J Med. 2014;370:2219–27. https://doi.org/10.1056/nejmra1402309.
Rahn EJ, Thakur GA, Wood JAT, Zvonok AM, Makriyannis A, Hohmann AG. Pharmacological characterization of AM1710, a putative cannabinoid CB 2 agonist from the cannabilactone class: antinociception without central nervous system side-effects. Pharmacol Biochem Behav. 2011;98:493–502. https://doi.org/10.1016/j.pbb.2011.02.024.
Deng L, Guindon J, Cornett BL, Makriyannis A, Mackie K, Hohmann AG. Chronic cannabinoid receptor 2 activation reverses paclitaxel neuropathy without tolerance or cannabinoid receptor 1-dependent withdrawal. Biol Psychiatry. 2015;77:475–87. https://doi.org/10.1016/j.biopsych.2014.04.009.
Calignano A, La Rana G, Piomelli D. Antinociceptive activity of the endogenous fatty acid amide, palmitylethanolamide. Eur J Pharmacol. 2001;419:191–8. https://doi.org/10.1016/S0014-2999(01)00988-8.
Lambert DM, Dipaolo FG, Sonveaux P, Kanyonyo M, Govaerts SJ, Hermans E, et al. Analogues and homologues of N-palmitoylethanolamide, a putative endogenous CB2 cannabinoid, as potential ligands for the cannabinoid receptors. Biochim Biophys Acta - Mol Cell Biol Lipids. 1999;1440:266–74. https://doi.org/10.1016/S1388-1981(99)00132-8.
Malan TP, Ibrahim MM, Lai J, Vanderah TW, Makriyannis A, Porreca F. CB2 cannabinoid receptor agonists: pain relief without psychoactive effects? Curr Opin Pharmacol. 2003;3:62–7. https://doi.org/10.1016/S1471-4892(02)00004-8.
Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997;74:129.
Dunn SL, Wilkinson JM, Crawford A, Bunning RAD, Le Maitre CL. Expression of cannabinoid receptors in human osteoarthritic cartilage: implications for future therapies. Cannabis Cannabinoid Res. 2016;1:3–15. https://doi.org/10.1089/can.2015.0001.
Sophocleous A, Landao-Bassonga E, Van’t Hof RJ, Idris AI, Ralston SH. The type 2 cannabinoid receptor regulates bone mass and ovariectomy-induced bone loss by affecting osteoblast differentiation and bone formation. Endocrinology. 2011;152:2141–9.
Musumeci G, Aiello FC, Szychlinska MA, Di Rosa M, Castrogiovanni P, Mobasheri A. Osteoarthritis in the XXIst century: Risk factors and behaviours that influence disease onset and progression. Int J Mol Sci. 2015;16:6093–112. https://doi.org/10.3390/ijms16036093.
Hunter DJ, Bierma-Zeinstra S. Osteoarthr Lancet. 2019;393:1745–59. https://doi.org/10.1016/S0140-6736(19)30417-9.
Wojdasiewicz P, Poniatowski ŁA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediat Inflamm. 2014;2014:1–19.
Sohn DH, Sokolove J, Sharpe O, Erhart JC, Chandra PE, Lahey LJ, et al. Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via toll-like receptor 4. Arthritis Res Ther. 2012;14:R7. https://doi.org/10.1186/ar3555.
Bondeson J, Wainwright SD, Lauder S, Amos N, Hughes CE. The role of synovial macrophages and macrophage-produced cytokines in driving aggrecanases, matrix metalloproteinases, and other destructive and inflammatory responses in osteoarthritis. Arthritis Res Ther. 2006;8:R187. https://doi.org/10.1186/ar2099.
Scanzello CR. Chemokines and inflammation in osteoarthritis: insights from patients and animal models. J Orthop Res. 2017;35:735–9. https://doi.org/10.1002/jor.23471.
Mabey T, Honsawek S. Cytokines as biochemical markers for knee osteoarthritis. World J Orthop. 2015;6:95–105. https://doi.org/10.5312/wjo.v6.i1.95.
Favero M, Belluzzi E, Trisolino G, Goldring MB, Goldring SR, Cigolotti A, et al. Inflammatory molecules produced by meniscus and synovium in early and end-stage osteoarthritis: a coculture study. J Cell Physiol. 2019;234:11176–87. https://doi.org/10.1002/jcp.27766.
Mathiessen A, Conaghan PG. Synovitis in osteoarthritis: current understanding with therapeutic implications. Arthritis Res Ther. 2017. https://doi.org/10.1186/s13075-017-1229-9.
Hu W, Chen Y, Dou C, Dong S. Microenvironment in subchondral bone: predominant regulator for the treatment of osteoarthritis. Ann Rheum Dis. 2020;80:413–22. https://doi.org/10.1136/annrheumdis-2020-218089.
Steinert AF, Ghivizzani SC, Rethwilm A, Tuan RS, Evans CH, Nöth U. Major biological obstacles for persistent cell-based regeneration of articular cartilage. Arthritis Res Ther. 2007. https://doi.org/10.1186/ar2195.
Kon E, Filardo G, Drobnic M, Madry H, Jelic M, van Dijk N, et al. Non-surgical management of early knee osteoarthritis. Knee Surgery, Sport Traumatol Arthrosc. 2012;20:436–49. https://doi.org/10.1007/s00167-011-1713-8.
Dong R, Wu Y, Xu S, Zhang L, Ying J, Jin H, et al. Is aquatic exercise more effective than land-based exercise for knee osteoarthritis? Medicine (Baltimore). 2018. https://doi.org/10.1097/MD.0000000000013823.
Abbott JH, Robertson MC, Chapple C, Pinto D, Wright AA, de Barra LS, et al. Manual therapy, exercise therapy, or both, in addition to usual care, for osteoarthritis of the hip or knee: a randomized controlled trial.1: clinical effectiveness. Osteoarthr Cartil. 2013;21:525–34.
Juhl C, Christensen R, Roos EM, Zhang W, Lund H. Impact of exercise type and dose on pain and disability in knee osteoarthritis: a systematic review and meta-regression analysis of randomized controlled trials. Arthritis Rheumatol. 2014;66:622–36. https://doi.org/10.1002/art.38290.
Karatosun V, Unver B, Gocen Z, Sen A, Gunal I. Intra-articular hyaluranic acid compared with progressive knee exercises in osteoarthritis of the knee: a prospective randomized trial with long-term follow-up. Rheumatol Int. 2006;26:277–84. https://doi.org/10.1007/s00296-005-0592-z.
Karatosun V, Unver B, Ozden A, Ozay Z, Gunal I. Intra-articular hyaluronic acid compared to exercise therapy in osteoarthritis of the ankle. A prospective randomized trial with long-term follow-up. Clin Exp Rheumatol. 2008;26:288–94.
Petersen SG, Miller BF, Hansen M, Kjaer M, Holm L. Exercise and NSAIDs: effect on muscle protein synthesis in patients with knee osteoarthritis. Med Sci Sports Exerc. 2011;43:425–31. https://doi.org/10.1249/MSS.0b013e3181f27375.
Holsgaard-Larsen A, Christensen R, Clausen B, Søndergaard J, Andriacchi TP, Roos EM. One year effectiveness of neuromuscular exercise compared with instruction in analgesic use on knee function in patients with early knee osteoarthritis: the EXERPHARMA randomized trial. Osteoarthr Cartil. 2018;26:28–33. https://doi.org/10.1016/j.joca.2017.10.015.
Scott DL, Wolfe F, Huizinga TWJ. Rheumatoid arthritis. Lancet. 2010;376:1094–108. https://doi.org/10.1016/S0140-6736(10)60826-4.
Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I Arthritis Rheum. 2008;58:15–25. https://doi.org/10.1002/art.23177.
Sokka T, Toloza S, Cutolo M, Kautiainen H, Makinen H, Gogus F, et al. Women, men, and rheumatoid arthritis: analyses of disease activity, disease characteristics, and treatments in the QUEST-RA Study. Arthritis Res Ther. 2009;11:R7. https://doi.org/10.1186/ar2591.
Crowson CS, Matteson EL, Myasoedova E, Michet CJ, Ernste FC, Warrington KJ, et al. The lifetime risk of adult-onset rheumatoid arthritis and other inflammatory autoimmune rheumatic diseases. Arthritis Rheum. 2011;63:633–9. https://doi.org/10.1002/art.30155.
Wasserman AM. Diagnosis and management of rheumatoid arthritis. Am Fam Physician. 2011;84:1245–52. https://doi.org/10.1016/0304-3959(84)90075-7.
Frisell T, Saevarsdottir S, Askling J. Family history of rheumatoid arthritis: an old concept with new developments. Nat Rev Rheumatol. 2016;12:335–43. https://doi.org/10.1038/nrrheum.2016.52.
Okada Y, Wu D, Trynka G, Raj T, Terao C, Ikari K, et al. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature. 2014;506:376–81. https://doi.org/10.1038/nature12873.
Deane KD, Demoruelle MK, Kelmenson LB, Kuhn KA, Norris JM, Holers VM. Genetic and environmental risk factors for rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2017;31:3–18. https://doi.org/10.1016/j.berh.2017.08.003.
Guerreiro CS, Calado Â, Sousa J, Fonseca JE. Diet, microbiota, and gut permeability-the unknown triad in rheumatoid arthritis. Front Med. 2018;5:349. https://doi.org/10.3389/fmed.2018.00349.
Konig MF, Abusleme L, Reinholdt J, Palmer RJ, Teles RP, Sampson K, et al. Aggregatibacter actinomycetemcomitans-induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis. Sci Transl Med. 2016. https://doi.org/10.1126/scitranslmed.aaj1921.
Mathews CJ, Weston VC, Jones A, Field M, Coakley G. Bacterial septic arthritis in adults. Lancet. 2010;375:846–55. https://doi.org/10.1016/S0140-6736(09)61595-6.
Hassan AS, Rao A, Manadan AM, Block JA. Peripheral bacterial septic arthritis: review of diagnosis and management. J Clin Rheumatol. 2017;23:435–42. https://doi.org/10.1097/RHU.0000000000000588.
Mínguez S, Molinos S, Mateo L, Gimenez M, Mateu L, Cabello J, et al. Septic arthritis due to methylcyllin-resistant Staphylococcus aureus in adults. Reumatol Clínica. 2015;11:381–6.
Noack M, Miossec P. Selected cytokine pathways in rheumatoid arthritis. Semin Immunopathol. 2017;39:365–83. https://doi.org/10.1007/s00281-017-0619-z.
Ridgley LA, Anderson AE, Pratt AG. What are the dominant cytokines in early rheumatoid arthritis? Curr Opin Rheumatol. 2018;30:207–14. https://doi.org/10.1097/BOR.0000000000000470.
Azizieh FY, Al Jarallah K, Shehab D, Gupta R, Dingle K, Raghupathy R. Patterns of circulatory and peripheral blood mononuclear cytokines in rheumatoid arthritis. Rheumatol Int. 2017;37:1727–34. https://doi.org/10.1007/s00296-017-3774-6.
Nell VPK, Machold KP, Eberl G, Stamm TA, Uffmann M, Smolen JS. Benefit of very early referral and very early therapy with disease-modifying anti-rheumatic drugs in patients with early rheumatoid arthritis. Rheumatology. 2004;43:906–14. https://doi.org/10.1093/rheumatology/keh199.
Zhang A, Lee YC. Mechanisms for joint pain in rheumatoid arthritis (RA): from cytokines to central sensitization. Curr Osteoporos Rep. 2018;16:603–10. https://doi.org/10.1007/s11914-018-0473-5.
Basavarajappa BS, Shivakumar M, Joshi V, Subbanna S. Endocannabinoid system in neurodegenerative disorders. J Neurochem. 2017;142:624–48. https://doi.org/10.1111/jnc.14098.
Cristino L, Bisogno T, Di Marzo V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat Rev Neurol. 2020;16:9–29. https://doi.org/10.1038/s41582-019-0284-z.
Morena M, Patel S, Bains JS, Hill MN. Neurobiological interactions between stress and the endocannabinoid system. Neuropsychopharmacology. 2016;41:80–102. https://doi.org/10.1038/npp.2015.166.
Woodhams SG, Sagar DR, Burston JJ, Chapman V. The role of the endocannabinoid system in pain. Handb Exp Pharmacol. 2015;227:119–43. https://doi.org/10.1007/978-3-662-46450-2_7.
Cabral GA, Rogers TJ, Lichtman AH. Turning over a new leaf: cannabinoid and endocannabinoid modulation of immune function. J Neuroimmune Pharmacol. 2015;10:193–203. https://doi.org/10.1007/s11481-015-9615-z.
Chevalier G, Siopi E, Guenin-Macé L, Pascal M, Laval T, Rifflet A, et al. Effect of gut microbiota on depressive-like behaviors in mice is mediated by the endocannabinoid system. Nat Commun. 2020;11:6363. https://doi.org/10.1038/s41467-020-19931-2.
Di Marzo V, Bisogno T, De Petrocellis L. The biosynthesis, fate and pharmacological properties of endocannabinoids. In: Pertwee RG, editor. Cannabinoids. Berlin Heidelberg: Springer; 2005. p. 147–85.
Richardson D, Pearson RG, Kurian N, Latif ML, Garle MJ, Barrett DA, et al. Characterisation of the cannabinoid receptor system in synovial tissue and fluid in patients with osteoarthritis and rheumatoid arthritis. Arthritis Res Ther. 2008. https://doi.org/10.1186/ar2401.
Vaughan CW, Christie MJ. Retrograde signalling by endocannabinoids. In: Pertwee RG, editor. Cannabinoids. Berlin Heidelberg: Springer; 2005. p. 367–83.
Kienzl M, Kargl J, Schicho R. The immune endocannabinoid system of the tumor microenvironment. Int J Mol Sci. 2020;21:8929. https://doi.org/10.3390/ijms21238929.
Turcotte C, Chouinard F, Lefebvre JS, Flamand N. Regulation of inflammation by cannabinoids, the endocannabinoids 2-arachidonoyl-glycerol and arachidonoyl-ethanolamide, and their metabolites. J Leukoc Biol. 2015;97:1049–70. https://doi.org/10.1189/jlb.3ru0115-021r.
Duncan M, Davison JS, Sharkey KA. Review article: endocannabinoids and their receptors in the enteric nervous system. Aliment Pharmacol Ther. 2005;22:667–83. https://doi.org/10.1111/j.1365-2036.2005.02648.x.
Kulkarni-Narla A, Brown DR. Localization of CB1-cannabinoid receptor immunoreactivity in the porcine enteric nervous system. Cell Tissue Res. 2000;302:73–80. https://doi.org/10.1007/s004410000261.
Kress M, Kuner R. Mode of action of cannabinoids on nociceptive nerve endings. Exp Brain Res. 2009;196:79–88. https://doi.org/10.1007/s00221-009-1762-0.
Bisogno T. Endogenous cannabinoids: structure and metabolism. J Neuroendocrinol. 2008;20(Suppl 1):1–9. https://doi.org/10.1111/j.1365-2826.2008.01676.x.
Baggelaar MP, Maccarrone M, van der Stelt M. 2-Arachidonoylglycerol: a signaling lipid with manifold actions in the brain. Prog Lipid Res. 2018;71:1–17. https://doi.org/10.1016/j.plipres.2018.05.002.
Guindon J, De Léan A, Beaulieu P. Local interactions between anandamide, an endocannabinoid, and ibuprofen, a nonsteroidal anti-inflammatory drug, in acute and inflammatory pain. Pain. 2006;121:85–93. https://doi.org/10.1016/j.pain.2005.12.007.
Malek N, Starowicz K. Dual-acting compounds targeting endocannabinoid and endovanilloid systems-a novel treatment option for chronic pain management. Front Pharmacol. 2016;7:257. https://doi.org/10.3389/fphar.2016.00257.
Malek N, Mrugala M, Makuch W, Kolosowska N, Przewlocka B, Binkowski M, et al. A multi-target approach for pain treatment: dual inhibition of fatty acid amide hydrolase and TRPV1 in a rat model of osteoarthritis. Pain. 2015;156:890–903. https://doi.org/10.1097/j.pain.0000000000000132.
Hanna VS, Hafez EAA. Synopsis of arachidonic acid metabolism: a review. J Adv Res. 2018;11:23–32. https://doi.org/10.1016/j.jare.2018.03.005.
Izzo AA, Deutsch DG. Unique pathway for anandamide synthesis and liver regeneration. Proc Natl Acad Sci USA. 2011;108:6339–40. https://doi.org/10.1073/pnas.1103566108.
Mukhopadhyay B, Cinar R, Yin S, Liu J, Tam J, Godlewski G, et al. Hyperactivation of anandamide synthesis and regulation of cell-cycle progression via cannabinoid type 1 (CB1) receptors in the regenerating liver. Proc Natl Acad Sci USA. 2011;108:6323–8. https://doi.org/10.1073/pnas.1017689108.
Piscitelli F, Di Marzo V. “Redundancy” of endocannabinoid inactivation: new challenges and opportunities for pain control. ACS Chem Neurosci. 2012;3:356–63. https://doi.org/10.1021/cn300015x.
Di Marzo V, De Petrocellis L. Why do cannabinoid receptors have more than one endogenous ligand? Philos Trans R Soc B Biol Sci. 2012;367:3216–28. https://doi.org/10.1098/rstb.2011.0382.
Fernández-Ruiz J, Romero J, Velasco G, Tolón RM, Ramos JA, Guzmán M. Cannabinoid CB2 receptor: a new target for controlling neural cell survival? Trends Pharmacol Sci. 2007;28:39–45. https://doi.org/10.1016/j.tips.2006.11.001.
Ye L, Cao Z, Wang W, Zhou N. New insights in cannabinoid receptor structure and signaling. Curr Mol Pharmacol. 2019;12:239–48. https://doi.org/10.2174/1874467212666190215112036.
Morales P, Reggio PH. An update on non-CB 1, non-CB 2 cannabinoid related G-protein-coupled receptors. Cannabis Cannabinoid Res. 2017;2:265–73. https://doi.org/10.1089/can.2017.0036.
Muller C, Morales P, Reggio PH. Cannabinoid ligands targeting TRP channels. Front Mol Neurosci. 2019;11:487. https://doi.org/10.3389/fnmol.2018.00487.
Pertwee RG. Pharmacological actions of cannabinoids. In: Pertwee RG, editor. Cannabinoids. Berlin Heidelberg: Springer; 2005. p. 1–51.
Onaivi ES, Ishiguro H, Gong JP, Patel S, Meozzi PA, Myers L, et al. Functional expression of brain neuronal CB2 cannabinoid receptors are involved in the effects of drugs of abuse and in depression. Ann NY Acad Sci. 2008;1139:434–49. https://doi.org/10.1196/annals.1432.036.
Ashton JC, Friberg D, Darlington CL, Smith PF. Expression of the cannabinoid CB2 receptor in the rat cerebellum: an immunohistochemical study. Neurosci Lett. 2006;396:113–6. https://doi.org/10.1016/j.neulet.2005.11.038.
Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–32.
Núñez E, Benito C, Pazos MR, Barbachano A, Fajardo O, González S, et al. Cannabinoid CB2 receptors are expressed by perivascular microglial cells in the human brain: an Immunohistochemical Study. Synapse. 2004;53:208–13. https://doi.org/10.1002/syn.20050.
Burston JJ, Sagar DR, Shao P, Bai M, King E, Brailsford L, et al. Cannabinoid CB2 receptors regulate central sensitization and pain responses associated with osteoarthritis of the knee joint. PLoS ONE. 2013;8:e80440. https://doi.org/10.1371/journal.pone.0080440.
Aso E, Ferrer I. CB2 cannabinoid receptor as potential target against Alzheimer’s disease. Front Neurosci. 2016;10:243. https://doi.org/10.3389/fnins.2016.00243.
Li C, Shi J, Wang B, Li J, Jia H. CB2 cannabinoid receptor agonist ameliorates novel object recognition but not spatial memory in transgenic APP/PS1 mice. Neurosci Lett. 2019;707:134286. https://doi.org/10.1016/j.neulet.2019.134286.
Ishiguro H, Horiuchi Y, Tabata K, Liu QR, Arinami T, Onaivi ES. Cannabinoid CB2 receptor gene and environmental interaction in the development of psychiatric disorders. Molecules. 2018;23:1863. https://doi.org/10.3390/molecules23081836.
Bahi A, Al Mansouri S, Al Memari E, Al Ameri M, Nurulain SM, Ojha S. β-Caryophyllene, a CB2 receptor agonist produces multiple behavioral changes relevant to anxiety and depression in mice. Physiol Behav. 2014;135:119–24. https://doi.org/10.1016/j.physbeh.2014.06.003.
Rentsch P, Stayte S, Egan T, Clark I, Vissel B. Targeting the cannabinoid receptor CB2 in a mouse model of l-dopa induced dyskinesia. Neurobiol Dis. 2020;134:104646. https://doi.org/10.1016/j.nbd.2019.104646.
Nasehi M, Hajikhani M, Ebrahimi-Ghiri M, Zarrindast MR. Interaction between NMDA and CB2 function in the dorsal hippocampus on memory consolidation impairment: an isobologram analysis. Psychopharmacology. 2017;234:507–14. https://doi.org/10.1007/s00213-016-4481-9.
Li Y, Kim J. CB2 cannabinoid receptor knockout in mice impairs contextual long-term memory and enhances spatial working memory. Neural Plast. 2016;2016:9817089. https://doi.org/10.1155/2016/9817089.
Pajak A, Kostrzewa M, Malek N, Korostynski M, Starowicz K. Expression of matrix metalloproteinases and components of the endocannabinoid system in the knee joint are associated with biphasic pain progression in a rat model of osteoarthritis. J Pain Res. 2017;10:1973–89. https://doi.org/10.2147/JPR.S132682.
Ismail M, Khawaja G. Study of cannabinoid receptor 2 Q63R gene polymorphism in Lebanese patients with rheumatoid arthritis. Clin Rheumatol. 2018;37:2933–8. https://doi.org/10.1007/s10067-018-4217-9.
Bellini G, Olivieri AN, Grandone A, Alessio M, Gicchino MF, Nobili B, et al. Association between cannabinoid receptor type 2 Q63R variant and oligo/polyarticular juvenile idiopathic arthritis. Scand J Rheumatol. 2015;44:284–7. https://doi.org/10.3109/03009742.2015.1020863.
Sophocleous A, Börjesson AE, Salter DM, Ralston SH. The type 2 cannabinoid receptor regulates susceptibility to osteoarthritis in mice. Osteoarthr Cartil. 2015;23:1586–94. https://doi.org/10.1016/j.joca.2015.04.020.
Fechtner S, Singh AK, Ahmed S. Role of cannabinoid receptor 2 in mediating interleukin-1β-induced inflammation in rheumatoid arthritis synovial fibroblasts. Clin Exp Rheumatol. 2019;37:1026–35.
Mugnaini C, Kostrzewa M, Bryk M, Mahmoud AM, Brizzi A, Lamponi S, et al. Design, synthesis, and physicochemical and pharmacological profiling of 7-hydroxy-5-oxopyrazolo[4,3- b ]pyridine-6-carboxamide derivatives with antiosteoarthritic activity in vivo. J Med Chem. 2020;63:7369–91. https://doi.org/10.1021/acs.jmedchem.0c00595.
Irrera N, D’ascola A, Pallio G, Bitto A, Mazzon E, Mannino F, et al. β-Caryophyllene mitigates collagen antibody induced arthritis (CAIA) in mice through a cross-talk between CB2 and PPAR-γ receptors. Biomolecules. 2019;9:326.
Malek N, Kucharczyk M, Starowicz K. Alterations in the anandamide metabolism in the development of neuropathic pain. Biomed Res Int. 2014;2014:686908. https://doi.org/10.1155/2014/686908.
Bryk M, Chwastek J, Kostrzewa M, Mlost J, Pędracka A, Starowicz K. Alterations in anandamide synthesis and degradation during osteoarthritis progression in an animal model. Int J Mol Sci. 2020;21:1–19. https://doi.org/10.3390/ijms21197381.
Valastro C, Campanile D, Marinaro M, Franchini D, Piscitelli F, Verde R, et al. Characterization of endocannabinoids and related acylethanolamides in the synovial fluid of dogs with osteoarthritis: a pilot study. BMC Vet Res. 2017;13:309. https://doi.org/10.1186/s12917-017-1245-7.
Haudenschild DR, Carlson AK, Zignego DL, Yik JHN, Hilmer JK, June RK. Inhibition of early response genes prevents changes in global joint metabolomic profiles in mouse post-traumatic osteoarthritis. Osteoarthr Cartil. 2019;27:504–12. https://doi.org/10.1016/j.joca.2018.11.006.
La Porta C, Andreea Bura S, Llorente-Onaindia J, Pastor A, Navarrete F, García-Gutierrez MS, et al. Role of the endocannabinoid system in the emotional manifestations of osteoarthritis pain. Pain. 2015;156:2001–12. https://doi.org/10.1097/j.pain.0000000000000260.
Nicholson J, Azim S, Rebecchi MJ, Galbavy W, Feng T, Reinsel R, et al. Leptin levels are negatively correlated with 2-arachidonoylglycerol in the cerebrospinal fluid of patients with osteoarthritis. PLoS ONE. 2015;10:e0123132. https://doi.org/10.1371/journal.pone.0123132.
Petrovszki Z, Kovacs G, Tömböly C, Benedek G, Horvath G. The effects of peptide and lipid endocannabinoids on arthritic pain at the spinal level. Anesth Analg. 2012;114:1346–52. https://doi.org/10.1213/ANE.0b013e31824c4eeb.
Mbvundula EC, Bunning RAD, Rainsford KD. Effects of cannabinoids on nitric oxide production by chondrocytes and proteoglycan degradation in cartilage. Biochem Pharmacol. 2005;69:635–40. https://doi.org/10.1016/j.bcp.2004.11.018.
Kinsey SG, Naidu PS, Cravatt BF, Dudley DT, Lichtman AH. Fatty acid amide hydrolase blockade attenuates the development of collagen-induced arthritis and related thermal hyperalgesia in mice. Pharmacol Biochem Behav. 2011;99:718–25. https://doi.org/10.1016/j.pbb.2011.06.022.
Sasso O, Bertorelli R, Bandiera T, Scarpelli R, Colombano G, Armirotti A, et al. Peripheral FAAH inhibition causes profound antinociception and protects against indomethacin-induced gastric lesions. Pharmacol Res. 2012;65:553–63. https://doi.org/10.1016/j.phrs.2012.02.012.
Philpott HT, McDougall JJ. Combatting joint pain and inflammation by dual inhibition of monoacylglycerol lipase and cyclooxygenase-2 in a rat model of osteoarthritis. Arthritis Res Ther. 2020;22:9. https://doi.org/10.1186/s13075-020-2096-3.
Pertwee RG, Howlett AC, Abood ME, Alexander SPH, Di Marzo V, Elphick MR, et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev. 2010;62:588–631.
Soethoudt M, Grether U, Fingerle J, Grim TW, Fezza F, De Petrocellis L, et al. Cannabinoid CB2 receptor ligand profiling reveals biased signalling and off-target activity. Nat Commun. 2017;8:13958. https://doi.org/10.1038/ncomms13958.
Lauckner JE, Jensen JB, Chen HY, Lu HC, Hille B, Mackie K. GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc Natl Acad Sci USA. 2008;105:2699–704. https://doi.org/10.1073/pnas.0711278105.
Ryberg E, Larsson N, Sjögren S, Hjorth S, Hermansson N-O, Leonova J, et al. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. 2007;152:1092–101. https://doi.org/10.1038/sj.bjp.0707460.
Montecucco F, Burger F, Mach F, Steffens S. CB2 cannabinoid receptor agonist JWH-015 modulates human monocyte migration through defined intracellular signaling pathways. Am J Physiol - Hear Circ Physiol. 2008;294:H1145–55. https://doi.org/10.1152/ajpheart.01328.2007.
Fechtner S, Singh AK, Srivastava I, Szlenk CT, Muench TR, Natesan S, et al. Cannabinoid receptor 2 agonist JWH-015 inhibits interleukin-1βinduced inflammation in rheumatoid arthritis synovial fibroblasts and in adjuvant induced arthritis rat via glucocorticoid receptor. Front Immunol. 2019;10:1027. https://doi.org/10.3389/fimmu.2019.01027.
Henriquez JE, Crawford RB, Kaminski NE. Suppression of CpG-ODN-mediated IFNα and TNFα response in human plasmacytoid dendritic cells (pDC) by cannabinoid receptor 2 (CB2)-specific agonists. Toxicol Appl Pharmacol. 2019;369:82–9. https://doi.org/10.1016/j.taap.2019.02.013.
Bai J, Ge G, Wang Y, Zhang W, Wang Q, Wang W, et al. A selective CB2 agonist protects against the inflammatory response and joint destruction in collagen-induced arthritis mice. Biomed Pharmacother. 2019;116:109025. https://doi.org/10.1016/j.biopha.2019.109025.
Gui H, Liu X, Liu LR, Su DF, Dai SM. Activation of cannabinoid receptor 2 attenuates synovitis and joint distruction in collagen-induced arthritis. Immunobiology. 2015;220:817–22. https://doi.org/10.1016/j.imbio.2014.12.012.
Lowin T, Pongratz G, Straub RH. The synthetic cannabinoid WIN55,212-2 mesylate decreases the production of inflammatory mediators in rheumatoid arthritis synovial fibroblasts by activating CB2, TRPV1, TRPA1 and yet unidentified receptor targets. J Inflamm (United Kingdom). 2016;13:15. https://doi.org/10.1186/s12950-016-0114-7.
Schuelert N, Zhang C, Mogg AJ, Broad LM, Hepburn DL, Nisenbaum ES, et al. Paradoxical effects of the cannabinoid CB2 receptor agonist GW405833 on rat osteoarthritic knee joint pain. Osteoarthr Cartil. 2010;18:1536–43. https://doi.org/10.1016/j.joca.2010.09.005.
Zhu M, Yu B, Bai J, Wang X, Guo X, Liu Y, et al. Cannabinoid receptor 2 agonist prevents local and systemic inflammatory bone destruction in rheumatoid arthritis. J Bone Miner Res. 2019;34:739–51. https://doi.org/10.1002/jbmr.3637.
Fukuda S, Kohsaka H, Takayasu A, Yokoyama W, Miyabe C, Miyabe Y, et al. Cannabinoid receptor 2 as a potential therapeutic target in rheumatoid arthritis. BMC Musculoskelet Disord. 2014;15:275. https://doi.org/10.1186/1471-2474-15-275.
McDougall JJ, Yu V, Thomson J. In vivo effects of CB 2 receptor-selective cannabinoids on the vasculature of normal and arthritic rat knee joints. Br J Pharmacol. 2008;153:358–66. https://doi.org/10.1038/sj.bjp.0707565.
Idris AI, Sophocleous A, Landao-Bassonga E, Van’t Hof RJ, Ralston SH. Regulation of bone mass, osteoclast function, and ovariectomy-induced bone loss by the type 2 cannabinoid receptor. Endocrinology. 2008;149:5619–26.
Geusens P. The role of RANK ligand/osteoprotegerin in rheumatoid arthritis. Ther Adv Musculoskelet Dis. 2012;4:225–33. https://doi.org/10.1177/1759720X12438080.
Remuzgo-Martínez S, Genre F, López-Mejiás R, Ubilla B, Mijares V, Pina T, et al. Expression of osteoprotegerin and its ligands, RANKL and TRAIL, in rheumatoid arthritis. Sci Rep. 2016;6:29713. https://doi.org/10.1038/srep29713.
Gui H, Liu X, Wang ZW, He DY, Su DF, Dai SM. Expression of cannabinoid receptor 2 and its inhibitory effects on synovial fibroblasts in rheumatoid arthritis. Rheumatol (United Kingdom). 2014;53:802–9. https://doi.org/10.1093/rheumatology/ket447.
Kong Y, Wang W, Zhang C, Wu Y, Liu Y, Zhou X. Cannabinoid WIN-55,212-2 mesylate inhibits ADAMTS-4 activity in human osteoarthritic articular chondrocytes by inhibiting expression of syndecan-1. Mol Med Rep. 2016;13:4569–76. https://doi.org/10.3892/mmr.2016.5137.
Dunn SL, Wilkinson JM, Crawford A, Le Maitre CL, Bunning RAD. Cannabinoid WIN-55,212-2 mesylate inhibits interleukin-1β induced matrix metalloproteinase and tissue inhibitor of matrix metalloproteinase expression in human chondrocytes. Osteoarthr Cartil. 2014;22:133–44. https://doi.org/10.1016/j.joca.2013.10.016.
Mbvundula EC, Bunning RAD, Rainsford KD. Arthritis and cannabinoids: HU-210 and Win-55,212-2 prevent IL-1 α-induced matrix degradation in bovine articular chondrocytes in-vitro. J Pharm Pharmacol. 2006;58:351–8. https://doi.org/10.1211/jpp.58.3.0009.
Selvi E, Lorenzini S, Garcia-Gonzalez E, Maggio R, Lazzerini PE, Capecchi PL, et al. Inhibitory effect of synthetic cannabinoids on cytokine production in rheumatoid fibroblast-like synoviocytes. Clin Exp Rheumatol. 2008;26:574–81.
Idris AI, Vant Hof RJ, Greig IR, Ridge SA, Baker D, Ross RA, et al. Regulation of bone mass, bone loss and osteoclast activity by cannabinoid receptors. Nat Med. 2005;11:774–9.
Yao BB, Hsieh GC, Frost JM, Fan Y, Garrison TR, Daza AV, et al. In vitro and in vivo characterization of A-796260: a selective cannabinoid CB2 receptor agonist exhibiting analgesic activity in rodent pain models. Br J Pharmacol. 2008;153:390–401. https://doi.org/10.1038/sj.bjp.0707568.
Krustev E, Reid A, McDougall JJ. Tapping into the endocannabinoid system to ameliorate acute inflammatory flares and associated pain in mouse knee joints. Arthritis Res Ther. 2014;16:437. https://doi.org/10.1186/s13075-014-0437-9.
Schuelert N, Johnson MP, Oskins JL, Jassal K, Chambers MG, McDougall JJ. Local application of the endocannabinoid hydrolysis inhibitor URB597 reduces nociception in spontaneous and chemically induced models of osteoarthritis. Pain. 2011;152:975–81. https://doi.org/10.1016/j.pain.2010.11.025.
Burston JJ, Mapp PI, Sarmad S, Barrett DA, Niphakis MJ, Cravatt BF, et al. Robust anti-nociceptive effects of monoacylglycerol lipase inhibition in a model of osteoarthritis pain. Br J Pharmacol. 2016;173:3134–44.
Wiese B, Wilson-Poe AR. Emerging evidence for cannabis’ role in opioid use disorder. Cannabis Cannabinoid Res. 2018;3:179–89. https://doi.org/10.1089/can.2018.0022.
Grenald SA, Young MA, Wang Y, Ossipov MH, Ibrahim MM, Largent-Milnes TM, et al. Synergistic attenuation of chronic pain using mu opioid and cannabinoid receptor 2 agonists. Neuropharmacology. 2017;116:59–70. https://doi.org/10.1016/j.neuropharm.2016.12.008.
La Porta C, Bura SA, Aracil-Fernández A, Manzanares J, Maldonado R. Role of CB1 and CB2 cannabinoid receptors in the development of joint pain induced by monosodium iodoacetate. Pain. 2013;154:160–74. https://doi.org/10.1016/j.pain.2012.10.009.
Lowin T, Tingting R, Zurmahr J, Classen T, Schneider M, Pongratz G. Cannabidiol (CBD): a killer for inflammatory rheumatoid arthritis synovial fibroblasts. Cell Death Dis. 2020;11:714.
Malfait AM, Gallily R, Sumariwalla PF, Malik AS, Andreakos E, Mechoulam R, et al. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc Natl Acad Sci USA. 2000;97:9561–6. https://doi.org/10.1073/pnas.160105897.
Funding
This work was supported by the National Science Centre of Poland (grant number OPUS7 UMO-2014/13/B/NZ7/02311) and statutory funds of the Maj Institute of Pharmacology, Polish Academy of Sciences.
Author information
Authors and Affiliations
Contributions
Conception or design of the work, acquisition, analysis, interpretation of data, drafting the work and revising it critically for important intellectual content: MB and KS; final approval of the version to be published: KS. Authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Bryk, M., Starowicz, K. Cannabinoid-based therapy as a future for joint degeneration. Focus on the role of CB2 receptor in the arthritis progression and pain: an updated review. Pharmacol. Rep 73, 681–699 (2021). https://doi.org/10.1007/s43440-021-00270-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-021-00270-y