Abstract
The scientific interest in volatile fatty acids (VFAs) as an energy source and chemical precursor in ruminant diets has been longstanding, as it has significant implications for animal physiology and well-being. The present study explores the substitution of volatile fatty acids (VFAs) derived from agro-food residues via acidogenic fermentation as an alternative energy source in ruminant feed. Utilizing the gas production method, rumen digestibility assays were conducted, wherein the recovered VFA effluent from the acidogenic fermentation of apple pomace and potato protein liquor was substituted for 10%, 20%, and 30% of the total mixed ration (TMR) energy. Various parameters such as gas, VFA yield and composition, VFA peak intervals, changes in pH, and ammonium nitrogen content were investigated. Based on the results obtained, provision of 20% and 30% of the energy with VFAs did not increase methane production or did not cause significant pH alternations. Nevertheless, such supplementation resulted in increased production and accumulation of VFAs in the rumen media. The bioconversion of agro-food side streams into VFAs opens a new path in sustainable nutrient recovery and feed production from low value agro-industrial residues.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The synthesis, metabolism, and effects of volatile fatty acids (VFAs) on ruminants’ health and products have been extensively studied over the past decades [1]. In addition to serving as a primary energy source for ruminants, VFAs also have diverse effects on gastrointestinal development, milk yield and composition, dry matter intake, body weight gain, rumen microbiota, insulin and glucagon regulation, intramuscular fat, and adipose tissue yield [1]. VFAs could be administered as feed supplement by ruminal infusion [2], oral supplementation [3] or added directly to the total mixed ration (TMR) [4].
The use of TMR as a feed strategy enhances cow productivity, diminishes methane emissions, enhances feed intake and digestibility, and sustains optimal rumen health and pH by minimizing the number of individual feed choices [5, 6]. Cattle's energy requirements are influenced by age, sex, body size, physiological state, and environment. Energy-dense feeds are crucial for optimal performance in high-performance dairy cows. Energy feeds such as corn can be substituted with forage, but may increase feed costs and decrease forage intake [7]. Fibrous agro-food byproducts can be utilized without impacting feed intake or digestibility to a certain degree. However, it is important to take into account the local availability, nutrient content, and animal productivity when incorporating these feedstuffs into TMR [8]. Yet, the local availability, nutrient content, animal productivity of adding these feedstuffs to TMR must be considered. In this view, VFAs, with their similar digestible energy to metabolizable energy, lack ruminal fermentation, do not incorporate in urine as inorganic nitrogen compounds, making them a suitable energy supplement and building block for lipids and glucose synthesis [9].
Studies on VFA supplementation in ruminants often use synthetic VFAs derived from fossil-based sources and presented in the form of sodium, calcium, or potassium salts [10], which are unsustainable and have a significant environmental impact [11]. There is a unique opportunity for agro-food side streams and residues to meet the demand for VFAs with minimal carbon footprint through anaerobic digestion. Acidogenic fermentation of VFAs is built upon the anaerobic digestion approach of handling organic waste where methane production is inhibited. The particle -and microorganism- free VFA effluent obtained from acidogenic fermentation of feed-grade agricultural residues have great potential to replace synthetic VFAs. To the authors’ knowledge, no study has utilized the residue derived VFAs produced in processes such as acidogenic fermentation in ruminant diets.
However, the supplementation of a VFAs mixture in TMR may present certain challenges. VFAs serve as precursors for methane production during anaerobic digestion. Therefore, supplementing TMR with a VFAs mixture could potentially increase both the rate and volume of methane production during ruminal fermentation. Moreover, it is important to note that VFAs are carboxylic acids, and their accumulation in the rumen can lead to a decrease in pH, disturbing the buffering capacity of rumen fluid (RF). Another challenge associated with the supplementation of VFAs is the potential alteration in VFAs production and acid distribution in rumen.
The combination of TMR with varying levels of VFAs mixture may influence the production rate, peak value, and distribution of individual acids during ruminal fermentation. Novel alternative feeds such as VFAs solution should focus on ruminant animals' quantitative digestion and metabolism to modulate their response in feed, based on rumen degradability, and feed energy content changes [12]. Biological data from in vivo, in situ, and in vitro methods is needed to quantify digestive and metabolic processes. In vitro digestive models mimic in vivo digestion and manipulate animal state parameters. They can research animal responses to a single factor without additional variables affecting the primary impact. The Hohenheim gas test, also known as Menke's method, uses the correlation between rumen fermentation and gas generation to investigate fermentation kinetics on a single sample or multiple samples simultaneously [13]. This approach is effective for evaluating the energy value of agro-industrial byproducts [14] and complex feeds [15]. However, sample size, donor animal-affected inoculum source, pH, temperature, shaking, and incubation medium composition and buffering capability may affect the Hohenheim gas test [13].
This study aimed to investigate the challenges and potential opportunities associated with supplementing agro-feed residues derived VFAs as energy carriers in ruminant feed. Specifically, the study focused on examining the impact of VFAs on ruminal feed digestibility, gas production, and the composition of ruminal microbiota. To achieve this, in vitro trials were conducted using the Hohenheim gas test, which is a widely used technique for estimating VFAs and gas production in the rumen. Besides, the same amount of VFAs solution was added to the rumen media mixture at different intervals to investigate the effect of rumen fluid age effect on ruminal fermentation performance.
Materials and methods
Characterization and preparation of feed and VFAs mixture
The effluent of VFAs utilized in this study was sourced from the acidogenic fermentation process involving a combination of apple pomace and potato protein liquor as substrate. Rumen fluid was employed as the inoculum within a bioreactor. Apple pomace (AP) was provided by the Herrljunga cider AB (Herrljunga, Sweden). Potato protein liquid (PPL) was provided by Lyckeby (Kristianstad, Sweden). The Swedish University of Agricultural Sciences (SLU) supplied rumen fluid obtained from fistulated Swedish red cows (Lövsta, Sweden). Rumen fluid collection was carried out in accordance with the ethical approval obtained from Uppsala animal experiment ethics board, Sweden (diary no 5.8.8–11,182/2019). The reactor was outfitted with a peristaltic permeation pump, a temperature and pH probe, a programmable logic controller (PLC) unit, a pressure sensor, a flow meter, and a submerged flat sheet membrane panel that has a built-in gas sparger. The membrane bioreactor (MBR) utilized was a 4-L bioreactor with a working volume of 3.5 L. The immersed membrane bioreactor (iMBR) was supplied with a mixture of AP and PPL in a ratio of 1:1 based on the weight of volatile solids (VS). It was then inoculated with 300 mL of rumen fluid and filled to its operational volume with water. The bioreactor temperature was set at 39 °C. There was a daily pH adjustment to 6.5 after daily feeding. This research work was conducted using an Integrated Permeate Channel (IPC) flat sheet membrane designed and kindly supplied by the Flemish Institute for Technological Research (VITO NV) (Mol, Belgium). The effluent containing VFAs, devoid of particles and microorganisms, was effectively separated using a membrane that was integrated within the reactor [16]. Therefore, applying the VFAs mixture in the feed to pass through the rumen would not contradict the normal digestion process in the rumen. The specification of the VFAs bearing effluent is given in Table 1.
The experiment was designed within an isoenergetic scope. Therefore, a proportion of the total energy derived from dairy cattle feed was substituted with a specific volume of VFAs mixture with equivalent energy content. TMR of dairy cattle that was kindly provided by Husshållningssälskapet Sjuhärad (Länghem, Sweden) was dried and ground to pass a 2 mm screen. TMR consisted of corn silage, wheat, barley, and protein concentrate (rapeseed meal). The chemical compositions and energy content of the feed and VFAs mixture are presented in Table 1. The molar mass of acetate, propionate, and butyrate are 60.05 g/mol, 74.08 g/mol, and 88.11 g/mol, respectively. According to literature, each gram of acetate, propionate, and butyrate can provide 14.65, 20.93, 25.12 kJ of energy respectively [17].
In vitro experiment design
The total energy content of the VFA solution has been estimated by considering the distribution and concentration of each acid and their respective calorific values. However, the potential energy derived from other soluble constituents has not been considered due to their insignificant quantities. The experiment was designed in three treatments to replace 10%, 20%, and 30% of feed gross energy with a VFAs solution. About 400 mg TMR was used as a substrate in each treatment and based on the level of replaced energy, different amount TMR were replaced with different volume of VFAs mixture. Approximately 10% of the energy content derived from a 400 mg of TMR was estimated to be roughly 750.4 J. In order to substitute this amount of energy, it is necessary to substitute 40 mg of TMR with 1.99 mL of VFAs solution. Hence, this treatment is denoted as TMR1 + 10%VFAs. As a result, to substitute 20% and 30% of the feed samples energy, it is necessary to replace 80 and 120 mg of TMR with 3.98 and 5.97 mL of VFAs mixture, respectively. Additionally, the condition pertaining to the replacement of 20% energy of TMR is referred to as TMR2 + 20%VFAs, while the substitution of 30% energy of the TMR is denoted as TMR3 + 30%VFAs. Groups TMR1, TMR2, and TMR3 serve as controls for treatment groups in which VFAs solution is substituted for 10, 20, and 30% of TMR's energy, respectively. It is important to note that the control groups did not exhibit any distinct variations in their TMR formulation. However, each treatment group had its own respective control group and the only difference in TMR control groups (TMR1, TMR2, TMR3) was the initial pH level which was set according to the relevant treatment group. For studying the effect of time on the performance of rumen fluid in digestibility essays, the rumen fluid was sparged by CO2 and sealed with a one-way valve to keep it in an anaerobic condition, then placed in an oven set at 39 ℃. The rumen fluid used in the experiment had been stored for 1 day prior to the start of the study.
The 120 mL serum bottles were used as fermentation vessels containing 20 mL of rumen fluid and 40 mL of the medium mixture. The medium mixture was prepared based on the gas production method protocol, consisting of (added in order) 400 mL H2O, 0.1 mL solution A (13.2 g CaCl2.2H2O, 10 g MnCl4. 4H2O, 1 g CoCl2. 6H2O, 8 g FeCl3. 6H2O and made up to 100 mL with H2O), 200 mL solution B (39 g NaHCO3/L H2O), 200 mL solution C (5.7 g Na2HPO4, 6.2 g KH2PO4, 0.6 g MgSO4.7H2O and made up to 1000 mL with H2O), 1 mL resazurine (0.1%, w/v) and 40 mL reduction solution (95 mL H2O, 4 mL lM NaOH and 625 mg Na2S.9H2O). The mixture was kept under CO2 in a water bath at 39 °C and stirred by a magnetic stirrer [18]. Different amounts of TMR (400 mg, 360 mg, 320 mg, and 280 mg) and VFAs mixture (1.99 mL, 3.98 mL, and 5.97 mL) were added to serum bottles containing 60 mL of rumen medium mixture, and the pH of conditions in each trial was set at the pH of the mixture of feed and VFAs. To adjust the pH, an acid solution of 0.1 M HCl and a base solution of 0.1 M NaOH were used. In another trial, different volumes of VFAs (1.99 mL, 3.97 mL, 5.96 mL) were added to rumen medium mixture. The rumen fluid utilized in this trial was sourced from different age groups, comprising 2-, 6-, and 10-day-old samples. Three separate batches of VFAs media mixture were prepared, each utilizing rumen fluid of different ages: 2-day-old fluid was added to the first batch, 6-day-old fluid to the second batch, and 10-day-old fluid to the third batch. The bottles were sealed by aluminum crimp seal with rubber stoppers and flushed with nitrogen gas for two minutes to provide the anaerobic condition. Fermentation was conducted in serum bottles in a water bath (LSB12, Grant, Cambridgeshire, UK) at 37 °C and 100 rpm. Biogas was collected by a gas-tight syringe (VICI, Precision Sampling Inc., USA) to analyze its composition and volume. To analyze VFAs distribution and concentration, 1 mL of fermentation liquid was taken by syringe. The gas and VFAs samples were taken at hours 0, 4, 8, 12, 24, 32, 48, 56, 72, 80, and 96. Finally, the pH of the fermentation liquid and ammonium/ammonia content were measured at hours 0, 24, 48, 72, and 96. Table 2 shows the details of each condition.
Analytical method
Fiber analyzer A200 (ANKOM Technology, New York, USA) was used for acid detergent fiber (ADF), and neutral detergent fiber (NDF) analysis based on manufacturer’s provided protocol. For fat content analysis, fat was extracted from solid samples using an ST 255 Soxtec™ extractive system (FOSS, Hillerød, Denmark) and quantitated based on supplier’s protocol. Moisture, volatile solid, and ash contents were analyzed using the standard method by Eaton, Clesceri [19]. Bomb Calorimeter, IKA 2000 (IKA-Werke GmbH & Co. KG, Staufen im Breisgau, Germany), was used to estimate the energy value of the TMR [20]. Analyzing ammonium nitrogen (NH4+–N) was performed using the Ammonium 100 test kit (Nanocolor, MACHEREY–NAGEL GmbH & Co. KG, Germany). NH4+–N concentrations were measured using the Nanocolor 500D Photometer (MACHEREY–NAGEL GmbH & Co. KG, Germany). By using gas chromatography (GC) (Clarus 590; Perkin-Elmer, Norwalk, CT, USA) benefitting from a packed column (CarboxenTM 1000, 6 × 1.8 OD, 60/80 mesh, Supelco, Shelton, CT, USA), and a thermal conductivity detector (TCD) the volume and composition of gas (CH4, H2, and CO2) was analyzed. The injection temperature for the GC-TCD was set to 200 °C, and the carrier gas was nitrogen at a flow rate of 30 mL/min at 75 °C. Throughout the anaerobic digestion process, 0.25 mL of gas samples were taken daily using a gas syringe (VICI, Precision Sampling Inc., USA). In this study, VFAs were determined using GC (Clarus 590; Perkin-Elmer, Norwalk, CT, USA) coupled to a capillary column (Elite-WAX ETR, 30 m 0.32 mm 1.00 m, Perkin-Elmer, Shelton, CT, USA) and flame ionized detector (FID). In the GC-FID condition, injection and detection temperatures were 250 and 300 degrees Celsius, respectively. The carrier gas was nitrogen with a flow rate of 2 mL/min and a pressure of 20 psi. Prior to VFAs analysis, 500 µL liquid samples were mixed with 100 µL acid mix (25 percent (v/v) formic acid and 25 percent (v/v) ortho-phosphoric acid at a ratio of 1:3) and centrifuged at 10,000 × g for 5 min. To remove particles that were not dissolved, 0.2 m syringe filters were used to filter the supernatant from the experiment. VFAs concentrations and acid distributions are determined by mixing 250 µL of the supernatant with 250 µL butanol and 500 µL milli-Q water and analyzing with GC. Butanol at 1 g/L is used as an internal standard.
Statistical analysis
The design considered is a one-way experimental design since the effects of a single factor (VFAs replacement) on the dependent responses such as gas, VFAs production, alteration in pH, and ammonia production are being explored. All experiments and analyses were conducted in triplicates. MINITAB® 21 (Minitab Ltd., Coventry, UK) was used for the statistical analysis of the data with the one-way ANOVA (analysis of variance) and a confidence interval of 95%. Pairwise comparisons were carried out according to Tukey's test.
Results and discussion
In the current study, the ruminal fermentation TMR was investigated both individually and in combination with a VFAs solution through batch assays. The ruminal production of gas and VFAs were determined. The composition of the generated biogas was analyzed at different time intervals to evaluate the influence of VFAs supplementation on rumen microbial performance and as an indicator of feed digestibility.
Gas production
The amount of biogas produced in different combinations of TMR and VFAs mixture is presented in Fig. 1. In addition, the comparison between variations and responses is given in Table 3. Hydrogen (H2) has a central role in controlling methane (CH4) and VFAs production, therefore, it is of great importance to determine the variations in hydrogen generation in different conditions. TMR1 produced 0.8 mL H2 until hour 24, which was four-fold production compared with the amount produced by TMR1 + 10%VFAs (p = 0.02) (Fig. 1a). On the other hand, the mixture of TMR1 and 10%VFAs did not generate a high level of H2. The production was elevated until hour 8 and kept constant at around 0.1 mL until the trial ended. TMR2 produced significantly higher H2 than TMR1 (p = 0.03). TMR2 production rate kept increasing until 32 h before becoming stable. The mixture of TMR2 and 20%VFAs (TMR2 + 20%VFAs) produced less H2 (P = 0.04), but the rate was the same as TMR2. TMR3 produced more H2 compared to the mixture of TMR3 and 30%VFAs (TMR3 + 30%VFAs) but the difference was not significant (p = 0.08). It is worth noting that adding higher levels of VFAs (20%, and 30%) resulted in producing a higher amount of H2. Adding 30% VFAs to TMR produced more H2 than 20% VFAs addition, but the difference is not significant (p = 0.07). The accumulated H2 by TMR1 + 10%VFAs is at the lowest amount among all other treatments, which justifies that hydrogen does not usually accumulate in the rumen media and the combination of TMR and 10%VFAs provides a favorable condition for a rapid metabolization rate of hydrogen with stoichiometric production of methane [21]. Accordingly, methane was produced from the initiation of the trial without any lag for TMR + 10%VFAs, while in other conditions, a lag time of between 4 to 32 h was experienced.
The lag time could provide enough time for H2 production pathways to result in the accumulation of H2 in the system. Czerkawski, Harfoot [22] found a direct correlation between the rate of methane production and hydrogen uptake in the gas phase and the concentration of hydrogen. In their experiment where different amounts of hydrogen were sparged into the rumen fluid, it was observed that methane production was inversely related to hydrogen accumulation. Replacing higher levels of energy with VFAs (20%, and 30%) led to more hydrogen in the headspace (1.49 mL and 1.47 mL). Increase in hydrogen levels in rumen fluid, is reported to suppress the activity of NADH ferredoxin oxidoreductase crucial for producing hydrogen during continuous oxidation of reducing equivalents. In these conditions, there is a surge in hydrogen production in a 24-h period (diurnal hydrogen production), however, methanogenesis does not increase simultaneously. It could be said that, the amount of hydrogen that escapes from the fluid phase represents the amount of hydrogen that evades methanogenesis or any other hydrogen sink in the fluid phase [23].
Unlike the higher H2 production trend by TMR1 compared to TMR1 + 10%VFAs, the latter produced significantly higher amount of methane (p = 0.03) (Fig. 1b). While the CH4 synthesis process was identical for both conditions until hour 12, TMR1 + 10%VFAs exhibited a notable rise from 2 mL to over 8 mL, steadily increasing to reach 15 mL by the conclusion of the experiment. The trend was the same, but the methane production rate for TMR1 became slower from hour 12 until hour 96. This resulted in 4 mL less methane production compared to TMR1 + 10%VFAs (Table 3). TMR2 + 20%VFAs started to produce methane at hour 24 and produced around 8 mL by the end of the experiment. Moreover, this trend was similar for TMR3 + 30%VFAs. In general, adding higher amounts of VFAs resulted in less CH4 production in terms of rate and extent. Hence, VFAs do not act as precursors for methane production during anaerobic digestion.
The amount of produced gas is an indicator of feed digestion in the gas method [24]. At higher levels of added VFAs, VFAs supplemented treatments produced rather similar volume of gas as TMR alone, showing the same digestibility in both conditions. Considering the lower load of TMR in treatments with added VFAs, similar gas production as for TMR represents increase in digestibility in the presence of VFAs. This is also backed by higher VFAs levels in treatment groups with 20 and 30% VFAs replacement (6.88 g/L, and 5.79 g/L). Moreover, increasing the incubation time would increase the proportion of methane in the total gas produced. Getachew, Robinson [25] investigated the differences among TMR in methane production at 6, 24, 30, 48 and 72 h of in vitro incubation. They suggested that the slowly digestible fraction of feed, i.e., structural carbohydrate, is associated with higher CH4 production during longer incubation. In this study, the average CH4 production by 48 h incubation was 12.3 mL, 3.5 mL, and 1.6 mL for 10%, 20%, and 30% replaced VFAs, respectively. It could be concluded that by replacing higher amounts of energy with VFAs, the amount of structural carbohydrates in feed would be decreased, resulting in less methane production and longer incubation time. However, factors such as NDF content of the feed, dilution rate and pH of rumen and medium, and feed particle size, could also affect the amounts of produced methane [25].
pH is another determining factor that can influence methanogens, therefore, the changes in the mean pH were recorded every 24 h and presented in Fig. 2. For all treatments, upon incubation for 96 h, it was observed that the medium’s buffering capacity always maintained a pH within the range of 6.9–7.4 necessary for optimum cellulolytic bacteria and methanogens growth [26]. In in vitro techniques, incubation systems are designed to maintain the pH between 6.7 and 7.0, which is considered to be the physiological range for a healthy and active rumen [27]. In this study, the usual trend of gas production for TMR and TMR + VFAs shows no exhaustion of the buffering capacity in the media and, the pH is maintained at the range 6.9 to 7.4 that makes a correlation with gas production. Compared to other studies [28, 29], those from Menke et al. [18] and Beuvink and Spoelstra [30] have a higher concentration of bicarbonate ions in media. To investigate the critical differences in the final pH, up to 12 mmol propionic acid, based on the amount and composition of the buffer, was added to different media with the same initial pH to simulate rumen fermentation. Accordingly, for Menke et al. [18] buffer, pH values were above 5.9, while in Theodorou et al. [28] and Huntington et al. [29] buffers, pH values were 5.42 and 5.29, respectively. The present investigation demonstrates that maintaining an incubation pH over 6.5 could potentially create an appropriate fermentation environment. Bertipaglia, Fondevila [31] reported that the gas production from a mixed concentrate feed after 24 h of incubation was 26% lower at pH 5.8 than at pH 6.5. As the fermentation medium pH decreased below 6.2, gas release became non-linear, emphasizing that the buffering capacity to contain fermentation end-products is limited and affected [30].
As a result, adding VFAs at the mentioned levels did not affect the ruminal pH. In addition, VFAs effluent used in this study contains a significant nitrogen content in the form of ammonium that assists with buffering.
The changes in the ammonia and ammonium content of the media during gas method analysis are presented in Fig. 3. At the beginning of the experiment, TMR3 + 30%VFAs had the highest amount of ammonia at 370 mg/L, due to higher VFAs solution addition. TMR1 + 10%VFAs had a high ammonia consumption rate as it reduced from 265 mg/L to 195 mg/L in a 24 h interval followed by an increase in content from 48 to 96 h. Microorganisms could have used the supplied ammonia to proliferate in this period. Similar ammonia reduction trend was observed for TMR3 + 30%VFAs. There is a gradual increase in VFAs, and methane production confirms the feed fermentation and ammonia release in 48 h and 96 h by TMR1 + 10%VFAs. Also, fast-fermenting carbohydrates resulted in higher microbial synthesis [32]. Alternating higher levels of energy to higher levels of VFAs would remove a chunk of fast fermenting carbohydrate, hindering microbial growth.
VFAs production
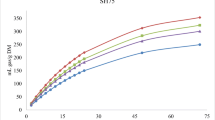
The changes in total VFAs production and distribution are represented in Fig. 4. Replacing 10% of TMR energy with VFAs increased the total produced VFAs in the system. The initial VFAs concentration for TMR1 + 10%VFAs was 2.84 g/L and reached 5.45 g/L by the end of the experiment. A higher production rate was recorded up to hour 48 and final VFAs concentration in the presence of 10% VFAs compared to TMR1 (p = 0.02). The difference in total VFAs is an indicator of VFAs production during 96 h that is calculated based on the deduction of initial total VFAs from the amount produced at the end of the experiment (Table 3). The difference in total VFAs was 3.01 g/L for TMR1 and 2.61 g/L for TMR1 + 10%VFAs. In both conditions, propionic and butyric acids gradually increased, but this was more pronounced for TMR1 + 10%VFAs. In contrast, TMR1 had higher acetic acid (3.63 g/L) but it was not significant (p = 0.06). Propionic and butyric acids are more favored as they have a broad range of applications in ruminant bodies and are important in different metabolic pathways, for instance propionate acts as glucose precursor in lactating cows [33]. Additionally, in high hydrogen generation situations propionic production can compete with methanogens on H2 uptake.
Over 50% of generated acetate and butyrate by TMR1 + 10%VFAs was produced in the first 24 h. The VFAs mixture had a high level of acetate and butyrate and by replacing 20% of energy with it in TMR, hydrogen was produced at a high rate until hour 32 (1.5 mL) and became stable after that. At the same time, methane started to increase at 32 h (1.14 mL) and reached 3.53 mL at 48 h. Notably, the acetate, propionate, and butyrate gradually increased by 48h (Fig. 4). Net hydrogen production is associated with acetate, and to a lesser extent, butyrate production from feed, respectively. As opposed to this, propionate production results in net hydrogen incorporation. Hydrogen was produced simultaneously in the acetate and butyrate production pathways. Based on the acetate and butyrate production rate, a hydrogen pool peak is expected by 24 h. However, as methanogenesis is the thermodynamically preferable approach for H2 sink, from hour 24 there was a sharp increase in methane production, resulting in decreasing the H2 production rate. In comparison, TMR1 has the same tendency in acetate and butyrate production and generates hydrogen at the same time. While the hydrogen uptake by methanogens in TMR1 after 24 h was not as significant as the hydrogen uptake by methanogens in TMR1 + 10%VFAs after 24 h, the methane production pattern remained unchanged. TMR2 + 20%VFAs produced the highest amount of VFAs during the 96-h experiment but the difference was not significant (p = 0.07) compared to the VFAs production by TMR2. The initial total VFAs were 3.43 g/L and increased to 5.68 g/L at 48 h, while total VFAs production was 4.77 g/L for TMR2. From 48 h until 96 h for TMR2 + 20%VFAs and TMR2 the VFAs generation soared to 6.89 g/L and 5.88 g/L, respectively. The butyric acid production by TMR2 fluctuated by around 0.5 g/L, while TMR2 + 20%VFAs experienced a gradual rise to reach 1.12 g/L at 96h. Furthermore, the TMR2 + 20%VFAs treatment resulted in a gradual increase in the production of propionic acid, with levels reaching 1.92 g/L by hour 80 producing the highest amount of propionic acid among all treatments.
In systems characterized by a high hydrogen concentration, such as TMR1, TMR2, and TMR2 + 20%VFAs, the process of hydrogen generation is constrained, resulting in a reduction in the flux through the H2 pool. In the presence of high H2 concentrations, pathways that produce H2 are less thermodynamically favorable than other pathways. A smaller amount of CH4 will be formed per unit of feed fermented in the media, as electrons will be channeled into other products, such as propionate. Consequently, lower H2 concentrations are required to maintain methanogens in the media, leading to the formation of more H2 and a reduction in propionic acid production [34]. A close correlation exists between the profile of VFAs produced and CH4 formation [34]. Propionic acid and CH4 compete as hydrogen sink, whereas acetate and butyrate produce hydrogen that methanogens can use it for CO2 reduction to methane [35]. In vitro balances of hydrogen production and incorporation in TMR1 + 10%VFAs show an inverse correlation between produced hydrogen and methane (Fig. 1a, e)[36].
TMR1 + 10%VFAs produced the highest amount of CO2 (24.2 mL) followed by TMR1. The energy replacement at higher percentages resulted in less CO2 production. The rate of CO2 production by TMR3 + 30%VFAs and TMR3 was noticeably fast until hour 24. After that, the production increased gradually by the end of the trial to reach 17.3 and 15.7 mL, respectively. The rate of CO2 production by TMR2 + 20%VFAs and TMR2 was the lowest compared to other treatments at 13.7 and 16.5 mL, respectively. CO2 is produced as a result of both microbial fermentation and the reaction between fermentation products and the sodium bicarbonate contained in buffer [30].
TMR1 + 10%VFAs produced 19.7 mL CO2 at 48 h, which was 80% of total generated CO2, while it was 12.3 mL CO2 for TMR1 fermentation at the same time. Respecting Fig. 4a, TMR1 + 10%VFAs produced more acetate (3.24 g/L) and butyrate (0.88 g/L) compared to TMR1 but the difference was not significant (p = 0.07). On the other hand, TMR1 + 10%VFAs produced less propionate compared to TMR1 (p = 0.02). Since substrate fermentation to propionate yields gas only from buffering in the medium, lower gas production is associated with higher propionate production [37]. In TMR2 + 20%VFAs the conversion of CO2 and H2 led to acetate production instead of CH4 and consequently reduction in the gas produced per mol of VFA [38], along with the uptake of net one mol of CO2. Therefore, it has lower CO2 level compared to TMR1 + 10%VFAs. It is noteworthy that inhibition or reduced activity of the methanogens results in hydrogen accumulation and inhibition of hydrogenases. Consequently, carbohydrate fermenting bacteria resort to other mechanisms to reduce equivalent disposal (e.g. dehydrogenases of propionate synthesis) [39]. Consequently, it is plausible that the elevated concentration of H2 in TMR2 + 20%VFAs, as compared to TMR1 + 10%VFAs, could potentially result in increased propionic acid synthesis and decreased CH4 and CO2 production in TMR2 + 20%VFAs. In addition to that, VFAs supplementation might have supported the growth of specific microorganisms such as fumarate-reducing enterococci, resulting in a rise in propionic acid production and reduction in methane production [40].
TMR3 + 30%VFAs and TMR3 had 3g/L acetic acid, the lowest amount among all conditions. Many authors have reported the significance of VFAs profile concerning H2 availability and methane production. Higher propionate and lower acetate and butyrate proportions contribute to lower H2 production and less methane output [41]. Conversely, TMR2 + 20%VFAs had higher acetic, propionic, and butyric acid but it produced methane at the same level as TMR2 with less hydrogen yield. Based on the amount of H2 and CH4 production, total VFAs generated and acids distribution, replacing 20% of TMR energy with VFAs solution is the most favorable condition which produces less gas and higher VFAs compared to its control group (TMR2).
Effect of rumen fluid age on gas and VFAs production
Introducing varying levels of VFAs (2 mL, 4 mL, 6 mL) into buffering media containing rumen fluid at different ages resulted in variations in gas and VFAs production which is presented in Fig. 5. The primary objective of this part of study was to examine the impact of the age of rumen fluid as an inoculum on ruminal fermentation when supplemented with VFAs in the absence of a TMR. Additionally, the study aimed to investigate the effect of time on the preservation of rumen fluid under natural rumen conditions. Keeping rumen fluid at 39℃ with sparging nitrogen to maintain the anaerobic condition for 5 and 10 days affected microbial performance.
The addition of VFA with 10% TMR energy equivalent to rumen fluid of 10 days aged (10%VFAs + 10D) produced the highest amount of H2 (0.15 mL) (Fig. 5a). It is worth noting that other percentages of VFAs with different rumen fluid ages produced negligible H2. However, the 20%VFAs in rumen fluid of 10 days old showed different trends. Hydrogen production started at hour 24 and stayed constant till 32 h. All three levels of added VFAs to 10-day-old rumen fluid produced the highest amount of methane. Besides 10%VFAs + 10D had the highest production rate, whereas the two other additional levels produced methane in a similar manner. However, the methane production rate of the addition of 30% energy of TMR in the form of VFAs to rumen fluid of 10 days aged (30%VFAs + 10D) was higher than the production rate of the addition of 20% energy of TMR in the form of VFAs to rumen fluid of 10 days aged (20%VFAs + 10D) at 52 h, producing 3.8 mL and 2.7 mL, respectively. Adding different levels of VFAs to fresh rumen fluid did not influence VFAs production. The difference between the initial VFAs and the final VFAs was negligible. Adding higher levels of VFAs increased the initial VFAs concentration in the system, but the changes in total VFAs production were the same for all VFAs levels. It is noteworthy that all VFAs levels had the same production trend but different VFAs distribution. The 10%VFAs + 10D was the only condition where acetic acid was produced during the incubation. The microbiota had not been fed for more than 10 days prior to the assay, therefore, only the last stage of anaerobic digestion, methanogenesis, was supplied with required precursors. Nevertheless, total VFAs increased drastically from 1.96 g/L at 4 h to 3.53 g/L at hour 8. VFAs production supports the sudden jump in H2 at 8 h, as there was a significant acetic and butyric acid production by that time. Considering the effect of ruminal fluid age on gas production, the trend for gas production from fresh and 5-day-old rumen fluid in this study was in line with Robinson, Mathews [42] findings. They found that the delays of up to 48 h from rumen fluid collection to the initiation of the fermentation experiment had no impact on the measured gas. Likewise, Cone, Van Gelder [43] observed that final gas production after storage of RF for 2, 4, 8, and 25 h at 39°C was similar for RF stored for up to 4 h. However, the amount of gas produced by RF stored for more than 25 h was significantly lower, which differs from the findings of this study. In addition to rumen enzyme activity being influenced by the preservation treatment and the donor animal’s diet, different enzymes are susceptible to changes by temperature and pH in different ways.
Conclusion
This study investigated the partial energy replacement of TMR with the VFAs solution at three different supplementation levels. Based on reduction in methane production, increase in VFAs generation, and more favored VFAs distribution, replacing 20% of TMR energy with VFAs derived from agro-food residues could be a promising approach for introducing a sustainable alternative ingredient for supplying energy in ruminants’ feed. Hence, VFAs solution could act as a promising player in balancing the ration for animals at special physiologic stage such as dairy cows at post-partum stage or cows with negative energy balance, however, follow up in vivo trials are necessary. Additionally, it is a convenient solution for farmers to bio convert agriculture residue to VFAs solution on site and step towards circularity in feed production.
Availability of data and materials
All the data results involved in this study have been presented in the article.
References
Mahboubi A, Agnihotri S, Uwineza C, Jomnonkhaow U, Taherzadeh MJ. Chapter 18 - Waste-derived volatile fatty acids for sustainable ruminant feed supplementation. In: Varjani S, Pandey A, Taherzadeh MJ, Ngo HH, Tyagi RD, editors. Biomass. Biofuels, Biochemicals: Elsevier; 2022. p. 407–30.
Shen Z, Kuhla S, Zitnan R, Seyfert H-M, Schneider F, Hagemeister H, et al. Intraruminal infusion of n-butyric acid induces an increase of ruminal papillae size independent of IGF-1 system in castrated bulls. J Archives of Animal Nutrition. 2005;59(4):213–25.
Gorka P, Kowalski Z, Pietrzak P, Kotunia A, Kiljanczyk R, Flaga J, et al. Effect of sodium butyrate supplementation in milk replacer and starter diet on rumen development in calves. J development. 2009;4(5):10–1.
Jones G. Volatile fatty acids in concentrate rations for lactating dairy cows. J Dairy Sci. 1971;54(8):1142–9.
Maekawa M, Beauchemin K, Christensen D. Effect of concentrate level and feeding management on chewing activities, saliva production, and ruminal pH of lactating dairy cows. J Dairy Sci. 2002;85(5):1165–75. https://doi.org/10.3168/jds.S0022-0302(02)74179-9.
Ben-Meir YA, Jami E, Portnik Y, Ya’acoby S, Chen Y, Ogunade IM, et al. Effect of silage inoculants on the quality of baled whole-crop wheat silages and milking cow performance. Grassl Sci. 2018;64(3):207–14. https://doi.org/10.1111/grs.12196.
Bohnert D, DelCurto T. Fundamentals of supplementing low-quality forage. CL317 in Cow–Calf Management Guide Producer’s Library Agricultural Communications, College of Agricultural. 2003;
Malenica D, Kass M, Bhat R. Sustainable Management and Valorization of Agri-Food Industrial Wastes and By-Products as Animal Feed: For Ruminants, Non-Ruminants and as Poultry Feed. Sustainability [Internet]. 2023; 15(1).
Loor JJ, Elolimy AA, McCann JC. Dietary impacts on rumen microbiota in beef and dairy production. Anim Front. 2016;6(3):22–9. https://doi.org/10.2527/af.2016-0030.
Cheng Z, Meng Z, Tan D, Datsomor O, Zhan K, Lin M, et al. Effects of supplementation of sodium acetate on rumen fermentation and microbiota in postpartum dairy cows. Front Microbiol. 2022;13:1053503.
Veluswamy G, Shah K, Ball A, Guwy A, Dinsdale R. A techno-economic case for volatile fatty acid production for increased sustainability in the wastewater treatment industry. Environ Sci Water Res Technol ENVIRON SCI-WAT RES. 2021;7(5):927–41. https://doi.org/10.1039/D0EW00853B.
López S. In vitro and in situ techniques for estimating digestibility. CABI Books. https://doi.org/10.1079/9780851998145.0087
Getachew G, Blümmel M, Makkar HPS, Becker K. In vitro gas measuring techniques for assessment of nutritional quality of feeds: a review. Anim Feed Sci Technol. 1998;72(3):261–81. https://doi.org/10.1016/S0377-8401(97)00189-2.
Aregheore EM. Chemical composition and nutritive value of some tropical by-product feedstuffs for small ruminants — in vivo and in vitro digestibility. Anim Feed Sci Technol. 2000;85(1):99–109. https://doi.org/10.1016/S0377-8401(00)00123-1.
Aiple KP, Steingass H, Drochner W. Prediction of the net energy content of raw materials and compound feeds for ruminants by different laboratory methods. Archiv für Tierernaehrung. 1996;49(3):213–20. https://doi.org/10.1080/17450399609381882.
Parchami M, Uwineza C, Ibeabuchi OH, Rustas B-O, Taherzadeh MJ, Mahboubi A. Membrane bioreactor assisted volatile fatty acids production from agro-industrial residues for ruminant feed application. Waste Manage. 2023;170:62–74. https://doi.org/10.1016/j.wasman.2023.07.032.
Kim HJ, Min DB. Tocopherol stability and prooxidant mechanisms of oxidized tocopherols in lipids. J Food Lipids. 2008;3:435–48.
Menke K, Raab L, Salewski A, Steingass H, Fritz D, Schneider W. The estimation of the digestibility and metabolizable energy content of ruminant feedingstuffs from the gas production when they are incubated with rumen liquor in vitro. J Agric Sci. 1979;93(1):217–22. https://doi.org/10.1017/S0021859600086305.
Eaton A, Clesceri L, Rice E, Greenberg A. Standard methods for the examination of water and wastewater. Washington, DC DC: American Public Health Association; 2005.
Vali N, Combres A, Hosseinian A, Pettersson A. The Effect of the Elemental Composition of Municipal Sewage Sludge on the Phosphorus Recycling during Pyrolysis, with a Focus on the Char Chemistry—Modeling and Experiments. Separations. 2023;10(1):31. https://doi.org/10.3390/separations10010031.
Carroll E, Hungate R. Formate dissimilation and methane production in bovine rumen contents. Arch Biochem Biophys. 1955;56(2):525–36. https://doi.org/10.1016/0003-9861(55)90272-1.
Czerkawski J, Harfoot C, Breckenridge G. The relationship between methane production and concentrations of hydrogen in the aqueous and gaseous phases during rumen fermentation in vitro. J appl bacteriol. 1972;35(4):537–51. https://doi.org/10.1111/j.1365-2672.1972.tb03735.x.
Hegarty R. Gerdes RJRaianiA. Hydrogen production and transfer in the rumen. 1999;12:37–44.
Getachew G, DePeters E, Robinson P. In vitro gas production provides effective method for assessing ruminant feeds. Calif Agric. 2004;58(1):54–8.
Getachew G, Robinson P, DePeters E, Taylor S, Gisi D, Higginbotham G, et al. Methane production from commercial dairy rations estimated using an in vitro gas technique. Anim Feed Sci Technol. 2005;123:391–402. https://doi.org/10.1016/j.anifeedsci.2005.04.056.
Doane P, Schofield P. Pell AJJoAS. Neutral detergent fiber disappearance and gas and volatile fatty acid production during the in vitro fermentation of six forages. 1997;75(12):3342–52.
Mould F, Morgan R, Kliem K, Krystallidou E. A review and simplification of the in vitro incubation medium. Anim Feed Sci Technol. 2005;123:155–72. https://doi.org/10.1016/j.anifeedsci.2005.05.002.
Theodorou MK, Williams BA, Dhanoa MS, McAllan AB, France J. A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim Feed Sci Technol. 1994;48(3–4):185–97. https://doi.org/10.1016/0377-8401(94)90171-6.
Huntington J, Rymer C, Givens D. The effect of host diet on the gas production profile of hay and high-temperature dried grass. Anim Sci. 1998;67(1):59–64. https://doi.org/10.1017/S1357729800009796.
Beuvink J, Spoelstra S. Interactions between substrate, fermentation end-products, buffering systems and gas production upon fermentation of different carbohydrates by mixed rumen microorganisms in vitro. Appl Microbiol Biotechnol. 1992;37(4):505–9. https://doi.org/10.1007/BF00180978.
Bertipaglia L, Fondevila M, Van Laar H, Castrillo C. Effect of pelleting and pellet size of a concentrate for intensively reared beef cattle on in vitro fermentation by two different approaches. Anim Feed Sci Technol. 2010;159(3–4):88–95. https://doi.org/10.1016/j.anifeedsci.2010.05.010.
Cone JW, Becker PM. Fermentation kinetics and production of volatile fatty acids and microbial protein by starchy feedstuffs. Anim Feed Sci Technol. 2012;172(1–2):34–41. https://doi.org/10.1016/j.anifeedsci.2011.12.006.
Aschenbach JR, Kristensen NB, Donkin SS, Hammon HM, Penner GB. Gluconeogenesis in dairy cows: the secret of making sweet milk from sour dough. IUBMB Life. 2010;62(12):869–77. https://doi.org/10.1002/iub.400.
Janssen PHJAFS. Influence of hydrogen on rumen methane formation and fermentation balances through microbial growth kinetics and fermentation thermodynamics. Anim Feed Sci Technol. 2010;160(1–2):1–22; https://doi.org/10.1016/j.anifeedsci.2010.07.002
Pereira AM, de Lurdes Nunes Enes Dapkevicius M, Borba AE. Alternative pathways for hydrogen sink originated from the ruminal fermentation of carbohydrates: Which microorganisms are involved in lowering methane emission? anim microbiome. 2022;4(1):5; https://doi.org/10.1186/s42523-021-00153-w
Ungerfeld EM. Shifts in metabolic hydrogen sinks in the methanogenesis-inhibited ruminal fermentation: a meta-analysis. Front Microbiol. 2015;6:37. https://doi.org/10.3389/fmicb.2015.00037.
Amanzougarene Z, Fondevila M. Fitting of the in vitro gas production technique to the study of high concentrate diets. Animals. 2020;10(10):1935. https://doi.org/10.3390/ani10101935.
Miller T. Ecology of methane production and hydrogen sinks in the rumen. In ‘Ruminant physiology: digestion, metabolism, growth and reproduction’.(Eds W von Engelhardt, S Leonhard-Marek, G Breves, D Giesecke) pp. . Ferdinand Enke Verlag: Stuttgart, Germany; 1995. p. 317–31.
Van Kessel JAS, Russell JB. The effect of pH on ruminal methanogenesis. FEMS Microbiol Ecol. 1996;20(4):205–10. https://doi.org/10.1111/j.1574-6941.1996.tb00319.x.
Kim S-H, Mamuad LL, Kim D-W, Kim S-K, Lee S-S. Fumarate reductase-producing enterococci reduce methane production in rumen fermentation in vitro. J Microbiol Biotechnol. 2016;26(3):558–66. https://doi.org/10.4014/jmb.1512.12008.
Bannink A, Kogut J, Dijkstra J, France J, Kebreab E, Van Vuuren A, et al. Estimation of the stoichiometry of volatile fatty acid production in the rumen of lactating cows. J Theor Biol. 2006;238(1):36–51. https://doi.org/10.1016/j.jtbi.2005.05.026.
Robinson P, Mathews MC, Fadel J. Influence of storage time and temperature on in vitro digestion of neutral detergent fibre at 48 h, and comparison to 48 h in sacco neutral detergent fibre digestion. Anim Feed Sci Technol. 1999;80(3–4):257–66. https://doi.org/10.1016/S0377-8401(99)00062-0.
Cone J, Van Gelder A, Bachmann H, editors. Influence of inoculum source, dilution and storage of rumen fluid on gas production profiles. Gas Production: Fermentation Kinetics for Feed Evaluation and to Assess Microbial Activity Proceedings of the EAAP Satellite Symposium on Gas Production, Wageningen, The Netherlands Proc Br Soc Anim Sci; 2000.
Acknowledgements
The authors would like to thank the University of Borås and Formas for the received technical and financial support. We also gratefully acknowledge the Swedish University of Agricultural Sciences for the kind provision of ruminal fluid.
Funding
Open access funding provided by University of Boras. This work has been funded by Formas (Sweden) grant number 2021–02458.
Author information
Authors and Affiliations
Contributions
MP: Data curation, Formal analysis, Methodology, Validation, Investigation, Writing—original draft, Writing—review & editing, Supervision; AM: Conceptualization, Investigation, Writing—review & editing, Supervision; BOR: Writing—review & editing, Methodology, Resources; MJT: Conceptualization, Funding acquisition, Resources, Supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Mohammad J Taherzadeh, a co-author on this paper, is the Editor of SMAB. He was blinded to this paper during the peer review and decision-making process.
Ethics approval and consent to participate
In this study no in vivo animal trials have been conducted. A small volume of rumen fluid (2 L) collected according to the ethical approval acquired from the Swedish Board of Agriculture (Jordbruksverket) and was provided by Swedish University of Agricultural Sciences (SLU) for the purpose of in vitro digestion investigation.
Consent for publication
Not applicable.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Parchami, M., Rustas, BO., Taherzadeh, M.J. et al. An in vitro evaluation of partial energy replacement in a total mixed ration with volatile fatty acids derived from agro-industrial residues. Syst Microbiol and Biomanuf (2024). https://doi.org/10.1007/s43393-024-00278-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43393-024-00278-4