Abstract
Purpose
Metallic spinal implants undergo wear and corrosion which liberates ionic or particulate metal debris. The purpose of this study was to identify and review studies that report the concentration of metal ions following multi-level spinal fusion and to evaluate the impact on clinical outcomes.
Methods
Databases (PubMed, EBSCO MEDLINE) were searched up to August 2019 for studies in English-language assessing metal ion levels [chromium (Cr), titanium (Ti), nickel (Ni)] in whole blood, serum, or plasma after spinal fusion using a specific search string. Study, patient, and implant characteristics, method of analysis, metal ion concentration, as well as clinical and radiographic results was extracted.
Results
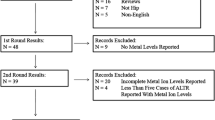
The systematic search yielded 18 studies encompassing 653 patients. 9 studies reported Ti ions, eight reported Cr, and six reported Ni. Ti levels were elevated compared to controls/reference range/preoperative baseline in seven studies with the other two reporting no difference. Cr levels were elevated compared to controls/reference range in seven studies with one reporting no difference. Ni levels showed no difference from controls/reference range in four studies with one reporting above normal and another elevated compared to controls. Radiographic evidence of corrosion, implant failure, pseudarthrosis, revision surgery and adverse reaction reporting was highly variable.
Conclusion
Metal ions are elevated after instrumented spinal fusion; notably Cr levels from stainless steel implants and Ti from titanium implants. The association between clinical and radiographic outcomes remain uncertain but is concerning. Further research with standardized reporting over longer follow-up periods is indicated to evaluate the clinical impact and minimizing risk.

Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Gilbert JL, Buckley CA, Jacobs JJ (1993) In vivo corrosion of modular hip prosthesis components in mixed and similar metal combinations. The effect of crevice, stress, motion, and alloy coupling. J Biomed Mater Res 27(12):1533–1544
Urban RM et al (2004) Accumulation in liver and spleen of metal particles generated at nonbearing surfaces in hip arthroplasty. J Arthroplasty 19(8 Suppl 3):94–101
Jacobs JJ, Gilbert JL, Urban RM (1998) Corrosion of metal orthopaedic implants. J Bone Joint Surg Am 80(2):268–282
Hallab NJ (2016) Biologic responses to orthopedic implants: innate and adaptive immune responses to implant debris. Spine (Phila Pa 1976) 41(Suppl 7):S30–S31
Dalal A et al (2012) Orthopedic implant cobalt-alloy particles produce greater toxicity and inflammatory cytokines than titanium alloy and zirconium alloy-based particles in vitro, in human osteoblasts, fibroblasts, and macrophages. J Biomed Mater Res A 100(8):2147–2158
Cunningham BW et al (2003) The effect of spinal instrumentation particulate wear debris. An in vivo rabbit model and applied clinical study of retrieved instrumentation cases. Spine J 3(1):19–32
Hallab NJ, Cunningham BW, Jacobs JJ (2003) Spinal implant debris-induced osteolysis. Spine (Phila Pa 1976) 28(20):S125–S138
Hallab NJ, Chan FW, Harper ML (2012) Quantifying subtle but persistent peri-spine inflammation in vivo to submicron cobalt-chromium alloy particles. Eur Spine J 21(12):2649–2658
Jacobs JJ, Hallab NJ (2006) Loosening and osteolysis associated with metal-on-metal bearings: a local effect of metal hypersensitivity? J Bone Joint Surg Am 88(6):1171–1172
Clark CE, Shufflebarger HL (1999) Late-developing infection in instrumented idiopathic scoliosis. Spine (Phila Pa 1976) 24(18):1909–1912
Cook S et al (2000) Reoperation after primary posterior instrumentation and fusion for idiopathic scoliosis Toward defining late operative site pain of unknown cause. Spine (Phila Pa 1976) 25(4):463–468
Ipach I et al (2012) The development of whole blood titanium levels after instrumented spinal fusion: is there a correlation between the number of fused segments and titanium levels? BMC Musculoskelet Disord 13:159
Kasai Y, Iida R, Uchida A (2003) Metal concentrations in the serum and hair of patients with titanium alloy spinal implants. Spine (Phila Pa 1976) 28(12):1320–1326
Cundy TP et al (2010) Chromium ion release from stainless steel pediatric scoliosis instrumentation. Spine (Phila Pa 1976) 35(9):967–974
Novak CC et al (2014) Metal ion levels in maternal and placental blood after metal-on-metal total hip arthroplasty. Am J Orthop (Belle Mead NJ) 43(12):E304–E308
MacDonald SJ (2004) Metal-on-metal total hip arthroplasty: the concerns. Clin Orthop Relat Res 429:86–93
Jacobs JJ et al (1998) Metal release in patients who have had a primary total hip arthroplasty. A prospective, controlled, longitudinal study. J Bone Joint Surg Am 80(10):1447–1458
Hussey DK et al (2016) Worse health-related quality of life and hip function in female patients with elevated chromium levels. Acta Orthop 87(5):485–491
Moher D et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097
Liberati A et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 6(7):e1000100
SIGN (2019) Critical appraisal notes and checklists. [cited 2020 January 22]; Available from: https://www.sign.ac.uk/checklists-and-notes.html.
Balshem H et al (2011) GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 64(4):401–406
MacDonald SJ, Brodner W, Jacobs JJ (2004) A consensus paper on metal ions in metal-on-metal hip arthroplasties. J Arthroplasty 19(8 Suppl 3):12–16
Cundy TP et al (2013) Serum titanium, niobium, and aluminum levels after instrumented spinal arthrodesis in children. Spine (Phila Pa 1976) 38(7):564–570
Cundy TP et al (2014) Serum titanium, niobium and aluminium levels two years following instrumented spinal fusion in children: does implant surface area predict serum metal ion levels? Eur Spine J 23(11):2393–2400
Cundy WJ et al (2015) Local and systemic metal ion release occurs intraoperatively during correction and instrumented spinal fusion for scoliosis. J Child Orthop 9(1):39–43
del Rio J, Beguiristain J, Duart J (2007) Metal levels in corrosion of spinal implants. Eur Spine J 16(7):1055–1061
Fernandez Bances I, Paz Aparicio J, Alvarez Vega MA (2019) Evaluation of titanium serum levels in patients after spine instrumentation: comparison between posterolateral and 360 masculine spinal fusion surgery. Cureus 11(8):e5451
Rackham MD et al (2010) Predictors of serum chromium levels after stainless steel posterior spinal instrumentation for adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 35(9):975–982
Richardson TD et al (2008) Serum titanium levels after instrumented spinal arthrodesis. Spine (Phila Pa 1976) 33(7):792–796
Lukina E et al (2016) Metal concentrations in the blood and tissues after implantation of titanium growth guidance sliding instrumentation. Spine J 16(3):380–388
McPhee IB, Swanson CE (2007) Metal ion levels in patients with stainless steel spinal instrumentation. Spine (Phila Pa 1976) 32(18):1963–1968
Savarino L et al (2015) Long-term systemic metal distribution in patients with stainless steel spinal instrumentation: a case-control study. J Spinal Disord Tech 28(3):114–118
Kim YJ et al (2005) Serum levels of nickel and chromium after instrumented posterior spinal arthrodesis. Spine (Phila Pa 1976) 30(8):923–926
Yilgor C et al (2018) Metal ion release during growth-friendly instrumentation for early-onset scoliosis: a preliminary study. Spine Deform 6(1):48–53
Richman SH et al (2017) Metallosis presenting as a progressive neurologic deficit four years after a posterior spinal fusion for adolescent idiopathic scoliosis: a case report. Spine (Phila Pa 1976) 42(1):E56–e59
Sherman B, Crowell T (2018) Corrosion of Harrington rod in idiopathic scoliosis: long-term effects. Eur Spine J 27(Suppl 3):298–302
Cheung JPY et al (2018) A randomized double-blinded clinical trial to evaluate the safety and efficacy of a novel superelastic nickel-titanium spinal rod in adolescent idiopathic scoliosis: 5-year follow-up. Eur Spine J 27(2):327–339
Hart AJ et al (2011) Sensitivity and specificity of blood cobalt and chromium metal ions for predicting failure of metal-on-metal hip replacement. J Bone Joint Surg Br 93(10):1308–1313
Kwon YM et al (2014) Risk stratification algorithm for management of patients with metal-on-metal hip arthroplasty: consensus statement of the American Association of Hip and Knee Surgeons, the American Academy of Orthopaedic Surgeons, and the Hip Society. J Bone Joint Surg Am 96(1):e4
Bower JJ, Leonard SS, Shi X (2005) Conference overview: molecular mechanisms of metal toxicity and carcinogenesis. Mol Cell Biochem 279:3–15
Shah KN et al (2018) Biomechanical comparison between titanium and cobalt chromium rods used in a pedicle subtraction osteotomy model. Orthop Rev (Pavia) 10(1):7541
Lamerain M et al (2014) CoCr rods provide better frontal correction of adolescent idiopathic scoliosis treated by all-pedicle screw fixation. Eur Spine J 23(6):1190–1196
Zartman KC et al (2011) Combining dissimilar metals in orthopaedic implants: revisited. Foot Ankle Spec 4(5):318–323
Eleswarapu A, Salib C, Mikhael M (2015) Radiographic lucency around pedicle screws with cobalt chromium rod constructs in the setting of lumbar fusion. MOJ Orthop Rheumatol 2(4):127–130
Funding
No funding was received for this work.
Author information
Authors and Affiliations
Contributions
OS: made substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data; or the creation of new software used in the work; drafted the work or revised it critically for important intellectual content; approved the version to be published; and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. JCU: made substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data; or the creation of new software used in the work; drafted the work or revised it critically for important intellectual content; approved the version to be published; and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. PR: made substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data; or the creation of new software used in the work; drafted the work or revised it critically for important intellectual content; approved the version to be published; and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
OS declares no conflict of interest; JCU declares no conflict of interest; PR declares no conflict of interest.
Ethics approval
This study did not require IRB approval since no patients were identified or directly involved in the analysis.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Siddiqi, O., Urquhart, J.C. & Rasoulinejad, P. A systematic review of metal ion concentrations following instrumented spinal fusion. Spine Deform 9, 13–40 (2021). https://doi.org/10.1007/s43390-020-00177-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43390-020-00177-3