Abstract
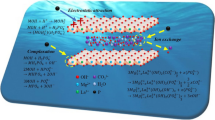
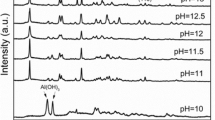
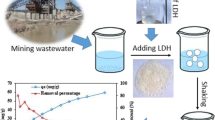
Layered double hydroxides (LDHs), known as a class of anionic clays, have attracted considerable attention recently due to their potential applications in different areas as catalyst materials, energy materials, and adsorbent materials for environmental remediation, especially for anionic pollutant removal. In this study, magnesium aluminum layered double hydroxide (MgAl-LDH) was synthesized by two methods: standard coprecipitation and urea hydrolysis. Their textural properties and morphologies were examined by X-ray powder diffraction (XRD), scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDS), Fourier transform infrared spectroscopy (FTIR), thermogravimetry (TG) and differential (DTG) analysis, and point of zero charge (pHpzc). The specific surface area was calculated from BET adsorption equation. The results indicated that the crystallinity and the regularity of the samples prepared by urea hydrolysis were much preferable to those prepared by the coprecipitation method. Their sorption properties toward phosphate were investigated and the experimental evidence showed that, at the initial concentration of 100 mg L−1 and at room temperature, the LDH synthesized by urea hydrolysis had a percentage removal of 94.3 ± 1.12% toward phosphate ions while 74.1 ± 1.34% were uptaked by LDH synthesized by coprecipitation method, suggesting that the crystallinity affects the sorption capability. The sorption mechanism indicates that phosphate ions could be sorbed onto LDHs via electrostatic attraction, ligand exchange, and ion exchange.









Similar content being viewed by others
References
Adachi-Pagano M, Forano C, Besse J-P (2003) Synthesis of Al-rich hydrotalcite-like compounds by using the urea hydrolysis reaction-control of size and morphology. J Mater Chem 13:1988–1993
Boclair JW, Braterman PS, Jiang J, Lou S, Yarberry F (1999) Layered double hydroxide stability. 2. Formation of Cr(III)-containing layered double hydroxides directly from solution. Chem Mater 11:303–307
Bruun Hansen HC, Koch CB (1995) Synthesis and characterization of pyroaurite. Appl Clay Sci 10:5–19
Carja G, Nakamura R, Niiyama H (2002) Copper and iron substituted hydrotalcites: properties and catalyst precursors for methylamines synthesis. Appl Catal A Gen 236:91–102
Cavani F, Trifirò F, Vaccari A (1991) Hydrotalcite-type anionic clays: preparation, properties and applications. Catal Today 11:173–301
Chiban M, Soudani A, Sinan F, Tahrouch S, Persin M (2011) Characterization and application of dried plants to remove heavy metals, nitrate, and phosphate ions from industrial wastewaters. Clean Soil Air Water 39:376–383
Chiban M, Zerbet M, Sinan F (2012) Low-cost materials for phosphate removal from aqueous solutions, Handbook of phosphates: sources, properties and applications. Nova Science Publishers, Inc, New York, pp 1–42
Costantino U, Marmottini F, Nocchetti M, Vivani R (1998) New synthetic routes to hydrotalcite-like compounds−characterisation and properties of the obtained materials. Eur J Inorg Chem 1998:1439–1446
Cullity BD (1978) Elements of X-ray diffraction, 2nd edn. Addison-Wesley, Reading, pp 284–501
Jitianu M, Zaharescu M, Bãlãsoiu M, Jitianu A (2003) The sol-gel route in synthesis of Cr(III)-containing clays. Comparison between Mg-Cr and Ni-Cr anionic clays. J Sol-Gel Sci Technol 26:217–221
Kang HR, Da Costa Fernandes CJ, Da Silva RA, Constantino VRL, Koh IHJ, Zambuzzi WF (2018) Mg-Al and Zn-Al layered double hydroxides promote dynamic expression of marker genes in osteogenic differentiation by modulating mitogen-activated protein kinases. Adv Healthc Mater:7
Khitous M, Salem Z, Halliche D (2016) Removal of phosphate from industrial wastewater using uncalcined MgAl-NO3 layered double hydroxide: batch study and modeling. Desalin Water Treat 57:15920–15931
Liu Z, Ma R, Osada M, Iyi N, Ebina Y, Takada K, Sasaki T (2006) Synthesis, anion exchange, and delamination of Co−al layered double hydroxide: assembly of the exfoliated nanosheet/polyanion composite films and magneto-optical studies. J Am Chem Soc 128:4872–4880
Liu H, Sun X, Yin C, Hu C (2008) Removal of phosphate by mesoporous ZrO2. J Hazard Mater 151:616–622
Mandal S, Tichit D, Lerner DA, Marcotte N (2009) azoic dye hosted in layered double hydroxide: physicochemical characterization of the intercalated materials. Langmuir 25:10980–10986
Miyata S (1980) Physico-chemical properties of synthetic hydrotalcites in relation to composition. Clay Clay Miner 28:50–56
Novillo C, Guaya D, Allen-Perkins Avendaño A, Armijos C, Cortina JL, Cota I (2014) Evaluation of phosphate removal capacity of Mg/Al layered double hydroxides from aqueous solutions. Fuel 138:72–79
Ogawa M, Kaiho H (2002) Homogeneous precipitation of uniform hydrotalcite particles. Langmuir 18:4240–4242. https://doi.org/10.1021/la0117045
Oh J-M, Hwang S-H, Choy J-H (2002) The effect of synthetic conditions on tailoring the size of hydrotalcite particles. Solid State Ionics 151:285–291
Rodier J, Legube B, Merlet N (2009) L’analyse de l’eau 9e édition Dunod, Paris
Sepehr MN, Al-Musawi TJ, Ghahramani E, Kazemian H, Zarrabi M (2017) Adsorption performance of magnesium/aluminum layered double hydroxide nanoparticles for metronidazole from aqueous solution. Arab J Chem 10:611–623
Tanasoi S, Tanchoux N, Urdă A, Tichit D, Săndulescu I, Fajula F, Marcu I-C (2009) New Cu-based mixed oxides obtained from LDH precursors, catalysts for methane total oxidation. Appl Catal A Gen 363:135–142
Titulaer MK, Jansen JBH, Geus JW (1994) The quantity of reduced nickel in synthetic takovite: effects of preparation conditions and calcination temperature. Clay Clay Miner 42:249–258
Wang F, Guo Z (2019) Facile synthesis of superhydrophobic three-metal-component layered double hydroxide films on aluminum foils for highly improved corrosion inhibition. New J Chem 43:2289–2298
Wei J, Gao Z, Song Y, Yang W, Wang J, Li Z, Mann T, Zhang M, Liu L (2013) Solvothermal synthesis of Li-Al layered double hydroxides and their electrochemical performance. Mater Chem Phys 139:395–402
Xing K, Wang H, Guo L, Song W, Zhao Z (2008) Adsorption of tripolyphosphate from aqueous solution by Mg–Al–CO3-layered double hydroxides. Colloids Surf A Physicochem Eng Asp 328:15–20
Xu ZP, Stevenson G, Lu C-Q, Lu GQM (2006a) Dispersion and size control of layered double hydroxide nanoparticles in aqueous solutions. J Phys Chem B 110:16923–16929. https://doi.org/10.1021/jp062281o
Xu ZP, Stevenson GS, Lu C-Q, Lu GQM, Bartlett PF, Gray PP (2006b) Stable suspension of layered double hydroxide nanoparticles in aqueous solution. J Am Chem Soc 128:36–37. https://doi.org/10.1021/ja056652a
Yang K, Yan L, Yang Y, Yu S, Shan R, Yu H, Zhu B, Du B (2014) Adsorptive removal of phosphate by Mg–Al and Zn–Al layered double hydroxides: kinetics, isotherms and mechanisms. Sep Purif Technol 124:36–42
Zeng H-Y, Deng X, Wang Y-J, Liao K-B (2009) Preparation of Mg-Al hydrotalcite by urea method and its catalytic activity for transesterification. AICHE J 55:1229–1235. https://doi.org/10.1002/aic.11722
Zhao Y, Li F, Zhang R, Evans DG, Duan X (2002) Preparation of layered double-hydroxide nanomaterials with a uniform crystallite size using a new method involving separate nucleation and aging steps. Chem Mater 14:4286–4291
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Benhiti, R., Ait Ichou, A., Zaghloul, A. et al. Synthesis, characterization, and comparative study of MgAl-LDHs prepared by standard coprecipitation and urea hydrolysis methods for phosphate removal. Environ Sci Pollut Res 27, 45767–45774 (2020). https://doi.org/10.1007/s11356-020-10444-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-10444-5