Abstract
Purpose
Vertebral body tethering (VBT) is a recent procedure to correct and reduce spinal curves in skeletally immature patients with adolescent idiopathic scoliosis (AIS). The purpose of this systematic review and meta-analysis is to determine the expected curve reduction and potential complications for adolescent patients after VBT.
Methods
PubMed, Embase, Google Scholar and Cochrane databases were searched until February 2022. Records were screened against pre-defined inclusion and exclusion criteria. Data sources were prospective and retrospective studies. Demographics, mean differences in Cobb angle, surgical details and complication rates were recorded. Meta-analysis was conducted using a random-effects model.
Results
This systematic review includes 19 studies, and the meta-analysis includes 16 of these. VBT displayed a statistically significant reduction in Cobb angle from pre-operative to final (minimum 2 years) measurements. The initial mean Cobb angle was 47.8° (CI 95% 42.9–52.7°) and decreased to 22.2° (CI 95% 19.9–24.5°). The mean difference is − 25.8° (CI 95% − 28.9–22.7) (p < 0.01). The overall complication rate was 23% (CI 95% 14.4–31.6%), the most common complication was tether breakage 21.9% (CI 95% 10.6–33.1%). The spinal fusion rate was 7.2% (CI 95% 2.3–12.1%).
Conclusion
VBT results in a significant reduction of AIS at 2 years of follow-up. Overall complication rate was relatively high although the consequences of the complications are unknown. Further research is required to explore the reasons behind the complication rate and determine the optimal timing for the procedure. VBT remains a promising new procedure that is effective at reducing scoliotic curves and preventing spinal fusion in the majority of patients.
Level of evidence
Systematic review of Therapeutic Studies with evidence level II–IV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
AIS is a three-dimensional spinal deformity demonstrating a lateral curve with a Cobb angle of > 10°, and exclusion of an underlying cause [1, 2]. AIS has an onset at puberty and female predominance [1,2,3]. AIS is painless in adolescence, but due to the progressive nature can cause issues with pain, cardiopulmonary function, cosmesis and early death in adulthood [4]. AIS is known to progress during the pubertal growth spurt, the risk of progression can be predicted using the Cobb angle, skeletal maturity and menarche status [5].
Current treatments are determined based on the major curve Cobb angle and the bone age. In skeletally immature patients, a major curve Cobb angle of < 25° can be managed conservatively with serial radiographs to assess progression [6]. A major curve Cobb angle 25–45° is expected to progress and is often managed with bracing [7]. A major curve ≥ 45° is likely to progress to cause disability and is expected to need surgical correction. Skeletally mature patients with a Cobb angle of ≤ 45° are unlikely to need surgical intervention as their risk of progression has significantly reduced [6].
Posterior spinal instrumented fusion (PSIF) is the gold standard surgical procedure, which reduces the deformity and fuses the spine to prevent progression [8]. The concern for early PSIF is it will halt remaining spinal growth and reduce the thorax volume, decreasing pulmonary function [9].
Another surgical option is growing rods, which require surgery every 4–6 months to manually lengthen as the spine grows [10]. Disadvantages include multiple general anaesthetics, risk of wound infections, spontaneous auto-fusion and eventual PSIF [11,12,13].
Young, skeletally immature patients with severe curves provide a unique challenge for surgical intervention. Surgeons must weigh up the curve severity and risk of progression, against preserving remaining growth of the thorax and spinal height. These patients may have been unable to tolerate a brace, or their curves may have progressed beyond the upper limit for bracing, yet they remain skeletally immature and therefore at risk of further progression of their spine deformity.
VBT is an emerging surgical procedure intending to reduce the curve and prevent progression with one surgery, whilst preventing a PSIF. VBT secures a polyethylene tether to the convex surface of the major curve with screws at multiple vertebral levels [14]. It is thought that VBT induces asymmetrical growth in the spine via the Hueter–Volkman law [15]. VBT does not involve spinal fusion and therefore allows the continued spinal growth. Since the concept was introduced off-label in 2010 [16], and FDA approved in 2019, multiple international centres have performed the VBT procedure.
The authors are not aware of a meta-analysis that compares outcomes from VBT exclusively. This is a systematic review and meta-analysis of studies solely investigating VBT outcomes in AIS. The primary purpose of this study was to determine if VBT was successful at reducing the spinal curvature. The second purpose was to determine the complication rate, and how many patients ultimately required a spinal fusion.
Materials and methods
Study selection
A systematic review was performed according to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines [17]. Studies met the inclusion criteria if they (1) investigated the treatment of AIS, (2) in patients with a main thoracic curve, by (3) performing anterior VBT, (4) in skeletally immature patients, (5) reported the pre- and post-operative major curve Cobb angle and (6) had minimum 2 years of follow-up.
Studies met the exclusion criteria if they (1) solely investigated non-idiopathic scoliosis e.g. neuromuscular (2) performed finite element analysis, (3) used non-human models, was a 4) review article, (5) conference abstract, (6) case report, or (7) only detailed surgical technique.
PubMed, Google Scholar, Embase and Cochrane were searched and retrieved all VBT clinical studies published in English. The following keywords were used: ‘vertebral body tethering’ and ‘adolescent idiopathic scoliosis’. Medical Subject Heading (MeSH) vocabularies were selected when retrieving articles. Databases were searched using the advanced function on 17 February 2022.
Data extraction
Two researchers (M.R. and J.P.L) screened all results against the inclusion and exclusion criteria. A list of studies from each source was printed and duplicates removed. Article abstracts were reviewed and discussed until a consensus was obtained. Full-text articles were retrieved, downloaded and manually organised, without automation tools. Citations were downloaded into EndNote.
A data extraction form was developed [18] and used by one researcher (M.R.). Corresponding authors of included studies were contacted for additional information when required.
Data outcomes
To determine the clinical success of the VBT procedure, the main measured outcome is the Cobb angle of the major curvature of the spine [19]. This was recorded pre-operatively and at final follow-up, which was mostly 2 years, but for some studies was longer than 2 years. The mean major curve Cobb angle, range of values and standard deviation (SD) were recorded. Other measured outcomes are surgical details and surgical complications.
Data synthesis
For the meta-analysis, a random-effects model was fitted using DerSimonian and Laird method (DL) for estimating heterogeneity [20]. Heterogeneity was examined using the Higgins I2 statistic [21]. I2 values of 25%, 50% and 75% indicated low, moderate and high heterogeneity. Forest and funnel plots were presented for visualisation of summary effect sizes and publication bias. All analyses were conducted using metafor package in R (version 4.0.4) (2021).
Given the homogeneity between the inclusion criteria of the studies, they were compared against each other for overall mean difference in major curve Cobb angle. In cases when there was no SD available, the study was excluded from the meta-analysis. Bar charts were created to visually display the mean change in major curve Cobb angle. The correlation coefficient was unable to be calculated. A conservative correlation coefficient was chosen using 0.4. The SD of the mean difference was calculated using the formula [22];
Small study effects owing to potential publication bias, poor methodological quality, true heterogeneity or chance were analysed with contour-enhanced funnel plots. To assess for selective reporting biases, the methods and the results sections were compared, and clinical knowledge was applied to assess for any discrepancy. The National Heart, Lung and Blood Institute (NHLBI) quality assessment tool for pre–post studies with no control group [23] was used to assess for the risk of bias in the selected studies.
Results
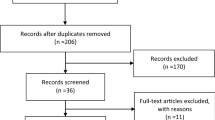
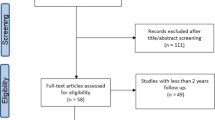
115 studies were identified using the above search strategy. 19 studies with a total of 677 patients met the inclusion criteria. Figure 1 displays the PRISMA flowchart for article selection. 83 articles were assessed for eligibility, 63 were excluded and the reasons are stated in Fig. 1.
Included studies
19 studies met the inclusion criteria (Table 1). 12 studies had a subset of patients that were excluded (appendix 1). 13 of the studies were rated as low, and 6 were rated as moderate risk of bias by the NHLBI criteria (appendix 2a and b).
Study cohort characteristics
Mean age was 12.2 years, mean follow-up was 34.1 months, 84.8% were female patients, and 74% were premenarchal. Table 1 displays pre-operative patient demographics. Appendix 3 displays the final follow-up demographics. All studies stated the pre-operative and a minimum 2-year post-operative Cobb angle. The median Risser score was 0, and the median Sanders was 3.
Results of individual studies
Initial mean major curve Cobb angle was 47.8° (CI 95% 42.9–52.7°) and decreased to 22.2° (CI 95% 19.9–24.5°) at minimum 2 years of post-operative. The mean main thoracic pre-operative major curve Cobb angle range was 31–81°, and at final follow-up, the range was − 26–62°. This indicates a mean reduction in major thoracic curve of 54% at the latest follow-up. The major curve Cobb angle results are displayed in Table 2 and Fig. 2.
Summary statistics
16 studies had sufficient data for a meta-analysis as displayed in Fig. 3. The mean difference in major curve Cobb angle was − 25.77° (CI95% − 28.9–22.65) with statistical significance (p < 0.01). I2 value of 89% and a significant Q-test for heterogeneity (Q = 140.18, df = 15, p < 0.01) indicate substantial heterogeneity.
Only one study [24] crossed the line of null effect, represented by the vertical line at 0 on the Forest Plot. This study had a small sample size with insufficient power, and an overall moderate risk of bias. There was no significant publication bias detected, as seen in Fig. 4.
Surgical details
Most studies used a thoracoscopic approach and the Zimmer Biomet tether. The mean number of vertebral levels instrumented was 7.6, the mean surgical time was 223 min, mean intra-operative blood loss was 144 mL and the mean hospital inpatient stay was 4.9 days.
Complications
17 studies reported complications, as seen in Table 3. The overall rate of complications was 23% (CI 95% 14.4–31.6%). The mean rate of tether breakage was 21.9% (CI 95% 10.6–33.1%), overcorrection was 11.4% (CI 95% 5.7–17.2%), re-operation was 11.4% (CI 95% 6.2–16.7%), spinal fusion rate was 7.2% (CI 95% 2.3–12.1%) and post-operative pulmonary complications was 6.7% (CI 95% 4–9.5%). Re-operations were most often removal of tether due to overcorrection, or replacement of a broken tether [25,26,27,28,29,30,31]. Complication rate was not reported in 2 studies [32, 33].
Discussion
Major curve Cobb angle reduction
All studies demonstrated a reduction in the mean major curve Cobb angle at a minimum 2 years of follow-up compared to the pre-operative angle. Scoliosis is a progressive condition and during the pubertal growth spurt, the curve is expected to increase [34]. The mean difference effect showed an overall reduction of 26° of the major curve Cobb angle, with clear statistical significance (p < 0.01). A decrease of this size is significant because it indicates that the major curve has been prevented from progressing, as well as the overall deformity reduction.
11 studies had patients with a major curve Cobb angle reduction to a negative value, indicating overcorrection [25,26,27,28,29, 32, 35,36,37,38,39]. Negative angles are likely to skew the mean result, causing the mean difference due to VBT surgery to be larger than what is truly accurate. Without the data detail to separate those patients, it is impossible to say to what extent the overcorrection skewed the final mean result given.
Newton et al. defined VBT success as a final thoracic major curve magnitude of < 35° and no spinal fusion indicated [26]. 9 papers used this definition or similar when defining success [28, 30,31,32, 35,36,37, 39, 40]. This meta-analysis has shown the mean main thoracic Cobb angle to be 22.2° 2 years postoperatively, which can be considered clinically successful as a PSIF is not indicated. Thoracic curves in AIS tend to rapidly progress during the first 2 years of puberty [34]. The minimum 2-year follow-up time point was chosen in the hope that these patients would have completed their rapid growth spurt and be nearing skeletal maturity. If these patients were skeletally mature, then a Cobb angle of 22° would not be expected to progress. However, there was limited evidence in the skeletal maturity status of these patients at the final follow-up. Whilst Yucekul et al. found that the median Sanders score was 7 and the median Risser grade was 5 [38], Newton et al. found that 47% of the cohort remained at Risser 0–1 at final follow-up [26]. It would be advantageous that studies have a longer follow-up, until confirmed completion of growth, to definitively state whether VBT had been successful at avoiding spinal fusion.
Negative surgical outcomes
The overall complication rate of 23% includes the most reported complications of tether breakages, overcorrection, revision, fusion and pulmonary complications. Tether breakage rate was 21.9%. As the tether is radiolucent, a break can be suspected if the angle between two adjacent screws increases by ≥ 5° between two time points [26]. The effect of a tether breakage has not been quantified, but several studies demonstrate some patients have progression of the curve after a breakage [26, 28, 36, 40, 41]. The clinical significance of the relatively high rate of tether breakages in this review is unknown and further research investigating the clinical outcomes after a breakage is required.
Whilst 21.9% of patients had a broken tether, 11.4% of patients experienced overcorrection, likely due to a greater growth potential at the time of surgery. These patients may have an undesirable outcome and a substantial deformity in the opposite direction. Further investigation correlating the demographics and surgical details between those with a broken tether and those who overcorrected may be useful to understand why these opposite consequences occur. As VBT is an emerging procedure, longer follow-up is required to see if the complication rate can be attributed to the surgical learning curve.
All patients had significant curves pre-operatively and the vast majority were destined towards a spinal fusion. After VBT, the reported rate of spinal fusion was 7%, indicating that the remaining 93% of patients were able to avoid a fusion at the minimum 2-year follow-up assessment. However, longer follow-up is essential to appreciate the true rate. Further correlation between patients that progressed and the timing of fusion would be beneficial, as slowing curve progression with VBT prior to PSIF may afford these young patients extra spinal height and avoid a more complex surgery.
There are currently two published systematic reviews on VBT [42, 43]. These papers used different inclusion and exclusion criteria to the current study. The study by Rialto et al. had a minimum follow-up period of 1 year [42], whereas the current study used 2 years as minimum follow-up to increase the likelihood of capturing changes seen within these growing patients. Bizzoca et al. included patients with lumbar only tethers [43], these patients were excluded in the current study as our aim was to directly compare thoracic VBT. Both of these systematic reviews did not include a meta-analysis, as the current study has done.
Limitations
This review is proposed as an overview of the current state of VBT although it contains several limitations that require further research. One limitation is the reporting of the final major curve Cobb angles, as some studies have excluded those patients who had curve progression and required a PSIF [32, 44]. It is unclear in the remainder of the studies how and if these patients were reported. As the SD of the mean difference in major curve Cobb angle was not given in most papers, this number was estimated using the Cochrane formula. Further research with published SD may be more accurate for meta-analysis in the future.
14 studies have a negative major curve Cobb range due to overcorrection and the effect this has on the true mean result is unknown. Further analysis separating negative from positive major curve Cobb angles would be beneficial. This review contains majority retrospective pre–post studies and no randomised controlled trials (RCTs) as none have yet been conducted and published. Further research by method of RCTs would be more accurate at examining the effect of VBTs than from pre–post studies alone.
9 studies have a subsection of patients who do not meet the selection criteria are excluded (appendix 1). However, 3 papers include patients as the data was unable to be separated—2 patients with an additional lumbar tether [28], 3 patients with concurrent lumbar stapling [25], and 10 patients who had not reached the 2-year follow-up [27]. As the additional information has not been retrieved, it is not possible to separate these patients from the data set. This review has endeavoured to present very narrow selection criteria and multiple authors have generously supplied additional details. These 15 patients represent 0.02% of the data presented, so it was deemed warranted to include them in the final analysis, despite their limitations.
Another limitation is the lack of a control group. As the inclusion and exclusion criteria for VBT surgery are for a specific subset of patients, there is no equivalent surgery currently being performed that would be suitable for objective comparison. PSIF has been proposed as a comparison for VBT [45]. However, we maintain VBT is beneficial for a different cohort of AIS patients than PSIF. VBT is indicated for young patients that have large curves, not suitable for bracing, yet remain too skeletally immature for fusion surgery. These patients have a spinal deformity that will continue to progress requiring complex fusion surgery by the time their growth rate has slowed to a point whereby PSIF is permissible. Complete replacement of PSIF with VBT is not expected, but rather VBT exists as an option for certain patients who meet the strict criteria for VBT. Therefore, we conclude it would not be appropriate to compare PSIF with VBT surgery directly as the patient demographics for the two types of operation are very different.
The authors acknowledge the multiple biases that can be inherent in a purely bibliographic review. However, a carefully performed systematic review and meta-analysis that strictly follows the PRISMA guidelines can provide important insights into the effectiveness and safety of this procedure in the population for which it is indicated. Attempted mitigation of potential biases has been performed through our comprehensive search strategy, and our strict inclusion criteria.
Implications for future practice
The results of this study demonstrate that VBT is effective at reducing and holding the scoliotic curve until 2 years of post-operative. Due to the nature of growing adolescents, studies examining the curves with longer follow-up are essential to determine the longitudinal effectiveness of this procedure into skeletal maturity. As the clinical effect from broken tethers has not yet been quantified, further studies that evaluate the change in major curve Cobb angle after a broken tether would also be beneficial. This will determine if a breakage is a complication resulting in continued curve progression, or if perhaps a tether breakage could be advantageous by contributing to the prevention of overcorrection. Further research into the timing of the procedure is necessary within a child’s growth, so that optimal reduction can be seen without overcorrection.
Further research is required to confirm the long-term effects of VBT, but with current knowledge, VBT remains an effective method to reduce the curve and prevent PSIF in skeletally immature patients with AIS.
Data availability
This study was registered with the Open Science Framework (URL https://osf.io/kx987/) and the review protocol can be accessed there. There were nil amendments to the registered protocol.
References
Sevastik JA, Diab KM (1997) Research into spinal deformities 1. IOS Press, Amsterdam
Choudhry MN, Ahmad Z, Verma R (2016) Adolescent idiopathic scoliosis. Open Orthop J 10:143–154
Konieczny MR, Senyurt H, Krauspe R (2013) Epidemiology of adolescent idiopathic scoliosis. J Child Orthop 7(1):3–9
Weinstein SL, Dolan LA, Spratt KF, Peterson KK, Spoonamore MJ, Ponseti IV (2003) Health and function of patients with untreated idiopathic scoliosis. J Am Med Assoc 289:559–567
Charles YP, Daures JP, de Rosa V, Diméglio A (2006) Progression risk of idiopathic juvenile scoliosis during pubertal growth. Spine 31(17):1933–1942
Sanders JO, Khoury JG, Kishan S, Browne RH, Mooney JF 3rd, Arnold KD, McConnell SJ, Bauman JA, Finegold DN (2008) Predicting scoliosis progression from skeletal maturity: a simplified classification during adolescence. J Bone Joint Surg Am 90(3):540–553. https://doi.org/10.2106/JBJS.G.00004
Weinstein SL, Dolan LA, Wright JG, Dobbs MB (2013) Effects of bracing in adolescents with idiopathic scoliosis. N Engl J Med 369(16):1512–1521
Hariharan AR, Shah SA, Petfield J, Baldwin M, Yaszay B, Newton PO et al (2022) Complications following surgical treatment of adolescent idiopathic scoliosis: a 10-year prospective follow-up study. Spine Deform. https://doi.org/10.1007/s43390-022-00508-6
Karol LA (2011) Early definitive spinal fusion in young children: what we have learned. Clin Orthop Relat Res 469(5):1323–1329
Yazici M, Olgun ZD (2013) Growing rod concepts: state of the art. Eur Spine J 22(Suppl 2):S118-130
Cahill PJ, Marvil S, Cuddihy L, Schutt C, Idema J, Clements DH et al (2010) Autofusion in the immature spine treated with growing rods. Spine 35(22):E1199–E1203
Shen TS, Schairer W, Widmann R (2019) In patients with early-onset scoliosis, can growing rods be removed without further instrumentation? An evidenced-based review. Hss J 15(2):201–204
Watanabe K, Uno K, Suzuki T, Kawakami N, Tsuji T, Yanagida H et al (2013) Risk factors for complications associated with growing-rod surgery for early-onset scoliosis. Spine 38(8):E464–E468
U. S. Food and Drug Administration (2019) Humanitarian Device Exemption (HDE). The Tether™ - Vertebral Body Tethering System H190005. Available at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfhde/hde.cfm?id=H190005
Hoh DJ, Elder JB, Wang MY (2008) Principles of growth modulation in the treatment of scoliotic deformities. Neurosurgery 63(3):A211–A221
Crawford CH 3rd, Lenke LG (2010) Growth modulation by means of anterior tethering resulting in progressive correction of juvenile idiopathic scoliosis: a case report. J Bone Joint Surg Am 92(1):202–209
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. https://doi.org/10.1136/bmj.n71
Effective Practice and Organisation of Care (EPOC) (2013) Data collection form. EPOC Resources for review authors. Norwegian Knowledge Centre for the Health Services, Oslo. Available at: http://epoc.cochrane.org/epoc-specific-resources-review-authors
Cobb JR (1948) Outlines for the study of scoliosis. Am Acad Orthop Surg Instr Course Lect 5:261–275
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (eds) (2022) Cochrane handbook for systematic reviews of interventions version 6.3 (updated February 2022). Cochrane. Available at: https://www.training.cochrane.org/handbook
National Institutes of Health (2021) Study quality assessment tools 2014. https://www.nhlbi.nih.gov/health‐topics/study‐quality‐assessment‐tools
Boudissa M, Eid A, Bourgeois E, Griffet J, Courvoisier A (2017) Early outcomes of spinal growth tethering for idiopathic scoliosis with a novel device: a prospective study with 2 years of follow-up. Childs Nerv Syst 33(5):813–818
Samdani AF, Ames RJ, Kimball JS, Pahys JM, Grewal H, Pelletier GJ et al (2014) Anterior vertebral body tethering for idiopathic scoliosis: two-year results. Spine 39(20):1688–1693
Newton PO, Kluck DG, Saito W, Yaszay B, Bartley CE, Bastrom TP (2018) Anterior spinal growth tethering for skeletally immature patients with scoliosis: a retrospective look two to four years postoperatively. J Bone Joint Surg Am 100(19):1691–1697
Alanay A, Yucekul A, Abul K, Ergene G, Senay S, Ay B et al (2020) Thoracoscopic vertebral body tethering for adolescent idiopathic scoliosis: follow-up curve behavior according to sanders skeletal maturity staging. Spine 45(22):E1483–E1492
Miyanji F, Pawelek J, Nasto LA, Simmonds A, Parent S (2020) Safety and efficacy of anterior vertebral body tethering in the treatment of idiopathic scoliosis; a multicentre review of 57 consecutive patients. Bone Joint J 102(12):1703–1708
Abdullah A, Parent S, Miyanji F, Smit K, Murphy J, Skaggs D et al (2021) Risk of early complication following anterior vertebral body tethering for idiopathic scoliosis. Spine Deform 9(5):1419–1431
Baker CE, Kiebzak GM, Neal KM (2021) Anterior vertebral body tethering shows mixed results at 2-year follow-up. Spine Deform 9(2):481–489
Samdani AF, Pahys JM, Ames RJ, Grewal H, Pelletier GJ, Hwang SW et al (2021) Prospective follow-up report on anterior vertebral body tethering for idiopathic scoliosis: interim results from an FDA IDE study. J Bone Joint Surg Am 103(17):1611–1619
McDonald TC, Shah SA, Hargiss JB, Varghese J, Boeyer ME, Pompliano M et al (2022) When successful, anterior vertebral body tethering (VBT) induces differential segmental growth of vertebrae: an in vivo study of 51 patients and 764 vertebrae. Spine Deform. https://doi.org/10.1007/s43390-022-00471-2
Hoernschemeyer DG, Boeyer ME, Tweedy NM, Worley JR, Crim JR (2021) A preliminary assessment of intervertebral disc health and pathoanatomy changes observed two years following anterior vertebral body tethering. Eur Spine J 30(12):3442–3449
Dimeglio A, Canavese F (2013) Progression or not progression? How to deal with adolescent idiopathic scoliosis during puberty. J Child Orthop 7(1):43–49
Wong H-K, Ruiz JNM, Newton PO, Liu GK-P (2019) Non-fusion surgical correction of thoracic idiopathic scoliosis using a novel, braided vertebral body tethering device: minimum follow-up of 4 years. JBJS Open Access 4(4):e0026
Newton PO, Bartley CE, Bastrom TP, Kluck DG, Saito W, Yaszay B (2020) Anterior spinal growth modulation in skeletally immature patients with idiopathic scoliosis: a comparison with posterior spinal fusion at 2 to 5 years postoperatively. J Bone Joint Surg Am 102(9):769–777
Rushton PRP, Nasto L, Parent S, Turgeon I, Aldebeyan S, Miyanji F (2021) Anterior vertebral body tethering for treatment of idiopathic scoliosis in the skeletally immature: results of 112 cases. Spine 46(21):1461–1467
Yucekul A, Akpunarli B, Durbas A, Zulemyan T, Havlucu I, Ergene G et al (2021) Does vertebral body tethering cause disc and facet joint degeneration? A preliminary MRI study with minimum two years follow-up. Spine J 21(11):1793–1801
Bernard J, Bishop T, Herzog J, Haleem S, Lupu C, Ajayi B et al (2022) Dual modality of vertebral body tethering : anterior scoliosis correction versus growth modulation with mean follow-up of five years. Bone Jt Open 3(2):123–129
Hoernschemeyer DG, Boeyer ME, Robertson ME, Loftis CM, Worley JR, Tweedy NM et al (2020) Anterior vertebral body tethering for adolescent scoliosis with growth remaining: a retrospective review of 2 to 5-year postoperative results. J Bone Joint Surg Am 102(13):1169–1176
Pehlivanoglu T, Oltulu I, Ofluoglu E, Sarioglu E, Altun G, Korkmaz M et al (2020) Thoracoscopic vertebral body tethering for adolescent idiopathic scoliosis : a minimum of 2 years’ results of 21 patients. J Pediatr Orthop 40(10):575–580
Raitio A, Syvänen J, Helenius I (2022) Vertebral body tethering: indications, surgical technique, and a systematic review of published results. J Clin Med 11(9):2576. https://doi.org/10.3390/jcm11092576.PMID:35566702;PMCID:PMC9099651
Bizzoca D, Piazzolla A, Moretti L, Vicenti G, Moretti B, Solarino G (2022) Anterior vertebral body tethering for idiopathic scoliosis in growing children: a systematic review. World J Orthop 13(5):481–493
Miyanji F, Fields MW, Murphy J, Matsumoto H, Fano AN, Roye BD et al (2021) Shoulder balance in patients with Lenke type 1 and 2 idiopathic scoliosis appears satisfactory at 2 years following anterior vertebral body tethering of the spine. Spine Deform 9(6):1591–1599
Mathew SE, Hargiss JB, Milbrandt TA, Stans AA, Shaughnessy WJ, Larson AN (2022) Vertebral body tethering compared to posterior spinal fusion for skeletally immature adolescent idiopathic scoliosis patients: preliminary results from a matched case-control study. Spine Deform 10(5):1123–1131. https://doi.org/10.1007/s43390-022-00519-3. (Epub 2022 May 24 PMID: 35610543)
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
MR: study conception and design, data collection and analysis, developed methodology, original draft manuscript preparation, editing and final approval of the manuscript. GA: study conception, editing and approval of the final manuscript. Agrees to be accountable for the work. RL: study conception, editing and approval of the final manuscript. SFZ: data analysis and approval of the final manuscript. MI: study conception and design, data collection, editing and approval of the final manuscript. JPL: study conception and design, data analysis, editing and approval of the final manuscript. Material preparation, data collection and analysis were performed by MR and MI. Statistics were performed by MR and SFZ. The first draft of the manuscript was written by MR and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no financial interests. Dr Roser’s Masters of Philosophy is sponsored by Queensland X-ray.
Ethical approval
Ethics approval for Spinal Deformity Management and Clinical Data Collection Project was granted from the Children’s Health Queensland HREC approval number: LNR/21/QCHQ/75249 QUT HREC approval number: QUT/4856.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Roser, M.J., Askin, G.N., Labrom, R.D. et al. Vertebral body tethering for idiopathic scoliosis: a systematic review and meta-analysis. Spine Deform 11, 1297–1307 (2023). https://doi.org/10.1007/s43390-023-00723-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43390-023-00723-9