Abstract
Compilation and scrutiny of all accessible specimen and observer records of the long-tailed woodnymph Thalurania watertonii, a hummingbird currently listed as ‘Endangered’ on the IUCN Red List, eliminates Guyana, Pará, Maranhão, Ceará, Rio Grande do Norte and Paraíba from its range and sets aside both Sergipe and Bahia as unproven, leaving 29 certain localities, 15 in Pernambuco and 14 in Alagoas, north-east Brazil, all of them in Atlantic Forest and not Cerrado or Caatinga. Among them are records from ten IUCN category I‒IV protected areas (seven in Pernambuco, two in Alagoas and one shared between the two). Remote sensing analysis shows all confirmed localities to contain a total of c.292 km2 of forest (with an extent of occurrence (EOO) and area of occupancy (AOO) of 16,090 and 910 km2, respectively), thus indicating the species qualifies for ‘Vulnerable’ (rather than ‘Endangered’) on the IUCN Red List. However, within the species’ range, we find a maximum total of 2568 km2 of forest, unexplored patches of which may host important populations of this and other threatened species endemic to the ‘Pernambuco Centre of Endemism’. Range-wide research is urgently needed into the condition of these sites and the status of the species within them as well as the general densities, ecology and true distribution of the species, which is now known to breed from October to March, to feed on at least 25 plant species and possibly to need shallow clean-water streams, in order to identify the key measures needed to ensure its survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The long-tailed woodnymph Thalurania watertonii is a species of hummingbird confined to lowland and hill forest (brejos de altitude) in north-eastern Brazil (ICMBio 2014; Piacentini 2018; Schuchmann et al. 2020; BirdLife International 2022). It is one of 4‒5 members of the genus Thalurania (following the transfer of T. ridgwayi to Eupherusa) that almost entirely replace each other allopatrically across South America into Central America (Schuchmann 1999; Stiles et al. 2017). Its type locality was long given as a site in Guyana, but a review of the evidence led to the judgement that this was based on an error of memory by the collector Charles Waterton and that the holotype had in reality been taken in Pernambuco, Brazil (Collar and Kirwan 2018). Since all other records of the species are from north-east Brazil, where it was first unambiguously shown to occur in Pernambuco (Salvin 1892; Gounelle 1909), the removal of Guyana from its range greatly reduced the size of the area over which it might be expected to occur.
In 2015, to match the national red list (ICMBio 2014), the global status of the long-tailed woodnymph was changed from IUCN category ‘Near Threatened’ (in which it was placed in 2004 after long treatment as of ‘Least Concern’) to ‘Endangered’, on account of a small population (judged < 2500 mature individuals) and its moderately small and severely fragmented range in Pernambuco, Alagoas and Sergipe. In the ICMBio (2014) assessment, Guyana had already been discounted as questionable, alongside reports of occurrence in Pará, while northern Bahia was treated as unconfirmed in the absence of ‘recent records’ (BirdLife International 2022; see also Roda 2008). In fact, as categorized by Bocalini et al. (2021), and as we discuss in detail below, T. watertonii is almost entirely confined to the Pernambuco Centre of Endemism north of the Rio São Francisco. Whatever its range size, the species was considered generally ‘common’ in both Schuchmann (1999) and the updated version of the same text (Schuchmann et al. 2020).
BirdLife’s most recent assessment essentially copied one by ICMBio (2014), but added that the long-tailed woodnymph is recorded in two private reserves—Pedra D’Antas and Frei Caneca—in Pernambuco, the transboundary Reserva Biológica de Pedra Talhada in Pernambuco/Alagoas and Monte Pascoal National Park in Bahia, along with recommendations to manage protected areas appropriately where the species occurs; study its ability to survive in degraded habitats; and attempt to determine its population status and trends (BirdLife International 2022). To render these objectives more practicable, we seek here to establish as fully as possible the true distribution of the long-tailed woodnymph and the evidence relating to both its general abundance and its potential resilience in secondary habitats.
Methods
We compiled all known records of long-tailed woodnymph up until July 2021 (eBird to April 2021) from museum specimens (n = 123 in 18 museums worldwide), museum sound archives (n = 1; Fonoteca Neotropical Jacques Vielliard, Universidade Estadual de Campinas), web-based repositories of citizen science observations (eBird [n = 759], iNaturalist [n = 17], Wiki-Aves [n = 320]) and online archives of photographs/sound recordings (Macaulay Library [n = 105], xeno-canto [n = 10]). Museum records were compiled by MAC, NJC and GMK using databases such as VertNet (http://www.vertnet.org/index.html) and GBIF (https://www.gbif.org/); personal communication with curators at institutions in Brazil, Europe and North America; an appeal for data on eBEAC (electronic Bulletin for European Avian Curators); and a published inventory (Lopes et al. 2021). Museums holding specimens with geographical information relevant to this work are American Museum of Natural History, New York (AMNH); Coleção de Aves Heretiano Zenaide, Universidade Federal da Paraíba, Areia (CAHZ); Field Museum of Natural History, Chicago (FMNH); Natural History Museum of Los Angeles County, Los Angeles (LACM); Museu Nacional, Rio de Janeiro (MNRJ); Museu de Zoologia da Universidade de São Paulo, São Paulo (MZUSP); Natural History Museum, Tring (NHMUK); and Universidade Federal de Pernambuco, Recife (UFPE). A file containing eBird records was supplied by Cornell Lab of Ornithology; all other non-specimen records were copied manually from their respective internet sources. Records attributed to the same observer at identical locations on the same date were considered duplicates and counted only once in analyses of occurrence.
All spatially referenced occurrence records were imported into a geographic information system (ArcGIS). To determine the degree of habitat protection, we identified blocks of forest in which these records were made and examined their coverage within designated protected areas. We also calculated the total area of forest in each block (as of 2020) using MapBiomas v6 (Souza et al. 2020; MapBiomas 2022), a project that uses the Google Earth Engine platform to determine the statistically most probable land use and land cover class of each cloud-free pixel in any given year.
To account for uncertainty introduced by a lack of comprehensive survey effort across the species’ range, we calculated minimum and maximum areas of likely occupied habitat, where the minimum is the total area of contiguous forest blocks with confirmed sightings and the maximum is the total area of forest blocks (larger than 50 ha) within the mapped range. Forest block sizes were calculated using areas coded ‘Formação Florestal [1.1]’ by MapBiomas (2022). Using this same layer, the total area of forest within the range of T. watertonii was compared between 2000 and 2020 to determine rates of forest loss.
For our determined minimum and maximum areas of likely occupied habitat, we calculated the key geographic range metrics Area of Occupancy (AOO) and Extent of Occurrence (EOO). Following IUCN Red List Guidelines (IUCN Standards and Petitions Committee 2019), AOO values were computed by overlaying and summing 2 × 2 km grid cells over forest blocks, and EOO values were calculated by drawing a minimum convex polygon around the outlying localities.
For four of the forest units (Estação Ecológica de Murici, the wider region around it, Reserva Biológica Saltinho and Parque Estadual Dois Irmãos) in which the species has most often been recorded, and which might reasonably be considered its strongholds, we compiled all complete eBird checklists (n = 96) to estimate the frequency with which individuals were encountered in appropriate habitat. Employing all the data we collected, an assessment of global conservation status against key IUCN Red List Criteria was carried out (IUCN 2012; IUCN Standards and Petitions Committee 2019).
Finally, to supplement existing autecological knowledge with a view to improving our perception of its degree of specialization, we collated the sparse information on breeding and foraging submitted to online databases, using associated images to identify food plants to genus and/or species level. We compiled photographs (n = 54) that included identifiable foodplants, and observations (n = 10) of nest-building, incubation and recently fledged juveniles.
Results
Confirmed localities
We itemize in bold each confirmed locality for Thalurania watertonii, numbered north to south within states (numbers corresponding to those on Fig. 1), with its geographical coordinates, total habitat area (THA; r = remnant, 0 = no forest left), Important Bird Area (IBA) number (codes from Bencke et al. 2006; Develey and Goerck 2009), Key Area (KA) number (codes from Wege and Long 1995) and formal protected area (FPA) status (categories below; long dash ‘—’ indicates no protection) and then briefly the source of records (with dates, months as numbers) in the sequence: specimens, publications and photographs/sightings (principally from eBird and WikiAves). Categories of protected area are indicated by abbreviations before their names: APA, Área de Proteção Ambiental (Environmental Protection Area); EE, Estação Ecológica (Ecological Station); PE, Parque Estadual (State Park); PNM, Parque Natural Municipal (Municipal Nature Park); RB, Reserva Biológica (Biological Reserve); RE, Reserva Ecológica (Ecological Reserve); RPPN, Reserva Particular do Patrimônio Natural (Private Natural Patrimony Reserve); REVIS, Refúgio de Vida Silvestre (Wildlife Refuge).
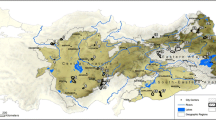
Confirmed localities of long-tailed woodnymph Thalurania watertonii (numbers correspond with those in text) circumscribed by a proposed range (pale pink) based on records and the distribution of Atlantic Forest (Global Forest Watch 2022). Red polygons with ascribed letters correspond to those in text that command more research. Grey = forest in 2020 (MapBiomas 2022)
Pernambuco — We identify 15 sites for the species, seven of them in protected areas with IUCN category I‒IV plus an eighth site shared with and listed under Alagoas (Pedra Talhada, number 21).
-
1.
Engenho Água Azul (= Engenho Croanji), eastern Serra do Mascarenhas, Timbaúba municipality (07.600 S; 35.383 W), THA 25.6 km2, IBA BR064, PE03, KA BR030, FPA —: 4 UFPE specimens (1 in 09.1968, 3 in 05.1999), 3 FMNH specimens from 1999 (month/s unknown); Roda (2002); eBird (11.2018).
-
2.
Mata do Estado, western Serra do Mascarenhas, São Vicente Férrer municipality (07.617 S; 35.500 W), THA 4.0 km2, IBA BR063, KA —, FPA —: 17 UFPE specimens (5 in 12.1998, 11 in 10.1999, 1 in 12.1999), 1 in MZUSP (02.2010), 4 in CAHZ (10.2015), 11 in FMNH (no dates); Roda (2002).
-
3.
Usina São José, Igarassu, Igarassu municipality (07.834 S; 35.004 W), THA 3.6 km2, IBA BR065, PE04, KA —, FPA REVIS Mata da Usina São José (IUCN cat III; 2.9 km2): 1 MNRJ specimen (03.1945, see Berla 1946).
-
4.
Parque Estadual Dois Irmãos, Recife municipality (08.010 S; 34.948 W), THA 4.3 km2, IBA —, KA —, FPA PE de Dois Irmãos (IUCN cat II; 3.87 km2): eBird (09.2003).
-
5.
Estação Ecológica do Tapacurá, São Lourenço da Mata, Paudalho and Chã de Alegria muncipalities (08.043 S; 35.201 W), THA 3.2 km2, IBA BR067, PE06, KA BR031, FPA EE do Tapacurá (IUCN cat Ia; 7.76 km2): Lyra-Neves et al. (2012); eBird (12.2018).
-
6.
Refúgio de Vida Silvestre Matas do Gurjaú, Cabo de Santo Agostinho, Moreno and Jabotão dos Guararapes municipalities (08.239 S; 35.050 W), THA 6.4 km2, IBA BR068, PE07, KA —, FPA REVIS Matas do Sistema Gurjaú (IUCN cat III; 10.77 km2): Lyra-Neves et al. (2004), Bencke et al. (2006).
-
7.
Parque Natural Municipal Professor João Vasconcelos Sobrinho and Reserva Ecológica Brejo dos Cavalos, Caruaru, São Caitano and Altinho muncipalities (08.370 S; 36.026 W), THA 3.6 km2, IBA BR070, PE09, KA BR032, FPA PNM Professor João Vasconcelos Sobrinho (IUCN cat II; 3.52 km2): 3 UFPE specimens (2 in 10.1974, 1 in 1976); Roda and Carlos (2004); eBird (11.2007, 07.2011, 02.2021).
-
8.
Brejão, Bonito municipality (08.546 S; 35.732 W), THA 2.5 km2, IBA —, KA —, FPA —: 2 or 3 CAHZ specimens (1 originally catalogued as ‘Thalurania cf. watertonii’, now just ‘Thalurania’); eBird, WikiAves (multiple records).
-
9.
Catende, Catende municipality (08.600 S; 35.783 W), THA 1.20 km2, IBA —, KA —, FPA —: eBird (03.2011).
-
10.
Usina Trapiche, Sirinhaém municipality (08.630 S; 35.183 W), THA 7.3 km2, IBA BR071, PE10, KA BR034, FPA APA Guadalupe (IUCN cat V; 439.21 km2): eBird (09.2003).
-
11.
Rio Formoso, Rio Formoso municipality (08.704 S; 35.118 W), THA r, IBA BR071, PE10, KA BR034, FPA —: eBird, WikiAves (many records).
-
12.
Serra do Urubu (RPPN Frei Caneca, Jaqueira, RPPN Pedra D’Antas, Lagoa dos Gatos), Jaqueira, Lagoa dos Gatos and São Benedito do Sul municipalities (08.717 S; 35.900 W), THA 20.7 km2, IBA BR074, PE13, KA —, FPA RPPN Frei Caneca (IUCN cat IV; 6.3 km2): 3 UFPE specimens (06.1999); eBird, WikiAves (many records).
-
13.
Reserva Biológica de Saltinho, Rio Formoso and Tamandaré municipalities (08.731 S; 35.176 W), THA 6.2 km2, IBA BR071, PE10, KA BR034, FPA RB de Saltinho (IUCN cat Ia; 5.63 km2), APA Guadalupe (IUCN cat V; 439.21 km2): 3 UFPE specimens (09.1979, 10.1980, 11.1996); eBird, WikiAves, xeno-canto (multiple records).
-
14.
Sertãozinho de Baixo, Maraial municipality (08.866 S; 35.759 W), THA r, IBA —, KA —, FPA —: eBird (04.2004).
-
15.
Eco Fazenda Morim, São José da Coroa Grande municipality (08.868 S; 35.209 W), THA 5.5 km2, IBA BR071, PE10, KA BR034, FPA APA Guadalupe (IUCN cat V; 439.21 km2): eBird (09.2020, 01.2021).
Alagoas — We identify 14 sites for the species, two of them in protected areas with IUCN category I‒IV plus a third site shared with Pernambuco.
-
16.
Mata do Açude do Pinto, Usina Serra Grande, Ibateguara municipality (08.979 S; 36.107 W), THA 6.2 km2, IBA —, KA —, FPA —: eBird (multiple records).
-
17.
Mata do Engenho Coimbra, Usina Serra Grande, Ibateguara municipality (09.007 S; 35.862 W), THA 20.2 km2, IBA BR121, AL01, KA —, FPA —: 2 FMNH specimens (07.2000), 2 UFPE specimens (06.2000, 11.2000); Silveira et al. (2003); eBird (multiple records).
-
18.
Serra da Barriga, União dos Palmares municipality (09.170 S; 36.088 W), THA r, IBA —, KA —, FPA —: eBird, WikiAves (multiple records).
-
19.
Quilombo Parque Hotel, União dos Palmares municipality (09.172 S; 35.999 W), THA r, IBA —, KA —, FPA —: eBird (multiple records).
-
20.
Estação Ecológica de Murici, Murici, Flexeiras, Branquinha and União dos Palmares municipalities (09.183 S; 35.883 W), THA 71.7 km2, IBA BR122, AL02, KA BR035, FPA EE Murici (IUCN cat Ia; 61.32 km2): 9 MNRJ specimens (11.1983, 05.1984, 01.1986); eBird, WikiAves (multiple records).
-
21.
Reserva Biológica de Pedra Talhada (transboundary Pernambuco/Alagoas), Quebrangulo, Chã Preta, Lagoa do Ouro and Correntes municipalities (09.200 S; 36.433 W), THA 46.2 km2, IBA BR123, AL/PE02, KA BR036, FPA RB de Pedra Talhada (IUCN cat Ia; 43.82 km2): 1 MZUSP specimen (11.1951); Grantsau (1988), Studer (2015); eBird, WikiAves (multiple records).
-
22.
Fazenda Santa Justina, Passo do Camaragibe municipality (09.221 S; 35.519 W), THA 8.5 km2, IBA —, KA —, FPA —: 1 MNRJ specimen (01.1988); WikiAves (multiple records).
-
23.
Mato do Faria, Passo do Camaragibe municipality (09.329 S; 35.456 W), THA 16.4 km2, IBA —, KA —, FPA —: two observations (Portes et al. 2018, F. I. Godoy pers. commun. 2022).
-
24.
Usina Santo Antônio I (09.383 S; 35.617 W) and Usina Santo Antônio II, São Luís do Quitunde municipality (09.383 S; 35.583 W), THA 12.5 km2, IBA —, KA —, FPA —: Silveira et al. (2003). The latter of the two sites has been included based on one 2001 record (Silveira et al. 2003) but is now deforested.
-
25.
Mata Bamburral II, Usina Cachoeira do Meirim, Maceió municipality (09.442 S; 35.735 W), THA 5.00 km2, IBA BR124, AL03, KA —, FPA —: Silveira et al. (2003).
-
26.
Reserva Particular do Patrimônio Natural Santa Tereza, Atalaia municipality (09.517 S; 35.978 W), THA 0.8 km2, IBA —, KA —, FPA RPPN Santa Tereza (IUCN cat IV; 1.01 km2): eBird (08.2018).
-
27.
São Miguel dos Campos, São Miguel dos Campos municipality (09.783 S; 36.083 W), THA 0, IBA —, KA BR037, FPA —: 2 MNRJ specimens (11.1983, 01.1987), 1 MZUSP specimen (09.1951).
-
28.
Usina Sinimbú, São Miguel dos Campos municipality (09.917 S; 36.133 W), THA r, IBA —, KA —, FPA —: 1 MZUSP specimen (03.1957). The forest at this and the preceding locality has been entirely transformed into sugarcane plantations (Lima et al. 2022).
-
29.
Usina Coruripe, Coruripe municipality (10.000 S; 36.267 W), THA 9.8 km2, IBA —, KA —, FPA —: Silveira et al. (2003), Pereira et al. (2014).
Unconfirmed and dubious localities
‘Guianas’ — Collar and Kirwan (2018) provided the rationale for regarding the holotype of Trochilus watertonii, NHMUK 1933.11.14.12, as originating in coastal Pernambuco, perhaps around Olinda, some time during 1816, rather than in Demerara (= Guyana) as stated by Loddiges (1826‒45; see also Butler 1926). However, three other specimens in NHMUK are labelled as from either ‘Guiana’ (NHMUK 1887.3.22.1010) or ‘British Guiana’ (NHMUK 1887.3.22.1009 and 1913.3.20.320), but this seems to have been entirely due to curatorial assumption based on the reported type locality rather than new or improved knowledge (Collar and Kirwan 2018).
Pará — Two male specimens (NHMUK 1888.7.25.294‒295), labelled ‘near mouth of Amazons?’, i.e., in the state of Pará, were first mentioned by Gould (1861), who stated that they came ‘not I believe [from] Demerara [= Guyana], but … probably near the embouchure of the great river Amazon’. This presumption became sufficiently embedded in the literature that it was repeated by numerous commentators (e.g. Pinto 1944; Peters 1945; Parker et al. 1996; Schuchmann 1999). Nevertheless, no evidence has ever been adduced for Pará to be considered part of the species’ range.
Maranhão — The north of the state was mapped in the woodnymph’s range by Schuchmann (1999), but we find no records to support this. The species was not listed by Carvalho et al. (2020).
Ceará — First cited for Ceará by Ruschi (1964a), the north of the state was included in the map furnished by Schuchmann (1999), and the species is mentioned as occurring in the Chapada do Araripe in the far south (Teixeira 1988; Nascimento 1996; Nascimento et al. 2000; Bencke et al. 2006; Major and Sales 2008). However, Teixeira never repeated the claim in any subsequent publication on the birds of north-east Brazil, while Nascimento et al. (2000) reported the species in cerrado, an extremely unlikely habitat (see ‘Ecology’ below), and Silva and Albano (2002) pointed out that records for the Araripe were incorrect. We have seen no documentation for any Thalurania species in this region, including on eBird or WikiAves, and all neighbouring records involve fork-tailed woodnymph T. furcata and seem to have been made in more humid forest, not Cerrado.
Rio Grande do Norte — Despite the map in Schuchmann (1999), the apparently exhaustive compilation by Sagot-Martin et al. (2020) did not list T. watertonii (or any congener) under any category, even hypothetical or rejected. Based on photographs supplied, a female specimen (UFPE 4291) purported to be from Salina Diamante Branco in Galinhos municipality, in the Caatinga biome on the north coast of Rio Grande do Norte, collected on 13 August 2000, is in our opinion unidentifiable to species.
Paraíba — The entire state was mapped by Schuchmann (1999), but there are no records (e.g. Marinho 2014).
Sergipe — The state was first included in the species’ range based on a sight record in the Reserva Biológica de Itabaiana (now Parque Nacional da Serra de Itabaiana) on 25 September 1991 (Pacheco and Whitney 1995). Sousa (2009) reported the species from the same site (repeated, still without evidence, in Bencke et al. 2006 and Silva et al. 2022) as well as in Cerrado and secondary forest around Santana (Pacatuba municipality) and Mata do Junco (Capela municipality) but provided no documentary evidence. Ruiz-Esparza et al. (2015) then claimed both T. watertonii and violet-capped woodnymph T. glaucopis for the Refúgio de Vida Silvestre Mata do Junco, although only the latter species was trapped during mist-netting surveys. Both eBird and WikiAves hold multiple well-documented records of T. glaucopis, including from the Parque Nacional da Serra de Itabaiana, where the species was also found by d’Horta et al. (2005). Given the rarity of unambiguous instances of sympatry between two species of Thalurania (see below), the potential difficulty in discriminating glaucopis from watertonii in the field (see also below) and the absence of supporting evidence for the records above, we treat T. watertonii as hypothetical for Sergipe.
Bahia — A number of specimens of T. watertonii are labelled ‘Bahia’ (e.g. AMNH 481563‒565), so the species has been widely treated as native to the state via sources as authoritative as Simon (1897, 1921), Pinto (1944, 1978), Peters (1945) and Schuchmann (1999), even though it is well established that the word ‘Bahia’ on specimens traded to America and Europe through the port of Salvador da Bahia is no guide to their true provenance (Jouanin 1944; Pacheco and Whitney 1995). However, several much more recent claims of its occurrence in the state have entered the (mainly grey) literature. Forrester (1993) listed T. watertonii for southern Bahia, specifically Parque Nacional Monte Pascoal (hence the latter’s mention in Schuchmann 1999 and BirdLife International 2022). Sargeant and Wall (1995), also cited by Schuchmann (1999), repeated Monte Pascoal, evidently based on Forrester, and added Santo Amaro and Boa Nova, based on ‘DWF’ (apparently Davis W. Finch), as well as their own observation at Reserva Biológica Una, mentioning that ‘In the poor light of the forest interior, the greener cap is not readily seen—the much longer tail (30%?) is a much better field mark. Beware also that Violet-capped occurs within forest interior.’ However, there are no documented records of T. watertonii at any of these (well-watched) sites, and Pacheco and Whitney (1995) already noted that the only Thalurania they and other experienced observers had encountered at the three southernmost localities (i.e. excluding Santo Amaro) was glaucopis. Citizen science data (eBird, WikiAves) and extensive fieldwork (by GMK) strongly suggest that only T. glaucopis occurs at these four sites. A sighting of watertonii in the Serra das Lontras by P. Cordeiro (Silveira et al. 2005) likewise lacks documentation and has never been replicated, including during several visits by GMK in the early 2000s.
While Pacheco and Whitney (1995) were relatively unambiguous in rejecting sightings of T. watertonii from southern Bahia, they judged that their own record in Sergipe (south of the Rio São Francisco) ‘corroborates the possibility of the existence of this hummingbird in northeastern Bahia at least, from whence probably came the “Bahia” trade skin(s)’. However, there are now tens of photographically documented records of male Thalurania in north-east Bahia and Sergipe (especially on WikiAves), and all of them are unequivocally T. glaucopis. As noted above, unambiguous documentation of sympatry between two species of Thalurania is distinctly uncommon, only seemingly occurring between glaucopis and furcata in Misiones in north-easternmost Argentina (Saibene et al. 1996; Savigny 2010; Martínez Gamba 2014; Pearman and Areta 2020; eBird 2021) and ‘central Brazil’ (Piacentini 2018).
Revised range and abundance
The minimum area of forest likely occupied by the woodnymph totals 292 km2 (Fig. 1) with an AOO and EOO of 910 km2 and 22,270 km2, respectively. The latter value reduces to 16,090 km2 when pre-2000 records are excluded. Combining all forest in the species’ range, the maximum area of suitable habitat is 2568 km2. Because of the severely fragmented nature of remaining forest in this region and its high perimeter:area ratio, the coarse 2 × 2 km grid generates a comparatively large maximum AOO of 21,864 km2. However, 52% of forest blocks are smaller than 1 km2 and 91% smaller than 5 km2 (Table 1), so many of them may not support populations—or at least viable populations—of T. watertonii (which is not to say that they might not have value as corridors or refuges for dispersing birds).
Between 2000 and 2020, the area of total suitable habitat reduced by 6.6% from 2748 to 2568 km2, and the number of fragments increased by 19% from 901 to 1072, indicating that the species’ habitat is undergoing steady attritional loss and fragmentation within its restricted range. According to Ruschi (1986), the long-tailed woodnymph bathes in clear streams like all Thalurania species, and this is confirmed by records of bathing at localities in the Serra do Urubu (ML211438551; COAG pers. obs.); it therefore bears consideration that extensive forest fragmentation may greatly reduce both the number and the quality of streams in the otherwise seemingly habitable AOO of the species.
Encounter rates
For four eBird ‘hotspots’ with a total of 96 complete checklists between them—Estação Ecológica de Murici, the wider region around it, Reserva Biológica de Saltinho and Parque Estadual Dois Irmãos—the maximum encounter rate was less than one bird per 10 h of observation. At PE Dois Irmãos, none was detected on any of the 18 complete checklists which jointly logged over 61 h of observation. In contrast, Silveira et al. (2003) recorded encounter rates of 2.0‒3.3 birds per 10 h between sites, roughly similar to those they derived for ‘common’ hummingbirds like reddish hermit Phaethornis ruber, blue-chinned sapphire Chlorestes notata and black-throated mango Anthracothorax nigricollis. In the Refúgio de Vida Silvestre Matas do Gurjaú, Pernambuco, Lyra-Neves et al. (2004) recorded T. watertonii at a similar frequency to A. nigricollis and about half as frequently as C. notata and four times less frequently than P. ruber.
Ecology
The species has been reported as occurring in ‘coastal rainforest, cerrado, semi-open clearings, plantations and parks’, with an elevational range from sea level to 550 m (Schuchmann 1999). A little earlier, however, it was said to range to at least 700 m, but to be probably almost wholly dependent on tropical lowland evergreen forest (Parker et al. 1996). Its occurrence in brejo de altitude in Reserva Ecológica Brejo dos Cavalos suggests that its upper elevational limit is probably around 800–1000 m, but it should not be assumed that this circumstance applies all year or throughout its range. All georeferenced localities compiled as part of this study refer to Atlantic Forest (per Global Forest Watch 2022), and we find no evidence that the species has ever occurred in Cerrado sensu stricto (a suggestion removed by GMK in Schuchmann et al. 2020), and definite evidence of its presence, permanent or otherwise, in plantations or urban parks also seems wanting.
Ruschi (1986) named 13 families of plants from whose flowers the woodnymph ‘most frequently’ feeds, alphabetically Anacardiaceae, Bignoniaceae, Bromeliaceae, Cactaceae, Leguminosaceae (= Leguminosae = Fabaceae), Loranthaceae, Malvaceae, Passerifloraceae, Proteaceae, Rubiaceae, Sterculiaceae (but this now subsumed in Malvaceae), Verbenaceae and Vochysiaceae. How dependable this list is cannot be gauged, but from photographs archived online and field observations by COAG, we identify the following plants being visited by the species (family alphabetically, number of records in brackets after each species): Acanthaceae Megaskepasma erythrochlamys (3) and Ruellia cearensis (endemic to north-east Brazil: Tripp and McDade 2012 [3]), the latter recorded being robbed (Studer 2015 and in 1 photograph consulted); Asteraceae Chresta pacourinoides (3) and Zinnia sp. (1); Bromeliaceae Aechmea fulgens (2), A. aquilega (2), A. costantinii (2), A. patentissima (1), A. leptantha (8), A. cephaloides (1) (the latter two both endemic to relatively small areas of north-east Brazil: Maciel et al. 2015), Canistrum aurantiacum (2), C. alagoanum (1), C. pickelii (1) (all three endemic to the Brazilian Atlantic Forest: Martinelli et al. 2008), Hohenbergia sp. (1), H. stellata (4), Quesnelia testudo (3), with several additional photographs showing the species at unidentified bromeliads); Caricaceae Carica papaya (1) (a now pantropical fruit-crop plant); Fabaceae Caesalpinia pulcherrima (2) (this and the preceding being exotic ornamentals commonly planted throughout the Neotropics); Gesneriaceae Paliavana tenuiflora (1); Heliconiaceae Heliconia psittacorum (2), H. bihai (1); Magnoliaceae Magnoliopsida indet. (1); Malvaceae non-native Hibiscus cf. rosa-sinensis (2 photographs showing probable robbing); Plantaginaceae Russelia equisetiformis (2); and Rubiaceae Tocoyena formosa (1).
The species’ breeding ecology is poorly documented. Only brief descriptions of nests, position, materials used, clutch size and incubation and nestling period are available (Ruschi 1986; Schuchmann 1999; Studer 2015; Piacentini and Ribenboim 2017; Schuchmann et al. 2020), supplemented by several photographs (Buzzetti and Silva 2005; Studer 2015; WikiAves below). Based on data from Recife, Ruschi (1964b) and Grantsau (1988), hence also Schuchmann (1999), indicated that the species breeds between November and February. Fitting with this is an observation of a female collecting nest material on 1 December 2020 (eBird S50336252; COAG pers. obs.) and a nest at Estação Ecológica de Murici that fledged young in January 2022 (D. Branch pers. commun. 2022). However, a search on WikiAves (WA) and Macaulay Library (ML) in February 2022 yielded two images of nests, on 12 October 2015 (WA1868696) and 4 March 2014 (WA1416596), while the span of records of males with juvenile characteristics (greyish rather than bright green feathering between throat and belly, duller dorsal colouration) covers 26 November 2012 (WA831370); 18, 19 and 24 January 2022 (WA4721238, ML412519931, ML412560881); 3 February 2010 (ML126770991); 18 February 2017 (WA2471810); 10 March 2020 (ML215576901); and 26 April 2014 (WA1317025). Therefore, the spread of records indicating reproductive activity involves a broader temporal range than mentioned in the available literature, covering 6 months, October to March, but this conflates many years and may not represent the situation in any single breeding season.
Discussion
Population size
Determining the population size of a species is a prerequisite for assessment under IUCN Red List Criteria C and D (IUCN 2012), but, to our knowledge, no species-specific density estimate/population size has been calculated for T. watertonii. A published population size of 1000‒2499 mature individuals (BirdLife International 2022; following ICMBio 2014 and upheld in ICMBio 2018) does not appear to be substantiated by traceable data, while mention of a density of ‘at least 2‒3 pairs/km2’ was erroneously attributed to T. watertonii in Schuchmann (1999) but refers instead to unpublished observations of T. glaucopis on the Brazilian side of Iguaçu Falls, in south-east Brazil (K.-L. Schuchmann pers. commun. 2022). Attempting to integrate existing abundance estimates with citizen science data, Callaghan et al. (2021) modelled the population of T. watertonii as 25,291 (95% CI: 238–2,261,123) mature individuals, but such broad confidence intervals (spanning almost four orders of magnitude) are not of practical use for conservation planning (see Robinson et al. 2022), and we discard these values here.
However, we can do little better ourselves. Density estimates of 6.0–20.4 birds/km2 have been reported for T. furcata (Thiollay 1986, 1992; Terborgh et al. 1990) and 6–64 birds/km2 for T. glaucopis (Marsden et al. 2001, K.-L. Schuchmann pers. commun. 2022). Applying these values (spanning 6–64 birds/km2) across the patches of forest with records of long-tailed woodnymph since 2000 (292 km2), as well as all suitable habitat within its revised range (2568 km2), yields a broad-spectrum estimate of c.1750–164,000 mature individuals. Even so, bird species of a similar mass, including congeners, can have widely divergent densities (Santini et al. 2018; Stephens et al. 2019), and the available encounter rate data indicate that T. watertonii is much scarcer than either T. furcata or T. glaucopis. While we therefore acknowledge that a population size cannot currently be estimated, we consider that it may be (potentially much) smaller than 10,000 mature individuals, the threshold for listing as threatened under Criterion C (see below).
Population trend
Detecting population declines in species that are scarce is intrinsically difficult without frequent and robust monitoring, as data are often too sparse to fit detectability functions and generate appropriate abundances, either relative or true (McCarthy et al. 2012). However, rarity and trend are not necessarily correlated (Daskalova et al. 2020), and T. watertonii cannot be assumed to be declining simply because it is scarce and/or has low detectability.
Nevertheless, we consider an ongoing decline in this species is plausible and indeed likely, with one confirmed historical locality (site 27) now cleared of suitable habitat and five (sites 11, 14, 18, 19 and 28) having only remnant forest. In Costa Rica, hummingbird abundance and species richness declined across a gradient of deforestation, with patch size the strongest predictor (Hadley et al. 2018). Within the range of T. watertonii, forest fragments have risen in number at the rate of almost 1% per year since the start of the century (see above), so they can only have become smaller. Localised extinction debt processes may therefore affect T. watertonii as they have other species in the Pernambuco Centre of Endemism: even in protected areas, and in the absence of ongoing forest loss, species in this bioregion have become locally and globally extinct (Develey and Phalan 2021), with the edge:core ratio of small forest patches rendering them less resistant to wind-drying effects and hence to fires (Loarie et al. 2011; Lees et al. 2014; Pereira et al. 2014) as well as the potential for as yet undetected Allee effects (Crates et al. 2017).
IUCN Red List status
The long-tailed woodnymph is currently assessed on the IUCN Red List as ‘Endangered’ (EN) B2ab(ii,iii,v); C2a(i). To qualify for ‘Endangered’ under Criterion B2, a species must have an AOO less than 500 km2 (IUCN 2012); ICMBio (2018) calculated a value of 456 km2 by overlaying a 2 × 2 km grid over recent records. Using a different methodology (overlaying the 2 × 2 km grid instead over confirmed forest blocks to account for record imprecision and data clumped by, for example, eBird hotspots), we generate a larger minimum AOO of 910 km2. We also calculate a much larger, maximum plausible AOO of 21,864 km2, but we caution against its use given the high percentage of fragments now possibly too small to support populations. We suggest that, even accounting for incomplete survey effort, there is a high probability that the species meets the threshold (< 2000 km2) for listing as ‘Vulnerable’ (VU) under Criterion B2. Our recalculated EOO (16,090 km2) meets the same category threshold for Criterion B1. Given that we know nothing of its ability to disperse between patches, we precautionarily assume that the species is severely fragmented (sensu IUCN Standards and Petitions Committee 2019).
By way of contrast, although the species is currently also listed under Criterion C, we find no compelling evidence that its population might fall below the requisite 2500 mature individuals. In the absence of appropriate data, T. watertonii cannot currently be assessed against Criterion C. From the evidence assembled here, it best merits listing as ‘Vulnerable’ B1ab(iii) + 2ab(iii) based on its small EOO and AOO, severely fragmented range (see Table 1), and presumed decline in area and habitat quality owing to attritional forest loss and dessication.
Research needs
Critical to elucidating the overall conservation status of this species is a need to determine its particular status in forest patches throughout its range. Localities with confirmed records since 2000 consist of only 292 km2 of forest, yet according to MapBiomas (2022), an order of magnitude more forest (2568 km2) is present within its range. Notwithstanding imperfections with remote sensing data, especially difficulties in distinguishing levels of degradation (Gao et al. 2020), it seems likely that at least some, perhaps many, unsurveyed areas of forest host T. watertonii while known sites may, with further effort, prove to hold healthy populations of the species.
To assist this endeavour, we identify five areas (letters correspond to those in Fig. 1) that ostensibly seem the strongest candidates to hold important populations of this species:
-
(A)
A large area (spanning > 200 km2 of forest) north-west of Recife encompassing Usina São José, Igarassu and Parque Estadual Dois Irmãos (localities 3 and 4 in Fig. 1). Despite the apparent expanse of suitable habitat, there has been no record of T. watertonii since an undocumented sighting in September 2003 (eBird), but if citizen science data are representative of survey effort in this region, it appears to be comparatively poorly known.
-
(B)
A forest area of approximately 50 km2, chiefly comprising Usina Serra Grande (locality 17 in Fig. 1). There are multiple recent records from this area, including single checklist counts of 8 and 6 in October 2019 and January 2020, respectively (eBird), considerably higher than those elsewhere.
-
(C)
Narrowly disconnected patches east of the main area of Reserva Biológica de Pedra Talhada (locality 21 in Fig. 1). Determining the woodnymph’s prevalence in them would be an informative exercise in this otherwise very isolated area of forest.
-
(D)
An extensive and partially fragmented area containing more than 150 km2 of nominally suitable forest, including the well-documented Estação Ecológica de Murici (locality 20; Fig. 1) and several smaller and variably isolated patches of forest. Repeated sightings (up to at least October 2020) of individuals at União dos Palmares (locality 19) are, notably, in heavily degraded habitat adjoining forest and could provide insight into this species’ habitat tolerance.
-
(E)
A highly fragmented area circumscribing localities 24 and 25 with no published records since October 2001 (Silveira et al. 2003). Many patches of forest here are tiny (none is larger than c.15 km2) but combine to cover nearly 200 km2.
The accumulation of presence/absence data from patches in these areas and others will enhance our ability to discriminate the features of occupied and unoccupied forest and conduct more robust predictive mapping exercises for the long-tailed woodnymph. For example, identifying the broad (forest size, condition, elevation, etc.) and narrow (food-plants and other features such as running water) habitat preferences of the species would make important improvements to population modelling and conservation management.
Surveys to determine the species’ status and true abundance in these areas also offer the opportunity to find hitherto unknown populations of other threatened species in the region, including Atlantic woodcreeper Xiphorhynchus atlanticus (VU), buff-throated purpletuft Iodopleura pipra (EN), Pinto’s spinetail Synallaxis infuscata (EN) and seven-coloured tanager Tangara fastuosa (EN). From this, strategic plans for conservation can steadily evolve and be implemented, taking full advantage of opportunities for enhanced management provided by protected areas. For the long-tailed woodnymph, in addition to the measures suggested above, early efforts would be welcome to survey reported areas of Sergipe to establish its status there more confidently.
References
Bencke GA, Mauricio GN, Develey PF, Goerck JM (2006) Áreas importantes para a conservação das aves no Brasil, Parte I—estados do domínio da Mata Atlântica. SAVE Brasil, São Paulo
Berla HF (1946) Lista das aves colecionadas em Pernambuco, com descrição de uma subespécie n., de um alótipo fêmea e notas de campo. Bol Mus Nac Zool 65:1–35
BirdLife International (2022) Species factsheet: Thalurania watertonii. http://www.birdlife.org. Accessed 14 January 2022
Bocalini F, Bolívar-Leguizamón SD, Silveira LF, Bravo GA (2021) Comparative phylogeographic and demographic analyses reveal a congruent pattern of sister relationships between bird populations of the northern and south-central Atlantic Forest. Mol Phylogenet Evol 154:106973. https://doi.org/10.1016/j.ympev.2020.106973
Butler AL (1926) Some notes on the humming-birds included in Chubb’s ‘Birds of British Guiana’. Ibis 68:333–339. https://doi.org/10.1111/j.1474-919X.1926.tb07585.x
Buzzetti D, Silva S (2005) Berços da vida. Editora Terceiro Nome, São Paulo
Callaghan C, Nakagawa S, Cornwell W (2021) Global abundance estimates for 9,700 bird species. Proc Natl Acad Sci USA 118:e2023170118. https://doi.org/10.1073/pnas.2023170118
Carvalho DL, Silva SM, Sousa-Neves T, Silva DP, Santos MPD (2020) An updated documented inventory and new records of bird species for the Brazilian state of Maranhão. Ornithol Res 28:77–85. https://doi.org/10.1007/s43388-020-00013-2
Collar NJ, Kirwan GM (2018) In support of Pinto: Pernambuco as the type locality of Thalurania watertonii. Bull Brit Ornithol Club 138:265–271. https://doi.org/10.25226/bboc.v138i3.2018.a7
Crates R, Rayner L, Stojanovic D, Webb M, Heinsohn R (2017) Undetected Allee effects in Australia’s threatened birds: implications for conservation. Emu-Austral Ornithol 117:207–221. https://doi.org/10.1080/01584197.2017.1333392
d’Horta FM, Gouveia SF, Rocha PA (2005) Aves. In: Carvalho CM, Vilar JC (eds) Parque Nacional Serra de Itabaiana, Levantamento da Biota. IBAMA, Aracaju
Daskalova G, Myers-Smith I, Godlee J (2020) Rare and common vertebrates span a wide spectrum of population trends. Nature Comm 11:4394. https://doi.org/10.1038/s41467-020-17779-0
Develey PF, Goerck J (2009) Brazil. In: Devenish C, Díaz Fernández DF, Clay RP, Davidson I, Yépez Zabala I (eds) Important Bird Areas Americas—priority sites for biodiversity conservation (BirdLife Conservation Series No. 16). BirdLife International, Quito, Ecuador, pp 99–112
Develey PF, Phalan BT (2021) Bird extinctions in Brazil’s Atlantic Forest and how they can be prevented. Front Ecol Evol 9:624587. https://doi.org/10.3389/fevo.2021.624587
eBird Basic Dataset (2021) Version: EBD_relApr-2021. Cornell Lab of Ornithology, Ithaca, New York. Accessed 14 January 2022
Forrester BC (1993) Birding Brazil. A check-list and site guide. Privately published, Irvine
Gao Y, Skutsch M, Paneque-Gálvez J, Ghilardi A (2020) Remote sensing of forest degradation: a review. Environ Res Lett 15:103001. https://doi.org/10.1088/1748-9326/abaad7
Global Forest Watch (2022) Brazil biomes. World Resources Institute. https://data.globalforestwatch.org/datasets/gfw::brazil-biomes. Accessed on 12 May 2022
Gould J (1861) A monograph of the Trochilidae, or family of humming-birds, vol 2. Taylor and Francis, London
Gounelle E (1909) Contribution à l’étude de la distribution géographique des trochilidés dans le Brésil central et oriental. Ornis 13:173–183
Grantsau R (1988) Os beija-flores do Brasil. Expressão e Cultura, Rio de Janeiro
Hadley A, Frey S, Robinson W, Betts M (2018) Forest fragmentation and loss reduce richness, availability, and specialization in tropical hummingbird communities. Biotropica 50:74–83. https://doi.org/10.1111/btp.12487
ICMBio (2014) https://www.icmbio.gov.br/portal/faunabrasileira/lista-de-especies/5737-especie-5737. Accessed 26 April 2022
ICMBio (2018) Livro vermelho da fauna brasileira ameaçada de extinção: Volume III—Aves. In: Instituto Chico Mendes de Conservação da Biodiversidade. Livro vermelho da fauna brasileira ameaçada de extinção. ICMBio, Brasília
IUCN (2012) IUCN Red List categories and criteria: version 3.1. Second edition. IUCN, Gland, Switzerland and Cambridge, UK
IUCN (2022) The IUCN Red List of threatened species. Version 2021–3. https://www.iucnredlist.org. Accessed 26 April 2022
IUCN Standards and Petitions Committee (2019) Guidelines for using the IUCN Red List categories and criteria. Version 14. Prepared by the Standards and Petitions Committee. http://www.iucnredlist.org/documents/RedListGuidelines.pdf. Accessed 26 April 2022
Jouanin C (1944) Liste des Trochilidés trouvés dans les collections commerciales de Bahia. Ois Rev France Ornithol 14:104–116
Lees AC, Albano C, Kirwan GM, Pacheco JF, Whittaker A (2014) The end of hope for Alagoas foliage-gleaner Philydor novaesi? Neotrop Birding 14:20–28
Lima RD, Silveira LF, de Amorim Lemos RC, Lobo-Araújo LW, de Andrade AB, Francisco MR, Efe MA (2022) An annotated avian inventory of the Brazilian state of Alagoas, one of the world’s most threatened avifaunas. Pap Avul Zool 62:e202262034. https://doi.org/10.11606/1807-0205/2022.62.034
Loarie SR, Lobell DB, Asner GP, Mu Q, Field CB (2011) Direct impacts on local climate of sugar-cane expansion in Brazil. Nat Clim Change 1:105–109. https://doi.org/10.1038/nclimate1067
Loddiges G (1826–1845) Manuscript collection held in Natural History Museum, Tring
Lopes RJ, Faria PMV, Gomes D, Freitas B, Málinger J (2021) The hummingbird collection of the Natural History and Science Museum of the University of Porto (MHNC-UP). Portugal Biodiversity Data J 9:e59913. https://doi.org/10.3897/BDJ.9.e59913
Lyra-Neves RM, Dias MM, Azevedo-Júnior SM, Telino-Júnior WR, Larrazábal MEL (2004) Comunidade de aves da Reserva Estadual de Gurjaú, Pernambuco, Brasil. Rev Bras Zool 21:581–592. https://doi.org/10.1590/S0101-81752004000300021
Lyra-Neves RM, Telino-Júnior WR, Azevedo-Júnior SM, Pereira GA (2012) Avifauna da Estação Ecológica do Tapacurá, São Lourenço da Mata, Pernambuco, Brasil. In: Moura GJB, Azevedo-Júnior SM, El-Deir ACA (eds) A biodiversidade da Estação Ecológica de Tapacurá: uma proposta de manejo e conservação. Editora Nupeea, Recife
Maciel JR, Louzada R, Alves M (2015) Aechmea Ruiz & Pavón from the northern portion of the Atlantic Forest. Rodriguésia 66:477–492. https://doi.org/10.1590/2175-7860201566215
Major I, Sales LG Jr (2008) Aves do Ceará. Gráfica LCR and Colégio Christus, Fortaleza
MapBiomas (2022) MapBiomas v6.0. mapbiomas.org. Accessed 28 January 2022
Marinho MFA (2014) Aves da Paraíba: uma revisão de informações históricas e atuais. BSc Monograph. Universidade Federal da Paraíba, João Pessoa
Marsden SJ, Whiffin M, Galetti M (2001) Bird diversity and abundance in forest fragments and Eucalyptus plantations around an Atlantic forest reserve, Brazil. Biodiversity Conserv 10:737–751. https://doi.org/10.1023/A:1016669118956
Martinelli G, Vieira CM, Gonzalez M, Leitman P, Piratininga A, Costa AF, Forzza RC (2008) Bromeliaceae da Mata Atlântica Brasileira: lista de espécies, distribuição e conservação. Rodriguésia 59:209–258. https://doi.org/10.1590/2175-7860200859114
Martínez Gamba R (2014) Lista de aves del Parque Natural Municipal Monte Seguín, Provincia de Misiones, Argentina. Not Faun, Seg Ser 163:1–10
McCarthy M, Moore J, Morris W, Parris K, Garrard G, Vesk P, Rumpff L, Giljohann K, Camac J, Bau S, Friend T, Harrison B, Yue B (2012) The influence of abundance on detectability. Oikos 122:717–726. https://doi.org/10.1111/j.1600-0706.2012.20781.x
Nascimento JLX (1996) Aves da Floresta Nacional do Araripe, Ceará. IBAMA, Brasília
Nascimento JLX, Nascimento ILS, Azevedo Júnior SM (2000) Aves da Chapada do Araripe (Brasil): biologia e conservação. Ararajuba 8:115–125
Pacheco JF, Whitney BM (1995) Range extensions for some birds in northeastern Brazil. Bull Brit Ornithol Club 115:157–163
Parker TA, Stotz DF, Fitzpatrick JW (1996) Ecological and distributional databases. In: Stotz DF, Fitzpatrick JW, Parker TA, Moskovits DK (eds) Neotropical birds: ecology and conservation. University of Chicago Press, Chicago, pp 115–436
Pearman M, Areta JI (2020) Birds of Argentina and the south-west Atlantic. Bloomsbury, London
Pereira GA, Dantas SM, Silveira LF, Roda SA, Albano C, Sonntag FA, Leal S, Periquito MC, Malacco GB, Lees AC (2014) Status of the globally threatened forest birds of northeast Brazil. Pap Avul Zool 54:177–194. https://doi.org/10.1590/0031-1049.2014.54.14
Peters JL (1945) Check-list of birds of the world, vol 5. Harvard Univ Press, Cambridge, MA
Piacentini VQ (2018) Thalurania watertonii (Bourcier, 1847). In: ICMBio. Livro vermelho da fauna brasileira ameaçada de extinção: Volume III—Aves. ICMBio, Brasília
Piacentini VQ, Ribenboim LCC (2017) Beija-flores do Brasil. Aves & Fotos Editora, São Paulo
Pinto OMO (1944) Catálogo das aves do Brasil. Segunda parte. Departamento de Zoologia, São Paulo
Pinto OMO (1978) Novo catálogo das aves do Brasil. Primeira parte. Conselho Nacional de Desenvolvimento Científico e Tecnológico, São Paulo
Portes CEB, Godoy FI, Kuniy AA (2018) Avifauna de três fragmentos de vegetação no litoral norte do estado de Alagoas, com ênfase em novos registros de aves ameaçadas. Atual Ornitol 204:33–42
Robinson OJ, Socolar JB, Stuber EF et al (2022) Extreme uncertainty and unquantifiable bias do not inform population sizes. Proc Natl Acad Sci USA 119:e2113862119. https://doi.org/10.1073/pnas.2113862119
Roda SA (2002) Aves endêmicas e ameaçadas de extinção do estado de Pernambuco. In: Tabarelli M, Silva, JMC (eds) Diagnóstico da biodiversidade de Pernambuco. Secretaria de Ciência, Tecnologia e Meio Ambiente. Editora Massangana, Recife
Roda SA (2008) Thalurania watertonii (Bourcier, 1847). In: Machado ABM, Drummond GM, Paglia AP (eds) Livro vermelho da fauna ameaçada de extinção no Brasil. Vol 2. Ministério do Meio Ambiente e Fundação Biodiversitas, Brasília
Roda SA, Carlos CJ (2004) Composição e sensitividade da avifauna dos brejos de altitude do Estado de Pernambuco. In: Porto KC, Cabral JJP, Tabarelli M (eds) Brejos de altitude em Pernambuco e Paraíba: história natural, ecologia e conservação. Ministerio do Meio Ambiente, Brasília, pp 211‒228
Ruiz-Esparza J, Santos CS, Cunha MA, Ruiz-Esparza DPB, Rocha PA, Beltrão-Mendes R, Ferrari SF (2015) Diversity of birds in the Mata do Junco State Wildlife Refuge, a remnant of the Atlantic Forest of northeastern Brazil. Check List 11:1647. https://doi.org/10.15560/11.3.1647
Ruschi A (1964a) Os nomes vulgares dos beija-flores do Estado do Ceará. Bol Mus Biol Prof Mello Leitão 16:1–3
Ruschi A (1964b) A estação ou período de reprodução nos beija-flores. Bol Mus Biol Prof Mello Leitão 42:1–9
Ruschi A (1986) Aves do Brasil, volume 5: beija-flores. Espressão e Cultura, Rio de Janeiro
Sagot-Martin F, Lima RD, Pacheco JF, Bañuelos Irusta J, Pichorim M, Hassett DM (2020) An updated checklist of the birds of Rio Grande do Norte, Brazil, with comments on new, rare, and unconfirmed species. Bull Brit Ornithol Club 140:218–298. https://doi.org/10.25226/bboc.v140i3.2020.a2
Saibene CA, Castelino MA, Rey NR, Herrera J, Caló J (1996) Inventario de las aves del Parque Nacional ‘Iguazú’, Misiones, Argentina. Monografía 9. Literature of Latin America, Buenos Aires
Salvin O (1892) Suborder Trochili. In: Hartert E, Salvin O (eds) Catalogue of the birds in the British Museum, vol 16. Trustees of the British Museum (Natural History), London, pp 27–433
Santini L, Isaac NJB, Maiorano L, Ficetola GF, Huijbregts MAJ, Carbone C, Thuiller W (2018) Global drivers of population density in terrestrial vertebrates. Glob Ecol Biogeogr 27:968–979. https://doi.org/10.1111/geb.12758
Sargeant DE, Wall JW (1995) Bahia. A birder’s guide to Bahia, NE Brazil. Privately published, Norfolk
Savigny C (2010) Aportes al conocimiento de la avifauna del Parque Nacional Iguazú y alrededores. Nuestras Aves 55:20–22
Schuchmann K-L (1999) Family Trochilidae (hummingbirds). In: del Hoyo J, Elliott A, Sargatal J (eds) Handbook of the birds of the world 5. Lynx Edicions, Barcelona, pp 468–680
Schuchmann K-L, Kirwan GM, Sharpe CJ (2020) Long-tailed woodnymph (Thalurania watertonii), version 1.0. In: del Hoyo J, Elliott A, Sargatal J, Christie DA, de Juana E (eds) Cornell Lab of Ornithology, Ithaca. https://doi.org/10.2173/bow.lotwoo2.01
Silva C, Ruiz-Esparza J, Azevedo CS, Souza Ribeiro A (2022) Avifauna of the Serra de Itabaiana National Park revisited: additions and deletions in a period of 15 years. Acta Sci Biol Sci 44:e59923. https://doi.org/10.4025/actascibiolsci.v44i1.59923
Silva WAG, Albano CG (2002) Lista remissiva da avifauna cearense. Observadores de Aves de Pernambuco, Recife
Silveira LF, Olmos F, Long AJ (2003) Birds in Atlantic Forest fragments in north-east Brazil. Cotinga 20:32–46
Silveira LF, Develey PF, Pacheco JF, Whitney BM (2005) Avifauna of the Serra das Lontras-Javi montane complex, Bahia, Brazil. Cotinga 24:45–54
Simon E (1897) Catalogue des espèces actuellement connues de la famille des trochilidés. Encyclopédie Roret, Paris
Simon E (1921) Histoire naturelle des trochilidés (synopsis et catalogue). Encyclopédie Roret, Paris
Sousa MC (2009) As aves de oito localidades do Estado de Sergipe. Atual Ornitol 149:33–57
Souza CM Jr, Shimbo JZ, Rosa MR et al (2020) Reconstructing three decades of land use and land cover changes in Brazilian biomes with Landsat archive and Earth Engine. Remote Sens 12:2735. https://doi.org/10.3390/rs12172735
Stephens P, Vieira M, Willis S, Carbone C (2019) The limits to population density in birds and mammals. Ecol Lett 22:654–663. https://doi.org/10.1111/ele.13227
Stiles FG, Remsen JV, McGuire JA (2017) The generic classification of the Trochilini (Aves: Trochilidae): reconciling taxonomy with phylogeny. Zootaxa 4353:401–424. https://doi.org/10.11646/zootaxa.4353.3.1
Studer A (2015) Aves (Aves) da Reserva Biológica de Pedra Talhada. In: Studer A, Nusbaumer L, Spichiger R (eds) Biodiversidade da Reserva Biológica de Pedra Talhada (Alagoas, Pernambuco - Brasil). Boissiera: mémoires des Conservatoire et jardim botaniques de la Ville de Genève 68:1–818
Teixeira DM (1988) Observações preliminares sobre a avifauna da Floresta Nacional de Araripe, município do Crato, Ceará. Annex to the final report of the project ‘Aves ameaçadas de extinção na Mata Atlântica do nordeste brasileiro’. WWF-US and FBCN. [Copy available on request from the authors.]
Terborgh J, Robinson SK, Parker TA, Munn CA, Pierpont N (1990) Structure and organization of an Amazonian forest bird community. Ecol Monogr 60:213–238. https://doi.org/10.2307/1943045
Thiollay J-M (1986) Structure comparée du peuplement avien dans trois sites de forêt primaire en Guyane. Rev Ecol (Terre Vie) 41:59–105
Thiollay J-M (1992) Influence of selective logging on bird species diversity in a Guianan rain forest. Conserv Biol 6:47–63. https://doi.org/10.1046/j.1523-1739.1992.610047.x
Tripp EA, McDade LA (2012) New synonymies for Ruellia (Acanthaceae) of Costa Rica and notes on other neotropical species. Brittonia 64:305–317. https://doi.org/10.1007/s12228-012-9244-2
Wege DC, Long AJ (1995) Key areas for threatened birds in the Neotropics. BirdLife Conservation Series 5. BirdLife International, Cambridge
Acknowledgements
We thank the curators of the museums we visited while researching this paper (Paul Sweet at AMNH, Marcos Raposo at MNRJ, Mark Adams and Hein van Grouw at NHMUK) for their help, as well as those others who responded to our inquiries (Helder F. P. de Araujo at CAHZ, Ricardo Jorge Lopes at MHNC-UP, Patrick Boussès at MNHN, Luís Fábio Silveira at MZUSP, Layse Albuquerque and Luciano N. Naka at UFPE, and Cordula Bracker at ZMH); also Cornell Lab of Ornithology for providing the eBird dataset; Nathan Ruser and Jemma Vine for assistance with mapping; K.-L. Schuchmann, Fernando Godoy and Dan Branch for answering inquiries; Dione Seripierri, Luiz Carlos Ramassotti, Wanieulli Pascoal and Weber Girão for bibliographic help; the SAVE Brasil team for the aid provided during fieldwork in the Serra do Urubu mountains; and Louis Nusbaumer, Alain Chautems, R. Louzada and Victor Farjalla Pontes for identifying many of the foodplants. Two anonymous referees kindly read the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Caio Graco Machado (Associated Editor)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Berryman, A.J., Collar, N.J., Crozariol, M.A. et al. The distribution, ecology and conservation status of the long-tailed woodnymph Thalurania watertonii. Ornithol. Res. 31, 1–12 (2023). https://doi.org/10.1007/s43388-022-00110-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43388-022-00110-4