Abstract
Purpose
To evaluate the impact of patients lost to follow-up on outcomes of surgery for adolescent idiopathic scoliosis (AIS) at 10-year postoperative.
Methods
Preoperative, 2-year, and 5-year postoperative demographic, radiographic, and SRS-22 data from a prospective multi-center registry were compared between patients with a 10-year follow-up visit versus those without. A second analysis utilized variables that were different between the groups, along with SRS scores, in a cohort of patients with preoperative, 2-, 5-, and 10-year postoperative SRS scores (complete cohort) to impute missing 10-year data (imputed cohort) utilizing Markov chain Monte Carlo simulation.
Results
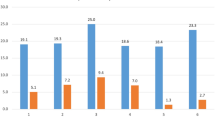
250 patients had 10-year follow-up (21%). Those with 10-year follow-up had a greater percentage of patients who underwent anterior procedures (p < 0.05). Radiographically, the groups were similar at all three time points. SRS-22 scores demonstrated slightly worse pain and function preoperatively and at 2 year in those lost to follow-up (effect size eta = 0.11–0.12), with no differences at 5 year. Imputed data analysis demonstrated similar trends over time in SRS-22 scores compared to the complete cohort for total score and all domains except pain. There was no significant difference in imputed versus complete 10-year SRS-22 scores (p > 0.05).
Conclusion
This study identified early differences between patients with 10-year follow-up and those without, though effect sizes were small and non-existent at 5 years. SRS-22 scores at 10 year between the complete and imputed data sets did not differ. Clinically relevant outcomes of the subset who followed-up at 10 year are likely generalizable to the entire eligible AIS population.
Similar content being viewed by others
References
Little RJ, D’Agostino R, Cohen ML, Dickersin K, Emerson SS, Farrar JT, Frangakis C, Hogan JW, Molenberghs G, Murphy SA, Neaton JD, Rotnitzky A, Scharfstein D, Shih WJ, Siegel JP, Stern H (2012) The prevention and treatment of missing data in clinical trials. N Engl J Med 367:1355–1360. https://doi.org/10.1056/NEJMsr1203730
Jørgensen AW, Lundstrøm LH, Wetterslev J, Astrup A, Gøtzsche PC (2014) Comparison of results from different imputation techniques for missing data from an anti-obesity drug trial. PLoS ONE 9:e111964. https://doi.org/10.1371/journal.pone.0111964
Clark TG, Altman DG (2003) Developing a prognostic model in the presence of missing data: an ovarian cancer case study. J Clin Epidemiol 56:28–37. https://doi.org/10.1016/s0895-4356(02)00539-5
Deng Y, Chang C, Ido MS, Long Q (2016) Multiple imputation for general missing data patterns in the presence of high-dimensional data. Sci Rep 6:21689. https://doi.org/10.1038/srep21689
Office of the Commissioner, Office of Clinical Policy and Programs, Food and Drug Administration guidance for the use of Bayesian statistics in medical device clinical trials. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-use-bayesian-statistics-medical-device-clinical-trials. Accessed 30 Jul 2020
Schafer JL, Yucel RM (2002) Computational strategies for multivariate linear mixed-effects models with missing values. J Comput Graph Stat 11:437–457. https://doi.org/10.1198/106186002760180608
Schunk D (2008) A Markov chain Monte Carlo algorithm for multiple imputation in large surveys. AStA Adv Stat Anal 92:101–114. https://doi.org/10.1007/s10182-008-0053-6
Lin TH (2010) A comparison of multiple imputation with EM algorithm and MCMC method for quality of life missing data. Qual Quant 44:277–287. https://doi.org/10.1007/s11135-008-9196-5
Lonner BS, Ren Y, Yaszay B, Cahill PJ, Shah SA, Betz RR, Samdani AF, Shufflebarger HL, Newton PO (2018) Evolution of surgery for adolescent idiopathic scoliosis over 20 years: have outcomes improved? Spine 43:402–410. https://doi.org/10.1097/BRS.0000000000002332
Parai C, Hägg O, Willers C, Lind B, Brisby H (2020) Characteristics and predicted outcome of patients lost to follow-up after degenerative lumbar spine surgery. Eur Spine J. https://doi.org/10.1007/s00586-020-06528-y
Endler P, Ekman P, Hellström F, Möller H, Gerdhem P (2020) Minor effect of loss to follow-up on outcome interpretation in the Swedish spine register. Eur Spine J 29:213–220. https://doi.org/10.1007/s00586-019-06181-0
Højmark K, Støttrup C, Carreon L, Andersen MO (2016) Patient-reported outcome measures unbiased by loss of follow-up. Single-center study based on DaneSpine, the Danish spine surgery registry. Eur Spine J 25:282–286. https://doi.org/10.1007/s00586-015-4127-3
Elkan P, Lagerbäck T, Möller H, Gerdhem P (2018) Response rate does not affect patient-reported outcome after lumbar discectomy. Eur Spine J 27:1538–1546. https://doi.org/10.1007/s00586-018-5541-0
Solberg TK, Sørlie A, Sjaavik K, Nygaard ØP, Ingebrigtsen T (2011) Would loss to follow-up bias the outcome evaluation of patients operated for degenerative disorders of the lumbar spine?: a study of responding and non-responding cohort participants from a clinical spine surgery registry. Acta Orthop 82:56–63. https://doi.org/10.3109/17453674.2010.548024
Djurasovic M, Glassman SD, Sucato DJ, Lenke LG, Crawford CH, Carreon LY (2018) Improvement in Scoliosis Research Society-22R pain scores after surgery for adolescent idiopathic scoliosis. Spine 43:127–132. https://doi.org/10.1097/BRS.0000000000001978
Carreon LY, Sanders JO, Diab M, Sturm PF, Sucato DJ, Spinal Deformity Study Group (2011) Patient satisfaction after surgical correction of adolescent idiopathic scoliosis. Spine 36:965–968. https://doi.org/10.1097/BRS.0b013e3181e92b1d
Pellegrino LN, Avanzi O (2014) Prospective evaluation of quality of life in adolescent idiopathic scoliosis before and after surgery. J Spinal Disord Tech 27:409–414. https://doi.org/10.1097/BSD.0b013e3182797a5e
Daubs MD, Hung M, Neese A, Hon SD, Lawrence BD, Patel AA, Annis P, Smith J, Brodke DS (2014) Scoliosis research society-22 results in 3052 healthy adolescents aged 10 to 19 years. Spine 39:826–832. https://doi.org/10.1097/BRS.0000000000000280
Baldus C, Bridwell K, Harrast J, Shaffrey C, Ondra S, Lenke L, Schwab F, Mardjetko S, Glassman S, Edwards C, Lowe T, Horton W, Polly D (2011) The Scoliosis research society health-related quality of life (SRS-30) age-gender normative data: an analysis of 1346 adult subjects unaffected by scoliosis. Spine 36:1154–1162. https://doi.org/10.1097/BRS.0b013e3181fc8f98
Semple JL, Sharpe S, Murnaghan ML, Theodoropoulos J, Metcalfe KA (2015) Using a mobile app for monitoring post-operative quality of recovery of patients at home: a feasibility study. JMIR mHealth uHealth 3:e18. https://doi.org/10.2196/mhealth.3929
Lang M, Zawati MH (2018) The app will see you now: mobile health, diagnosis, and the practice of medicine in Quebec and Ontario. J Law Biosci 5:142–173. https://doi.org/10.1093/jlb/lsy004
Martínez-Ramos C, Cerdán MT, López RS (2009) Mobile phone-based telemedicine system for the home follow-up of patients undergoing ambulatory surgery. Telemed e-Health 15:531–537. https://doi.org/10.1089/tmj.2009.0003
Goz V, Spiker WR, Brodke D (2019) Mobile messaging and smartphone apps for patient communication and engagement in spine surgery. Ann Transl Med 7:S163. https://doi.org/10.21037/atm.2019.08.10
Dattilo J, Gittings D, Sloan M, Hardaker W, Deasey M, Sheth N (2017) “Is there an app for that?” Orthopaedic patient preferences for a smartphone application. Appl Clin Inf 08:832–844. https://doi.org/10.4338/ACI-2017-04-RA-0058
Acknowledgements
This study was supported in part by grants to the Setting Scoliosis Straight Foundation in support of Harms Study Group research from DePuy Synthes Spine, EOS imaging, Stryker Spine, Medtronic, NuVasive, Zimmer Biomet and the Food and Drug Administration. Harms Study Group Investigators: Aaron Buckland, MD; Royal Children’s Hospital—Melbourne Australia, Amer Samdani, MD; Shriners Hospitals for Children—Philadelphia, Amit Jain, MD; Johns Hopkins Hospital, Baron Lonner, MD; Mount Sinai Hospital, Benjamin Roye, MD; Columbia University, Burt Yaszay, MD; Rady Children’s Hospital, Chris Reilly, MD; BC Children’s Hospital, Daniel Hedequist, MD; Boston Children’s Hospital, Daniel Sucato, MD; Texas Scottish Rite Hospital, David Clements, MD; Cooper Bone & Joint Institute New Jersey, Firoz Miyanji, MD; BC Children’s Hospital, Harry Shufflebarger, MD; Paley Orthopedic & Spine Institute, Jack Flynn, MD; Children’s Hospital of Philadelphia, John Asghar, MD; Paley Orthopedic & Spine Institute, Jean Marc Mac Thiong, MD; CHU Sainte-Justine, Joshua Pahys, MD; Shriners Hospitals for Children—Philadelphia, Juergen Harms, MD; Klinikum Karlsbad-Langensteinbach, Karlsbad, Keith Bachmann, MD; University of Virginia, Lawrence Lenke, MD; Columbia University, Lori Karol, MD; Children’s Hospital, Denver Colorado, Mark Abel, MD; University of Virginia, Mark Erickson, MD; Children’s Hospital, Denver Colorado, Michael Glotzbecker, MD; Rainbow Children’s Hospital, Cleveland, Michael Kelly, MD; Washington University, Michael Vitale, MD; Columbia University, Michelle Marks, PT, MA; Setting Scoliosis Straight Foundation, Munish Gupta, MD; Washington University, Nicholas Fletcher, MD; Emory University, Noelle Larson, MD; Mayo Clinic Rochester Minnesota, Patrick Cahill, MD; Children’s Hospital of Philadelphia, Paul Sponseller, MD; Johns Hopkins Hospital, Peter Gabos, MD: Nemours/Alfred I. duPont Hospital for Children, Peter Newton, MD; Rady Children’s Hospital, Peter Sturm, MD; Cincinnati Children’s Hospital, Randal Betz, MD; Institute for Spine & Scoliosis, Stefan Parent, MD: CHU Sainte-Justine, Stephen George, MD; Nicklaus Children's Hospital, Steven Hwang, MD; Shriners Hospitals for Children—Philadelphia, Suken Shah, MD; Nemours/Alfred I. duPont Hospital for Children, Sumeet Garg, MD; Children’s Hospital, Denver Colorado, Tom Errico, MD; Nicklaus Children's Hospital, Vidyadhar Upasani, MD; Rady Children’s Hospital.
Funding
This study was supported in part by grants to the Setting Scoliosis Straight Foundation in support of Harms Study Group research from DePuy Synthes Spine, EOS imaging, Stryker Spine, Medtronic, NuVasive, Zimmer Biomet and the Food and Drug Administration.
Author information
Authors and Affiliations
Contributions
RH: design, data acquisition, analysis and/or interpretation of work, manuscript drafting and/or critically revising, final approval. LL: design, data acquisition, analysis and/or interpretation of work, manuscript drafting and/or critically revising, final approval. PS: design, data acquisition, analysis and/or interpretation of work, manuscript drafting and/or critically revising, final approval. TB: design, data acquisition, analysis and/or interpretation of work, manuscript drafting and/or critically revising, final approval. CB: design, data acquisition, analysis and/or interpretation of work, manuscript drafting and/or critically revising, final approval. PON: design, data acquisition, analysis and/or interpretation of work, manuscript drafting and/or critically revising, final approval. HS: design, data acquisition, analysis and/or interpretation of work, manuscript drafting and/or critically revising, final approval. BL: design, data acquisition, analysis and/or interpretation of work, manuscript drafting and/or critically revising, final approval. SS: design, data acquisition, analysis and/or interpretation of work, manuscript drafting and/or critically revising, final approval. BY: design, data acquisition, analysis and/or interpretation of work, manuscript drafting and/or critically revising, final approval. RB: design, data acquisition, analysis and/or interpretation of work, manuscript drafting and/or critically revising, final approval.
Corresponding author
Ethics declarations
Conflict of interest
One or more of the authors reports funding to their institution related to the submitted work and financial relationships with entities outside of the submitted work. Details of which can be found in the individual author disclosure forms submitted with this manuscript.
Ethical approval
IRB approval was obtained for this study.
Rights and permissions
About this article
Cite this article
Bastrom, T.P., Howard, R., Bartley, C.E. et al. Are patients who return for 10-year follow-up after AIS surgery different from those who do not?. Spine Deform 10, 527–535 (2022). https://doi.org/10.1007/s43390-021-00458-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43390-021-00458-5