Abstract
Background and context
In patients with adolescent idiopathic scoliosis (AIS) of main thoracic and lumbar spine regions, combined anterior thoracic vertebral body tethering and posterior lumbar spine tethering (ATVBT/PLST) is a novel non-fusion treatment option for growth modulation and conservation of motion.
Methods
Fourteen patients with AIS who underwent ATVBT/PLST with at least 2-year follow-up were included. Primary outcomes included quality of life as assessed by SRS-22 instruments, radiographic analysis, and revision operations. We secondarily reported perioperative metrics and post-operative opiate morphine equivalents (OME). Clinical success was defined as patients who achieved skeletal maturity with ≤ 30° curve magnitude of both their main thoracic and thoracolumbar/lumbar curves and who did not undergo posterior spine instrumentation and fusion (PSIF).
Results
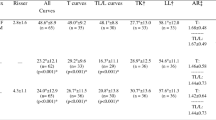
Patients had a mean age of 11.6 years (range 10–14 years), majority were girls (92%), and mean follow-up was 3.0 years (range 2–4.8 years). All patients were skeletally immature with a Risser ≤ 2. Included curves were Lenke 1C, 3C, or 6C. Mean preoperative curve magnitudes were 53° ± 8° (range 45°–65°) main thoracic and 49° ± 9° (range 40°–62°) thoracolumbar/lumbar curves. At most recent follow-up, patients had a mean main thoracic curve of 29° ± 8° (range 15°–40°) and a mean thoracolumbar/lumbar curve of 20° ± 15° (range 4°–35°). 50% required a revision operation. Cable breakage occurred in 43%, which did not always require revision. One patient progressed to thoracic fusion, but no patient underwent lumbar fusion. Patients had a mean SRS-22 outcome score of 4.2 ± 0.4.
Conclusions
ATVBT/PLST is a potential alternative to spine fusion for select immature patients with AIS at a minimum 2-year follow-up. ATVBT/PLST potentially offers motion conservation at the cost of a higher revision rate. Further study and reporting of results are necessary to refine indications and techniques, which in turn will improve outcomes of this procedure.
Level of evidence
Level IV—Case series without comparative group.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The standard surgical treatment for AIS is posterior spine instrumentation and fusion (PSIF). Recently, spine tethering has become a treatment option for a select cohort of skeletally immature patients, to conserve spine motion [1]. Although PSIF is effective, it eliminates motion and growth [2,3,4,5,6]. Spine tethering is a non-fusion approach that conserves motion and may guide growth to correct deformity in the child [1, 2, 7,8,9,10]. AIS patients undergoing thoracic PSIF with an additional thoracolumbar/lumbar structural curve or skeletally immature patients who are at risk for progression or junctional phenomena may require extension into the lumbar spine [11,12,13,14]. Tethering candidates are at risk for lumbar decompensation or developing a future lumbar curve due to significant growth remaining [11,12,13, 15,16,17,18]. Posterior lumbar spine tethering (PLST) conserves mobility and has the potential to allow for growth modulation compared with PSIF [2, 19,20,21,22]. PLST conserves spine motion and has the potential to allow for growth modulation compared with PSIF. It has the potential to improve the sagittal plane by restoring lordosis through compression by tensioning the cable between screws, which effect would be amplified by concave growth. By comparison, in vertebral body tethering (VBT), convex compression with concave growth is advantageous in the thoracic spine by being kyphosing but potentially disadvantageous in the lumbar spine. PLST also is lower morbidity compared with fusion [23,24,25].
We report clinical and radiographic outcomes of a novel treatment approach for skeletally immature patients with thoracic and lumbar scoliosis, treated by combined anterior thoracic vertebral body tethering and posterior lumbar spine tethering (ATVBT/PLST).
Materials and methods
Following approval from the institutional review board (IRB), we conducted a single-center and single-surgeon retrospective cohort study of skeletally immature patients with idiopathic scoliosis who underwent ATVBT/PLST. Inclusion criteria were skeletally immature patients (Risser ≤ 2), main thoracic curve and lumbar curve ≥ 40°, combined ATVBT/PLST and a minimum of 2-year follow-up. Patients with Lenke 1C lumbar curves ≥ 40° standing but ≤ 25° bending were instrumented with posterior lumbar tethering if there was concern for distal adding on phenomenon [11,12,13,14]. Seventeen patients were available, of which 14 met inclusion criteria (Fig. 1). Patients with prior spine surgery, neuromuscular or syndromic scoliosis, history of infection, tumor or trauma were excluded.
Patient demographics included age at index operation, sex, menarchal status (for female patients), height, weight, BMI, curve magnitudes, Risser staging, triradiate cartilage, and Lenke classification.
Perioperative data included levels instrumented with anterior and posterior tether, operative complications, American Society of Anesthesiology (ASA) classification, case duration, estimated blood loss (EBL), removal of Foley catheter, days to solid food, length of stay. Perioperative opiate morphine equivalent (OME) use was recorded and converted to mg/kg to account for differences in weight.
Post-operative assessment included revision procedure and 30-day readmission. Functional outcomes were assessed with the Scoliosis Research Society 22-item Questionnaire (SRS-22).
Radiographic data were recorded preoperatively, as well as at 1st-, 2-year, and most recent post-operative visits. We measured curve magnitude by the standard method of Cobb at all time points, including in the event of cable breakage. Other radiographic measurements were thoracic kyphosis (sagittal view, T5–T12), lumbar lordosis (sagittal view, L1–S1), coronal imbalance (C7–CSVL), and shoulder height differences. For ATVBT/PLST patients who progressed to fusion, measurement data were not included in comparative analysis. To evaluate for cable breakage, we measured curve magnitude and inter-screw distance. We assumed cable breakage if there was progression as > 5° for an instrumented curve, consistent with spine deformity literature, and additional separation of screw heads > 2 mm compared with immediate surgical result.
Primary outcomes included quality of life as assessed by SRS-22 instruments, radiographic analysis and revision operations. Secondary outcomes included perioperative metrics and total post-operative OME use. Consistent with previous literature, clinical success was defined as patients who achieved skeletal maturity and ≤ 30° magnitude of both the main thoracic and lumbar curves at most recent post-operative visit [4]. Subsequent PSIF was considered a clinical failure.
Statistical analysis was performed in R (The R Foundation) v4.0.2 and RStudio v.1.3.1093. We expressed continuous data as mean ± standard deviation. Dependent t-tests were utilized to compare radiographic data over time. Dichotomous variables and categorical variables are expressed as values with percentages. The threshold of statistical significance was set at α = 0.05.
Surgical technique
The senior author performed all ATVBT/PLST procedures at a single institution (Fig. 2). Selection of curves was based on radiographic magnitude and patient immaturity. Standard Stryker® multaxial pedicle screws and rods were used for fusion, and Zimmer Biomet tethering system were used for both anterior and posterior tethering. Upper (UIV) and lower (LIV) instrumented vertebrae were selected as stable in coronal and sagittal planes based upon criteria established for spine fusion [26].
Patient with Lenke 3C classification with pre-operative 61o main thoracic curve and 47o lumbar curve (left images). Patient had an anterior tether from T5 to T11 and posterior tether from T12 to L4. At final post-op (right images), both curves have remained stable, and the patient is considered a clinical success
For ATVBT, an open muscle sparing approach was used on the side of curve convexity. This approach has been described in a previous study [27]. For posterior lumbar tethering, a Wiltse approach was used toward the curve convexity [28, 29]. Facet joints were spared in the dissection. Pedicle screws were started at the base of transverse process for a far lateral to medial trajectory, to avoid violating the facet joint and to keep screw heads away from this motion segment. The cable was attached by set screws, tightening sequentially to partially correct the deformity, to allow for future growth to continue correction and to guard against overcorrection.
Post-operative management included admission for pain control and mobilization, as well as a brace for 3 months to maximize the effect of the hydroxyapatite coating of the tethering screws.
Results
Patient demographics
Fourteen patients with AIS who underwent ATVBT/PLST were included in this study. Patient characteristics are summarized in Table 1. Mean age was 11.6 ± 1.7 years (range 10–14 years), 93% were girls, and mean follow-up time was 3.0 years (range 2–4.8 years). Mean preoperative height was 154.6 cm and 162.3 cm at most recent follow-up. Preoperative Risser staging distribution for 0/1/2/3/4/5 was 72%/ 14%/ 14%/ 0% / 0%/ 0% and post-operatively was 0%/ 7%/ 0%/ 7%/ 50%/ 36%. Preoperatively, 64% patients had an open triradiate cartilage and 7% post-operatively. Lenke classifications among patients were 1C (28%)/3C (43%)/6C (29%).
Comparison of spine curvatures, coronal imbalance, and shoulder height differences in the ATVBT/PLST group
Cobb angles (main thoracic and thoracolumbar/lumbar) were recorded preoperatively and compared at 1st-, 2-year and most recent post-operative visits (Table 2). Main thoracic curve magnitude decreased significantly from mean 52° ± 8° (range 45°–65°) preoperatively to 25 ± 5° (range 13°–33°) at 1st post-operative visit, representing a 51% correction, 25° ± 14° (range 8°–30°) at 2-year post-operative visit (p < 0.001) and 29° ± 8° (range 15°–40°) at most recent post-operative visit (p < 0.001), representing a 43% correction. Similarly, the thoracolumbar/lumbar curve decreased from an average 49° ± 9° (range 40°–62°) preoperatively to 20° ± 10° (range 4°–30°) at 1st post-operative visit, representing a 59% correction, 15° ± 9° (range 5°–25°) at 2-year post-operative visit (p < 0.001) and 20° ± 15° (range 4°–35°) at most recent post-operative visit (p < 0.001), representing a 60% correction. There were no statistically significant changes to kyphotic angle. Lordotic curvature remained stable across preoperative, as well as 1st-, 2-year and most recent post-operative visits (p > 0.05).
We compared coronal imbalance and shoulder height differences between preoperative and all post-operative visits. Coronal imbalance did not show significant differences (p > 0.05). Shoulder height significantly increased from average preoperative height of 0.7 ± 0.6 cm to 1.1 ± 0.7 cm at 2 years (p = 0.03) but was not found to be significant at most recent follow-up.
Perioperative metrics following ATVBT/PLST
Perioperative metrics were recorded following ATVBT/PLST (Table 3). Mean number of levels tethered was 11.8 with an average of 7.2 for anterior tether and 5.1 for posterior tether. For ATVBT, T5 was the upper instrumented vertebrae (UIV) in 8/14 (57%) patients and the lowest instrumented vertebrae (LIV) was T12 in 8/14 (57%) patients. In PLST, the UIV was T12 in 8/14 (57%) and L4 was the LIV in 8/12 (67%) of patients. There were no intraoperative complications and no 30-day readmissions.
Mean case length was 418.9 min and estimated blood loss was 173 mL. No patient required a blood transfusion. Mean Foley catheter duration was 57 h and days to solid food was 1.1. Mean length of stay (LOS) was 4.1 days.
During the intraoperative and post-operative period, opioid morphine equivalent (OME) was measured and recorded. Mean OME use intraoperatively was 7.2 mg/kg and post-operatively, patients required an average of 4.3 mg/kg. Two (14%) patients had an epidural infusion post-operatively and nine (64%) patients used gabapentin on post-operative day 0.
Revisions, complications, and clinical outcomes of ATVBT/PLST
Revisions, complications, and clinical outcomes for patients who underwent ATVBT/PLST are summarized in Table 4. Revision surgery occurred in 7/14 (50%) and cable breakage occurred in 6/14 (43%) of patients. Patient 1 is currently considering revision fusion after undergoing 2 revision procedures for cable breakage and lumbar overcorrection. Patient 2 underwent cable replacement and extension 2.1 years after initial surgery due to cable breakage. Patients 4 and 6 had revision surgeries due to lumbar overcorrection but have now been considered clinical successes with main thoracic and lumbar curves ≤ 30°. Patient 5 underwent a minor revision due to an unstable L4 set screw. Patients 7 (skeletally immature) and 9 (skeletally mature) underwent thoracic PSIF for thoracic curve progression, but the lumbar curves have remained stable with the original posterior tether. No patients treated with ATVBT/PLST have undergone or have indication for lumbar fusion.
Clinical success
At most recent follow-up, 12 patients achieved skeletal maturity as defined by Risser stage 4/5 and closed triradiate cartilage. Among these patients, one underwent PSIF. Among the 11 patients who did not undergo PSIF, 10 patients (91%) achieved ≤ 30° correction of their lumbar curve with posterior tethering and 7 (64%) of these patients have also achieved ≤ 30° correction of their main thoracic curve with anterior tethering. Therefore, among the patients who did not require PSIF and were Risser 4 or 5 at most recent follow-up, 7/11 (64%) achieved clinical success with ≤ 30 correction of both their main thoracic and lumbar curves.
Patient-reported outcomes
SRS-22r data are summarized in Table 5. SRS-22r was available at most recent follow-up for 13 of 14 patients. One patient did not participate because of a cancer diagnosis and treatment. Mean total score following ATVBT/PLST was 4.2 ± 0.4.
Discussion
This is the first study to describe a combined anterior and posterior tethering approach for skeletally immature patients with AIS. We included 14 skeletally immature patients who underwent ATVBT/PLST and had a minimum 2-year follow-up.
Patients undergoing ATVBT/PLST achieved 51% main thoracic and 59% thoracolumbar/lumbar percent curve corrections at 1st post-operative visit and maintained this correction at the most recent post-operative visit with an average 43% main thoracic and 60% thoracolumbar/lumbar curve reduction. Mean curve correction is consistent with successful spine tethering reported by other studies [2, 4, 8, 9]. Additionally, ATVBT/PLST patients had a mean SRS-22 score of 4.2 which also is consistent with prior spine tethering and PSIF reports [2, 8].
As a principle, it is important to report all results, including if success rate is low or even in the event that results are negative. In our ATVBT/PLST group, 43% had cable breakage at an average of 2.7 years (2–3.5 years). Cable breakage was similar in both the main thoracic and lumbar curves. In the 23 cases reported by Newton et al., 44% had cable breakage, while Hoerneschmeyer et al. reported a rate of 54% at a minimum of 2 years after operation [8, 9]. A more recent study of single and double anterior lumbar tethers reported a cable breakage rate of approximately 73% [30]. It has become clear that the polyethylene terephthalate cable is the Achilles heel of spine tethering, given the rates of breakage [8]. Although 43% of patients in our cohort had cable breakage, it is important to recognize that some patients do not require revision surgery following cable breakage—as seen in four of our patients—because curve progression is not significant at the level(s) of breakage.
Newton et al. reported a revision rate of 30% in 23 patients, three of which were PSIF due to curve progression [8]. In the 29-case cohort reported by Hoerneschmeyer et al., rate of revision was lower at 21% with two patients progressing to PSIF [9]. The most common reasons for revision in our cohort were lumbar overcorrection, showing the concept validity and potency of the procedure in the lumbar spine. Previous literature by Miyanji et al. attributed lumbar overcorrection to patients with an open triradiate cartilage [7]. All of our patients who had lumbar overcorrection had an open triradiate cartilage [7].
An up to 50% revision rate means that half of patients will benefit from the procedure. For a novel procedure, there is value in recognizing the success rate at all time points in the procedure’s lifespan. This will aid surgeons in refining indications and techniques, which in turn will raise the success rate. Furthermore, if a procedure is low morbidity with potentially high reward, there can be a greater tolerance of failure. PLST is low morbidity as the approach is after Wiltse [28, 29], so blood loss and tissue dissection are minimal,as opposed to a retroperitoneal approach with or without a thoracotomy and diaphragmatic takedown for anterior tethering. Compared with fusion, there is no joint excision, no decortication, half the implants, less blood loss, and no graft agents. However, we realize we do not have data in this study to support these claims. Perhaps, the best statement to the lower morbidity is that we have performed isolated PLST when indicated as an outpatient procedure. Furthermore, there is significant value to conservation of spine motion, as opposed to its elimination [19,20,21,22].
Defining clinical success for our cohort of patients was based upon skeletal maturity, ≤ 30° residual magnitude of thoracic and lumbar curves, and avoidance of PSIF. In our cohort, we defined Risser ≥ 4 patients as skeletally mature. Progression after Risser 4 is critical in decision-making for braced curves. At our institution, we have not seen growth modulation, relevant to tethered curves, after Risser 4.
We have followed the early literature’s definition of success for spine tethering, namely, a mobile spine with up to 30° of deformity [31,32,33]. If a tether is a temporary intervention to conserve spine motion for a child during growth, while keeping the curve < 50°, then leaving the child with a curve of 30° is a success, as it would be for a brace. If a tether has to support the spine regardless of maturity, then barring cable breakage there will be no progression and no concern for worsening deformity into adulthood. With this in mind, 8/12 (67%) skeletally mature patients avoided fusion and were considered clinical successes with ≤ 30° magnitude of both main thoracic and lumbar curves at a minimum 2-year follow-up. One skeletally immature patient underwent thoracic fusion 12 months after the index operation. One skeletally mature patient underwent thoracic fusion 3.3 years following index operation. No patient has received or has indication for fusion of the lumbar spine.
Our mean EBL (mean 173 mL) is consistent with published literature for VBT [7, 8, 34, 35]. A functional assessment of blood loss is that none of our spine tethering patients has required a transfusion. Furthermore, no spine tethering patient has had an infection, screw failure or neural signal change, supporting that from a perioperative perspective the procedure is a safe alternative to fusion.
This study has limitations. Given the novelty of the procedure, our study is limited by sample size and retrospective design. While we have a minimum 2-year follow-up on all patients, we recognize that medium- and long-term follow-ups are essential to understanding a condition and a procedure. Such follow-up should be through maturity—which may be defined by Risser 5, by the distal physes of ulna and radius, or other radiographic method (e.g., of Sanders)—when growth can deform the spine and thereby challenge any system that would resist such deformation. Long-term follow-up will give experience and perspective that can aid comparison with other methods of treatment. Long-term follow-up also will expose unanticipated consequences (such as the health of squeezed and partially immobilized intervertebral discs), bring focus to technical issues such as cable failure, and determine whether the intervention is temporary during growth (like a brace—in which case cable breakage may not matter and may even be an advantage) or must be durable as long as possible.
In conclusion, ATVBT/PLST is a potential alternative to spine fusion for select immature patients with AIS at a minimum 2-year follow-up. ATVBT/PLST potentially offers motion conservation at the cost of a higher revision rate. As we and others continue to study and report results, indications and techniques will be refined, which in turn will improve outcomes of this procedure.
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.
References
Newton PO (2020) Spinal growth tethering: indications and limits. Ann Transl Med 8:27–27. https://doi.org/10.21037/atm.2019.12.159
Pehlivanoglu T, Oltulu I, Erdag Y, Akturk UD, Korkmaz E, Yildirim E, Sarioglu E, Ofluoglu E, Aydogan M (2021) Comparison of clinical and functional outcomes of vertebral body tethering to posterior spinal fusion in patients with adolescent idiopathic scoliosis and evaluation of quality of life: preliminary results. Spine Deform 9:1175–1182. https://doi.org/10.1007/s43390-021-00323-5
Mehkri Y, Hernandez J, McQuerry JL, Carmona J, Ihnow S (2021) Global spine range of motion in patients with adolescent idiopathic scoliosis before and after corrective surgery. Cureus 13:e19362. https://doi.org/10.7759/cureus.19362
Newton PO, Kluck DG, Saito W, Yaszay B, Bartley CE, Bastrom TP (2018) Anterior spinal growth tethering for skeletally immature patients with scoliosis: a retrospective look two to four years postoperatively. J Bone Jt Surg 100:1691–1697. https://doi.org/10.2106/JBJS.18.00287
Eyvazov K, Samartzis D, Cheung JPY (2017) The association of lumbar curve magnitude and spinal range of motion in adolescent idiopathic scoliosis: a cross-sectional study. BMC Musculoskelet Disord 18:51. https://doi.org/10.1186/s12891-017-1423-6
Helenius I, Remes V, Yrjönen T, Ylikoski M, Schlenzka D, Helenius M, Poussa M (2002) Comparison of long-term functional and radiologic outcomes after Harrington instrumentation and spondylodesis in adolescent idiopathic scoliosis: a review of 78 patients. Spine 27:176–180. https://doi.org/10.1097/00007632-200201150-00010
Miyanji F, Pawelek J, Nasto LA, Rushton P, Simmonds A, Parent S (2020) Safety and efficacy of anterior vertebral body tethering in the treatment of idiopathic scoliosis: a multicentre review of 57 consecutive patients. Bone Jt J 102-B:1703–1708. https://doi.org/10.1302/0301-620X.102B12.BJJ-2020-0426.R1
Newton PO, Bartley CE, Bastrom TP, Kluck DG, Saito W, Yaszay B (2020) Anterior spinal growth modulation in skeletally immature patients with idiopathic scoliosis: a comparison with posterior spinal fusion at 2 to 5 years postoperatively. J Bone Jt Surg 102:769–777. https://doi.org/10.2106/JBJS.19.01176
Hoernschemeyer DG, Boeyer ME, Robertson ME, Loftis CM, Worley JR, Tweedy NM, Gupta SU, Duren DL, Holzhauser CM, Ramachandran VM (2020) Anterior vertebral body tethering for adolescent scoliosis with growth remaining: a retrospective review of 2 to 5-year postoperative results. J Bone Jt Surg 102:1169–1176. https://doi.org/10.2106/JBJS.19.00980
Baker CE, Kiebzak GM, Neal KM (2021) Anterior vertebral body tethering shows mixed results at 2-year follow-up. Spine Deform 9:481–489. https://doi.org/10.1007/s43390-020-00226-x
Qin X, Xia C, Xu L, Sheng F, Yan H, Qiu Y, Zhu Z (2018) Natural history of postoperative adding-on in adolescent idiopathic scoliosis: what are the risk factors for progressive adding-on? BioMed Res Int 2018:1–8. https://doi.org/10.1155/2018/3247010
Matsumoto M, Watanabe K, Hosogane N, Kawakami N, Tsuji T, Uno K, Suzuki T, Ito M, Yanagida H, Yamaguchi T, Minami S, Akazawa T (2013) Postoperative distal adding-on and related factors in lenke type 1A curve. Spine 38:737–744. https://doi.org/10.1097/BRS.0b013e318279b666
Wang Y, Hansen ES, Høy K, Wu C, Bünger CE (2011) Distal adding-on phenomenon in Lenke 1A scoliosis: risk factor identification and treatment strategy comparison. Spine 36:1113–1122. https://doi.org/10.1097/BRS.0b013e3181f51e95
Fujii T, Daimon K, Fujita N, Yagi M, Michikawa T, Hosogane N, Nagoshi N, Tsuji O, Kaneko S, Tsuji T, Nakamura M, Matsumoto M, Watanabe K (2020) Risk factors for postoperative distal adding-on in Lenke type 1B and 1C and its influence on residual lumbar curve. J Pediatr Orthop 40:e77–e83. https://doi.org/10.1097/BPO.0000000000001399
Hefti F, McMaster M (1983) The effect of the adolescent growth spurt on early posterior spinal fusion in infantile and juvenile idiopathic scoliosis. J Bone Jt Surg Br 65-B:247–254. https://doi.org/10.1302/0301-620X.65B3.6841390
Lykissas MG, Jain VV, Nathan ST, Pawar V, Eismann EA, Sturm PF, Crawford AH (2013) Mid- to long-term outcomes in adolescent idiopathic scoliosis after instrumented posterior spinal fusion: a meta-analysis. Spine 38:E113–E119. https://doi.org/10.1097/BRS.0b013e31827ae3d0
Fischer CR, Kim Y (2011) Selective fusion for adolescent idiopathic scoliosis: a review of current operative strategy. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc 20:1048–1057. https://doi.org/10.1007/s00586-011-1730-9
Lenke LG, Betz RR, Bridwell KH, Harms J, Clements DH, Lowe TG (1999) Spontaneous lumbar curve coronal correction after selective anterior or posterior thoracic fusion in adolescent idiopathic scoliosis. Spine 24:1663. https://doi.org/10.1097/00007632-199908150-00007
Nicolini LF, Kobbe P, Seggewiß J, Greven J, Ribeiro M, Beckmann A, Da Paz S, Eschweiler J, Prescher A, Markert B, Stoffel M, Hildebrand F, Trobisch PD (2022) Motion preservation surgery for scoliosis with a vertebral body tethering system: a biomechanical study. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc 31:1013–1021. https://doi.org/10.1007/s00586-021-07035-4
Trobisch P, Mahoney JM, Eichenlaub EK, Antonacci CL, Cuddihy L, Amin DB, Razo-Castaneda D, Orbach MR, McGuckin JP, Bucklen BS, Antonacci MD, Betz RR (2023) An investigation of range of motion preservation in fusionless anterior double screw and cord constructs for scoliosis correction. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. https://doi.org/10.1007/s00586-023-07608-5
Mathew SE, Milbrandt TA, Larson AN (2022) Measurable lumbar motion remains 1 year after vertebral body tethering. J Pediatr Orthop 42:e861–e867. https://doi.org/10.1097/BPO.0000000000002202
Buyuk AF, Milbrandt TA, Mathew SE, Larson AN (2021) Measurable thoracic motion remains at 1 year following anterior vertebral body tethering, with sagittal motion greater than coronal motion. J Bone Jt Surg Am 103:2299–2305. https://doi.org/10.2106/JBJS.20.01533
Carreon LY, Puno RM, Lenke LG, Richards BS, Sucato DJ, Emans JB, Erickson MA (2007) Non-neurologic complications following surgery for adolescent idiopathic scoliosis. J Bone Jt Surg-Am 89:2427–2432. https://doi.org/10.2106/00004623-200711000-00013
Kwan MK, Loh KW, Chung WH, Chiu CK, Hasan MS, Chan CYW (2021) Perioperative outcome and complications following single-staged Posterior Spinal Fusion (PSF) using pedicle screw instrumentation in Adolescent Idiopathic Scoliosis (AIS): a review of 1057 cases from a single centre. BMC Musculoskelet Disord 22:413. https://doi.org/10.1186/s12891-021-04225-5
Reames DL, Smith JS, Fu K-MG, Polly DW, Ames CP, Berven SH, Perra JH, Glassman SD, McCarthy RE, Knapp RD, Heary R, Shaffrey CI (2011) Complications in the surgical treatment of 19,360 cases of pediatric scoliosis: a review of the scoliosis research society morbidity and mortality database. Spine 36:1484–1491. https://doi.org/10.1097/BRS.0b013e3181f3a326
Beauchamp EC, Lenke LG, Cerpa M, Newton PO, Kelly MP, Blanke KM, Harms Study Group Investigators (2020) Selecting the “Touched Vertebra” as the lowest instrumented vertebra in patients with lenke type-1 and 2 curves: radiographic results after a minimum 5-year follow-up. J Bone Jt Surg 102:1966–1973. https://doi.org/10.2106/JBJS.19.01485
Siu J, Wu H-H, Saggi S, Allahabadi S, Katyal T, Diab M (2023) Perioperative outcomes of open anterior vertebral body tethering and instrumented posterior spinal fusion for skeletally immature patients with idiopathic scoliosis. J Pediatr Orthop. https://doi.org/10.1097/BPO.0000000000002320
Wiltse LL, Bateman JG, Hutchinson RH, Nelson WE (1968) The paraspinal sacrospinalis-splitting approach to the lumbar spine. J Bone Jt Surg Am 50:919–926
Wiltse LL, Spencer CW (1988) New uses and refinements of the paraspinal approach to the lumbar spine. Spine 13:696–706
Trobisch PD, Baroncini A (2021) Preliminary outcomes after vertebral body tethering (VBT) for lumbar curves and subanalysis of a 1- versus 2-tether construct. Eur Spine J 30:3570–3576. https://doi.org/10.1007/s00586-021-07009-6
Weinstein SL, Zavala DC, Ponseti IV (1981) Idiopathic scoliosis: long-term follow-up and prognosis in untreated patients. J Bone Jt Surg Am 63:702–712
Weinstein SL, Dolan LA, Spratt KF, Peterson KK, Spoonamore MJ, Ponseti IV (2003) Health and function of patients with untreated idiopathic scoliosis: a 50-year natural history study. JAMA 289:559–567. https://doi.org/10.1001/jama.289.5.559
Weinstein SL (2019) The natural history of adolescent idiopathic scoliosis. J Pediatr Orthop 39:S44–S46. https://doi.org/10.1097/BPO.0000000000001350
Rushton PRP, Nasto L, Parent S, Turgeon I, Aldebeyan S, Miyanji F (2021) Anterior vertebral body tethering for treatment of idiopathic scoliosis in the skeletally immature: results of 112 cases. Spine 46:1461–1467. https://doi.org/10.1097/BRS.0000000000004061
Abdullah A, Parent S, Miyanji F, Smit K, Murphy J, Skaggs D, Gupta P, Vitale M, Ouellet J, Saran N, Cho RH, El-Hawary R, Group PSS (2021) Risk of early complication following anterior vertebral body tethering for idiopathic scoliosis. Spine Deform 9:1419–1431. https://doi.org/10.1007/s43390-021-00326-2
Funding
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or material discussed in the manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by JS, H-HW, SS, SA, TK, and Mohammad D. The first draft of the manuscript was written by Jeremy Siu and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript, and agree to be accountable for the work.
Corresponding author
Ethics declarations
Conflict of interest
Hao-Hua Wu, Satvir Saggi, Sachin Allahabadi, Toshali Katyal, Mohammad Diab: No relevant financial or non-financial interests to disclose.
Ethics approval
This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Human Investigation Committee (IRB) of University of California, San Francisco approved this study.
Informed consent
Consent was not necessary for the use of X-rays in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Siu, J.W., Wu, HH., Saggi, S. et al. Radiographic and perioperative outcomes following anterior thoracic vertebral body tethering and posterior lumbar spine tethering: a pilot series. Spine Deform 11, 1399–1408 (2023). https://doi.org/10.1007/s43390-023-00717-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43390-023-00717-7