Abstract
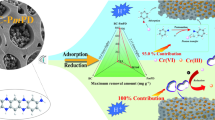
Heavy metal ion pollution of water resources is becoming increasingly serious, and adsorption is one of the most effective strategies for removing heavy metal ions. In the paper, hydrated hydrogen sodium vanadium oxide (HNaV6O16⋅4H2O) film developed for heavy metal ion adsorption was prepared directly via a low-temperature liquid-phase deposition approach. The prepared film shows an interesting porous flower-like morphology and has large spacing (d = 10.87 Å). The highest adsorption capacity of the obtained HNaV6O16⋅4H2O film for Pb2+, Cu2+, Cd2+ and Mn2+ is 513 mg/g (2565 mg/m2), 430 mg/g (2150 mg/m2), 134 mg/g (875 mg/m2) and 175 mg/g (670 mg/m2), respectively. The adsorption percentage of the sample decreased from 92.2 to 86.3% after 4 cycles. The adsorption process follows the Langmuir adsorption isotherm and the pseudo second-order dynamic model, indicating that heavy metal ion adsorption by the film is a single molecular layer chemical adsorption. In combination with various characterizations and comparison tests of samples after adsorption, the adsorption mechanisms include surface electrostatic attraction, complexation, and cation exchange. The results indicate that the film is a potential material to remove heavy metal ions from the aqueous solution.















Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this article.
References
X. He, T. Zhang, Q. Xue, Y. Zhou, H. Wang, N.S. Bolan, R. Jiang, D.C.W. Tsang, Enhanced adsorption of Cu(II) and Zn(II) from aqueous solution by polyethyleneimine modified straw hydrochar. Sci. Total. Environ. 778, 146116 (2021)
A. El-Sikaily, A. El Nemr, A. Khaled, O. Abdelwehab, Removal of toxic chromium from wastewater using green alga Ulva lactuca and its activated carbon. J. Hazard. Mater. 148(1–2), 216–228 (2007)
T.S. Anirudhan, S. Jalajamony, S.S. Sreekumari, Adsorption of heavy metal ions from aqueous solutions by amine and carboxylate functionalised bentonites. Appl. Clay. Sci. 65, 67–71 (2012)
D. Purchase, L.N.L. Scholes, D.M. Revitt, R.B.T. Shutes, Effects of temperature on metal tolerance and the accumulation of Zn and Pb by metal-tolerant fungi isolated from urban runoff treatment wetlands. J. Appl. Microbiol. 106(4), 1163–1174 (2008)
Z. Ahmad, B. Gao, A. Mosa, H. Yu, X. Yin, A. Bashir, H. Ghoveisi, S. Wang, Removal of Cu(II), Cd(II) and Pb(II) ions from aqueous solutions by biochars derived from potassium-rich biomass. J. Clean. Prod. 180, 437–449 (2018)
I. Chakraborty, S.M. Sathe, C.N. Khuman, M.M. Ghangrekar, Bioelectrochemically powered remediation of xenobiotic compounds and heavy metal toxicity using microbial fuel cell and microbial electrolysis cell. Mater. Sci. Energy. Technol. 3, 104–115 (2019)
M. Chen, D. Wang, S. Ding, X. Fan, Z. Jin, Y. Wu, Y. Wang, C. Zhang, Zinc pollution in zones dominated by algae and submerged macrophytes in Lake Taihu. Sci. Total. Environ. 670, 361–368 (2019)
C. McManamon, A.M. Burke, J.D. Holmes, M.A. Morris, Amine-functionalised SBA-15 of tailored pore size for heavy metal adsorption. J. Colloid Interf. Sci. 369(1), 330–337 (2011)
S. Das, V.V. Goud, Characterization of a low-cost adsorbent derived from agro-waste for ranitidine removal. Mater. Sci. Energy Technol. 3, 879–888 (2020)
K. Yin, Q. Wang, M. Lv, L. Chen, Microorganism remediation strategies towards heavy metals. Chem. Eng. J. 360, 1553–1563 (2018)
Q. Li, W. Song, M. Sun, J. Li, Z. Yu, Response of Bacillus vallismortis sp. EPS to exogenous sulfur stress/ induction and its adsorption performance on Cu(II). Chemosphere 251, 126343 (2020)
X. Luo, Z. Zhang, P. Zhou, Y. Liu, G. Ma, Z. Lei, Synergic adsorption of acid blue 80 and heavy metal ions (Cu2+/Ni2+) onto activated carbon and its mechanisms. J. Ind. Eng. Chem. 27, 164–174 (2014)
Z. Guan, J. Lv, P. Bai, X. Guo, Boron removal from aqueous solutions by adsorption—a review. Desalination 383, 29–37 (2015)
Y. Matsui, T. Yoshida, S. Nakao, D.R.U. Knappe, T. Matsushita, Characteristics of competitive adsorption between 2-methylisoborneol and natural organic matter on superfine and conventionally sized powdered activated carbons. Water Res. 46(15), 4741–4749 (2012)
E. Padilla-Ortega, R. Leyva-Ramos, J.V. Flores-Cano, Binary adsorption of heavy metals from aqueous solution onto natural clays. Chem. Eng. J. 225, 535–546 (2013)
Q. Huang, L.X. Zou, P. Lan, C. Yang, Z.Y. Jing, Y.Z. Xu, J.F. Xu, Synthesis of the Y nanometer zeolites from fly ash and its adsorption models for aqueous Cs+ ions. J. Radioanal. Nucl. Chem. 323(1), 65–72 (2019)
D. Panchal, A. Sharma, P. Mondal, Heterolayered TiO2@layered double hydroxide-MoS2 nanostructure for simultaneous adsorption-photocatalysis of co-existing water contaminants. Appl. Sur. Sci. 553, 149577 (2021)
W. Yu, F. Lian, G. Cui, Z.Q. Liu, N-doping effectively enhances the adsorption capacity of biochar for heavy metal ions from aqueous solution. Chemosphere 193, 8–16 (2018)
D.K. Chanda, D. Mukherjee, P.S. Das, C.K. Ghosh, A.K. Mukhopadhyay, Toxic heavy metal ion adsorption kinetics of Mg(OH)2 nanostructures with superb efficacies. Mater. Res. Express. 5(7), 075027 (2018)
A. Stafiej, K. Pyrzynska, Adsorption of heavy metal ions with carbon nanotubes. Sep. Purif. Technol. 58, 49–52 (2007)
L. Bai, L. Jia, Z.D. Yan, Z.C. Liu, Y.Q. Liu, Plasma-etched electrospun nanofiber membrane as adsorbent for dye removal. Chem. Eng. Res. Des 132, 445–451 (2018)
D.J. Yang, Z.F. Zheng, H.W. Liu, H.Y. Zhu, X.B. Ke, Y. Xu, D. Wu, Y. Sun, Layered titanate nanofibers as efficient adsorbents for removal of toxic radioactive and heavy metal ions from water. J. Phys. Chem. C. 112(42), 16275–16280 (2008)
J.K. Rama, K.N. Ashish, U.K. Moni, P. Anjali, Surfactant bilayer on chitosan bead surface for enhanced Ni(II) adsorption. Sustain. Mater. Technol. 18, e00077 (2018)
Z. Modrzejewska, K. Wladyslaw, Separation of Cr(VI) on chitosan membranes. Ind. Eng. Chem. Res. 38(12), 4946–4950 (2000)
M.U. Tahir, X.T. Su, M. Zhao, Y.N. Liao, R. Wu, D. Chen, Preparation of hydroxypropyl-cyclodextrin-graphene/Fe3O4 and its adsorption properties for heavy metals. Surf. Interfaces. 16, 43–49 (2019)
H.C. Wang, Z.W. Wang, R.R. Yue, F. Gao, R. Ren, J.F. Wei, X.L. Wang, Z.Y. Kong, Functional group-rich hyperbranched magnetic material for simultaneous efficient removal of heavy metal ions from aqueous solution. J. Hazard. Mater. 384, 121288 (2020)
J. Chen, H.Y. Xu, J.H. Ruan, Y.M. Xin, Y. Li, D.C. Li, A.G. Wang, D.S. Sun, Preparation and electrochemical performance of binder-free sodium vanadium bronze thin film electrodes based on a low temperature liquid phase deposition method. Mater. Chem. Phys. 249, 122935 (2020)
S. Kasap, I. kaya, E. Erdem, Superbat: battery-like supercapacitor utilized by graphene foam and zinc oxide (ZnO) electrodes induced by structural defects. Nanoscale Adv. 1(7), 2586–2597 (2019)
H.Y. Xu, J.H. Ruan, F.L. Liu, D.C. Li, F.J. Zhang, A.G. Wang, D.S. Sun, W.C. Oh, Preparation of lithium-doped NaV6O15 thin film cathodes with high cycling performance in SIBs. J. Korean Ceram. Soc. (2021). https://doi.org/10.1007/s43207-021-00127-3
R. Sun, X. Ji, C. Luo, S. Hou, P. Hu, X. Pu, L. Cao, L. Mai, C. Wang, Water-pillared sodium vanadium bronze nanowires for enhanced rechargeable magnesium ion storage. Small 16(30), 2000741 (2020)
M. Najdoski, V. Koleva, S. Demiri, Chemical bath deposition and characterization of electrochromic thin films of sodium vanadium bronzes. Mater. Res. Bull. 47(3), 737–743 (2011)
R. Dobrowolski, A. Szcześ, M. Czemierska, A. Jarosz-Wikołazka, Studies of cadmium(II), lead(II), nickel(II), cobalt(II) and chromium(VI) sorption on extracellular polymeric substances produced by Rhodococcus opacus and Rhodococcus rhodochrous. Bioresour. Technol. 225, 113–120 (2016)
L. Wei, Y. Li, D.R. Noguera, N. Zhao, Y. Song, J. Ding, Q. Zhao, F. Cui, Adsorption of Cu2+ and Zn2+ by extracellular polymeric substances (EPS) in different sludges: effect of EPS fractional polarity on binding mechanism. J. Hazard. Mater. 321, 473–483 (2016)
J. Mu, F. Teng, H. Miao, Y. Wang, X. Hu, In-situ oxidation fabrication of 0D/2D SnO2/SnS2 novel Step-scheme heterojunctions with enhanced photoelectrochemical activity for water splitting. Appl. Surf. Sci. 501, 143974 (2020)
W.J. Lee, J.S. Lee, D. Atarashi, Y.H. Kim, S.H.Lee, Pore structure and possibility of fine dust removal for bottom ash sand. J. Korean Ceram. Soc. 57(4), 378–384 (2020)
H. Choi, H.J. Kang, M.S. Song, E.D. Jung, J.S. Kim, Solidification of heavy metal ions using magnesia-phosphate cement. J. Korean Ceram. Soc. 48(1), 20–20 (2011)
S. Tajik, H. Beitollahi, F. Garkani Nejad, I. Sheikhshoaie, A.S. Nugraha, H.W. Jang, Y. Yamauchi, M. Shokouhimehr, Performance of metal–organic frameworks in the electrochemical sensing of environmental pollutants. J. Mater. Chem. A. 9(13), 8195–8220 (2021)
H. Liu, B. Lian, Quantitative evaluation of different fractions of extracellular polymeric substances derived from Paenibacillus mucilaginosus against the toxicity of gold ions. Colloids Surf. B. 175, 195–201 (2018)
W. Yu, F. Lian, G. Cui, Z. Liu, N-doping effectively enhances the adsorption capacity of biochar for heavy metal ions from aqueous solution. Chemosphere 193, 8–16 (2017)
F.A. Soria, C.D. Valentin, Binding group of oligonucleotides on TiO2 surfaces: Phosphate anions or nucleobases. Appl. Surf. Sci. 575, 151560 (2021)
F. Mei, K. Dai, J. Zhang, W. Li, C. Liang, Construction of Ag SPR-promoted step-scheme porous g-C3N4/Ag3VO4 heterojunction for improving photocatalytic activity. Appl. Surf. Sci. 488, 151–160 (2019)
J. Kim, J.Y. Jo, I.G. Choi, Y.C. Kim, Search for adsorption geometry of precursor on surface using genetic algorithm: MoO2Cl2 on SiO2 surface. J. Korean Ceram. Soc. 57(6), 669–675 (2020)
B. Nayebi, K.P. Niavol, B. Nayebi, S. Young Kim, K.T. Nam, H.W. Jang, R.S. Varma, M. Shokouhimehr, Prussian blue-based nanostructured materials: Catalytic applications for environmental remediation and energy conversion. Mol. Catal. 514, 11835 (2021)
P. Tan, Y.Y. Hu, Q. Bi, Competitive adsorption of Cu2+, Cd2+ and Ni2+ from an aqueous solution on graphene oxide membranes. Colloid Surf. A. 509, 56–64 (2016)
C.H. Xu, Y. Feng, H.R. Li, R.F. Wu, J.R. Ju, S.L. Liu, Y. Yang, B. Wang, Adsorption of heavy metal ions by iron tailings: Behavior, mechanism, evaluation and new perspectives. J. Clean. Prod. 344, 131065 (2022)
L. Ma, S.M. Isiam, C. Xiao, J. Zhao, H. Liu, M. Yuan, G. Sun, H. Li, S. Ma, M.G. Kanatzidis, Rapid simultaneous removal of toxic anions [HSeO3]-, [SeO3]2-, and [SeO4]2-, and metals Hg2+, Cu2+, and Cd2+ by MoS42- intercalated layered double hydroxide. J. Am. Chem. Soc. 139(36), 12745–12757 (2017)
A.N.A. Hosain, E. Nemr, E. Sikaily, M.E. Mahmoud, M.F. Amira, Surface modifications of nanochitosan coated magnetic nanoparticles and their applications in Pb (II), Cu (II) and Cd (II). Remov J. Environ. Chem. Eng. 8, 104316 (2020)
U.T. Uthappa, G. Sriram, O.R. Arvind, S. Kumar, Y.J. Ho, G.M. Neelgund, D. Losic, M.D. Kurkuri, Engineering MIL-100(Fe) on 3D porous natural diatoms as a versatile high performing platform for controlled isoniazid drug release, Fenton’s catalysis for malachite green dye degradation and environmental adsorbents for Pb2+ removal and dyes. Appl. Surf. Sci. 528, 146974 (2020)
C. Liu, T. Wu, P.C. Hsu, J. Xie, J. zhao, Direct/alternating current electrochemical method for removing and recovering heavy metal from water using graphene oxide electrode. ACS Nano 13(6), 6431–6437 (2019)
J. Sun, F. Lian, Z. Liu, L. Zhu, Z. Song, Biochars derived from various crop straws: characterization and Cd(II) removal potential. Ecotoxicol. Environ. Saf. 106, 226–231 (2014)
X.J. Tong, J.Y. Li, J.H. Yuan, R.K. Xu, Adsorption of Cu(II) by biochars generated from three crop straws. Chem. Eng. J. 172(2–3), 828–834 (2011)
Acknowledgements
Financial supports from National key R&D program of China (2019YFE03070001), Excellent Young Talents Fund Program of Higher Education Institutions of Anhui Province (gxyqZD2016150), and the Key Research and Development Projects of Anhui Province (202004b11020033) are acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest with this work.
Ethical approval
All data generated or analyzed during this study are included in this published article.
Research involving human and animals’ rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, H.Y., Yang, Y.C., Li, D.C. et al. A liquid phase deposited porous flower-like HNaV6O16⋅4H2O film developed for a novel adsorbent to remove Pb2+, Cu2+, Mn2+ and Cd2+. J. Korean Ceram. Soc. 60, 474–487 (2023). https://doi.org/10.1007/s43207-022-00224-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43207-022-00224-x