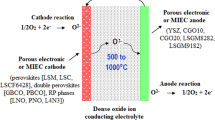
Abstract
Solid oxide fuel cells (SOFCs), which are widely viewed as the next-generation energy conversion devices, provide environmentally friendly power generation by direct conversion of chemical energy with high efficiency and less pollutant emissions. However, their high operating temperatures limit their usability in applications, such as distributed generation of electricity and heat, power plants, and transportation. At reduced temperatures, the electrolytes and electrodes used in SOFCs experience sluggish oxygen transport kinetics. Therefore, the development of materials with high oxygen ion conduction and unique cell designs is needed to achieve higher performance. This article provides an overview of the recent progress on solid oxide electrolyte materials, unique cell designs featuring bilayer electrolytes, and resulting microstructures at lower operating temperatures.

Copyright 2017 Wiley

Copyright 2019 RSC

Copyright 2006 PNAS

Copyright 1978 Elsevier

Copyright 1981 IOP publishing

Copyright 1996 IOP publishing

Copyright 2009 AAAS

Copyright 2012 Elsevier

Copyright 2012 Wiley

Copyright 2021 American Chemical Society

Copyright 2017 American Chemical Society

Copyright 1997 IOP publishing

Copyright 2011 AAAS

Copyright 2012 Elsevier

Copyright 2012 RSC

Copyright 2019 Springer

Copyright 2006 Elsevier

Copyright 2018 Elsevier

Copyright 2014 Elsevier

Copyright 2017 ACS. Copyright 2020 RSC
Similar content being viewed by others
References
E.D. Wachsman, C.A. Marlowe, K.T. Lee, Role of solid oxide fuel cells in a balanced energy strategy. Energy Environ. Sci. 5(2), 5498–5509 (2012)
E.D. Wachsman, K.T. Lee, Lowering the temperature of solid oxide fuel cells. Science 334(6058), 935–939 (2011)
R.M. Ormerod, Solid oxide fuel cells. Chem. Soc. Rev. 32(1), 17–28 (2003)
O. Yamamoto, Solid oxide fuel cells: fundamental aspects and prospects. Electrochim. Acta 45(15–16), 2423–2435 (2000)
S.C. Singhal, Solid oxide fuel cells for stationary, mobile, and military applications. Solid State Ionics 152, 405–410 (2002)
T.M. Gür, Review of electrical energy storage technologies, materials and systems: challenges and prospects for large-scale grid storage. Energy Environ. Sci. 11(10), 2696–2767 (2018)
P. Boldrin, N.P. Brandon, Progress and outlook for solid oxide fuel cells for transportation applications. Nat. Catal. 2(7), 571–577 (2019)
S.J. Skinner, Recent advances in Perovskite-type materials for solid oxide fuel cell cathodes. Int. J. Inorg. Mater. 3(2), 113–121 (2001)
A.B. Stambouli, E. Traversa, Solid oxide fuel cells (SOFCs): a review of an environmentally clean and efficient source of energy. Renew. Sustain. Energy Rev. 6(5), 433–455 (2002)
N.Q. Minh, Solid oxide fuel cell technology—features and applications. Solid State Ionics 174(1–4), 271–277 (2004)
J.H. Park, H.-N. Im, K.T. Lee, Understanding redox cycling behavior of Ni–YSZ anodes at 500° C in solid oxide fuel cells by electrochemical impedance analysis. J. Korean Ceram. Soc. 58(5), 606–613 (2021)
S. Jo, B. Sharma, D.-H. Park, J.-H. Myung, Materials and nano-structural processes for use in solid oxide fuel cells: a review. J. Korean Ceram. Soc. 57(2), 135–151 (2020)
H.-W. Lee, H.-I. Ji, J.-H. Lee, B.-K. Kim, K.J. Yoon, J.-W. Son, Powder packing behavior and constrained sintering in powder processing of solid oxide fuel cells (SOFCs). J. Korean Ceram. Soc. 56(2), 130–145 (2019)
D. Kim, J.W. Park, B.-H. Yun, J.H. Park, K.T. Lee, Correlation of time-dependent oxygen surface exchange kinetics with surface chemistry of La0. 6Sr0. 4Co0. 2Fe0. 8O3− δ catalysts. ACS Appl. Mate. Interfaces 11(35), 31786–31792 (2019)
H. Ullmann, N. Trofimenko, F. Tietz, D. Stöver, A. Ahmad-Khanlou, Correlation between thermal expansion and oxide ion transport in mixed conducting perovskite-type oxides for SOFC cathodes. Solid State Ionics 138(1–2), 79–90 (2000)
S.-I. Lee, J. Hong, H. Kim, J.-W. Son, J.-H. Lee, B.-K. Kim et al., Highly dense Mn-Co spinel coating for protection of metallic interconnect of solid oxide fuel cells. J. Electrochem. Soc. 161(14), F1389 (2014)
D.J. Brett, A. Atkinson, N.P. Brandon, S.J. Skinner, Intermediate temperature solid oxide fuel cells. Chem. Soc. Rev. 37(8), 1568–1578 (2008)
S.B. Adler, Factors governing oxygen reduction in solid oxide fuel cell cathodes. Chem. Rev. 104(10), 4791–4844 (2004)
J.M. Vohs, R.J. Gorte, High-performance SOFC cathodes prepared by infiltration. Adv. Mater. 21(9), 943–956 (2009)
C. Duan, J. Tong, M. Shang, S. Nikodemski, M. Sanders, S. Ricote et al., Readily processed protonic ceramic fuel cells with high performance at low temperatures. Science 349(6254), 1321–1326 (2015)
S. Choi, S. Yoo, J. Kim, S. Park, A. Jun, S. Sengodan et al., Highly efficient and robust cathode materials for low-temperature solid oxide fuel cells: PrBa 0.5 Sr 0.5 Co 2− x Fe x O 5+ δ. Sci. Rep. 3(1), 1–6 (2013)
Z. Shao, S.M. Haile, A high-performance cathode for the next generation of solid-oxide fuel cells, in Materials for sustainable energy: a collection of peer-reviewed research and review articles from Nature Publishing Group. (World Scientific, 2011), pp. 255–258
A. Sarkar, Q. Wang, A. Schiele, M.R. Chellali, S.S. Bhattacharya, D. Wang et al., High-entropy oxides: fundamental aspects and electrochemical properties. Adv. Mater. 31(26), 1806236 (2019)
A. Sarkar, L. Velasco, D. Wang, Q. Wang, G. Talasila, L. de Biasi et al., High entropy oxides for reversible energy storage. Nat. Commun. 9(1), 1–9 (2018)
J.H. Shim, Ceramics breakthrough. Nat. Energy 3(3), 168–169 (2018)
E. Wachsman, Functionally gradient bilayer oxide membranes and electrolytes. Solid State Ionics 152, 657–662 (2002)
E. Wachsman, P. Jayaweera, N. Jiang, D. Lowe, B. Pound, Stable high conductivity ceria/bismuth oxide bilayered electrolytes. J. Electrochem. Soc. 144(1), 233 (1997)
J.S. Ahn, D. Pergolesi, M.A. Camaratta, H. Yoon, B.W. Lee, K.T. Lee et al., High-performance bilayered electrolyte intermediate temperature solid oxide fuel cells. Electrochem. Commun. 11(7), 1504–1507 (2009)
K.L. Duncan, K.-T. Lee, E.D. Wachsman, Dependence of open-circuit potential and power density on electrolyte thickness in solid oxide fuel cells with mixed conducting electrolytes. J. Power Sources 196(5), 2445–2451 (2011)
J. Kilner, R. Brook, A study of oxygen ion conductivity in doped non-stoichiometric oxides. Solid State Ionics 6(3), 237–252 (1982)
B. Steele, J. Kilner, P. Dennis, A. McHale, M. Van Hemert, A. Burggraaf, Oxygen surface exchange and diffusion in fast ionic conductors. Solid State Ionics 18, 1038–1044 (1986)
J.W. Fergus, Electrolytes for solid oxide fuel cells. J Power Sources. 162(1), 30–40 (2006)
N. Mahato, A. Banerjee, A. Gupta, S. Omar, K. Balani, Progress in material selection for solid oxide fuel cell technology: a review. Prog. Mater Sci. 72, 141–337 (2015)
W. Quadakkers, J. Piron-Abellan, V. Shemet, L. Singheiser, Metallic interconnectors for solid oxide fuel cells–a review. Mater. High Temp. 20(2), 115–127 (2003)
Z. Gao, L.V. Mogni, E.C. Miller, J.G. Railsback, S.A. Barnett, A perspective on low-temperature solid oxide fuel cells. Energy Environ. Sci. 9(5), 1602–1644 (2016)
A. Chroneos, B. Yildiz, A. Tarancón, D. Parfitt, J.A. Kilner, Oxygen diffusion in solid oxide fuel cell cathode and electrolyte materials: mechanistic insights from atomistic simulations. Energy Environ. Sci. 4(8), 2774–2789 (2011)
N.Q. Minh, Ceramic fuel cells. J Am Ceram Soc. 76(3), 563–588 (1993)
V. Kharton, F. Marques, A. Atkinson, Transport properties of solid oxide electrolyte ceramics: a brief review. Solid State Ionics 174(1–4), 135–149 (2004)
A. Mai, M. Becker, W. Assenmacher, F. Tietz, D. Hathiramani, E. Ivers-Tiffée et al., Time-dependent performance of mixed-conducting SOFC cathodes. Solid State Ion. 177(19–25), 1965–1968 (2006)
L. Adijanto, R. Küngas, F. Bidrawn, R. Gorte, J. Vohs, Stability and performance of infiltrated La0. 8Sr0. 2CoxFe1− xO3 electrodes with and without Sm0. 2Ce0. 8O1. 9 interlayers. J Power Sources. 196(14), 5797–5802 (2011)
D.A. Andersson, S.I. Simak, N.V. Skorodumova, I.A. Abrikosov, B. Johansson, Optimization of ionic conductivity in doped ceria. Proc. Natl. Acad. Sci. 103(10), 3518–3521 (2006)
S. Omar, E.D. Wachsman, J.C. Nino, Higher conductivity Sm3+ and Nd3+ co-doped ceria-based electrolyte materials. Solid State Ion. 178(37–38), 1890–1897 (2008)
K. Venkataramana, C. Madhuri, C. Madhusudan, Y.S. Reddy, G. Bhikshamaiah, C.V. Reddy, Investigation on La3+ and Dy3+ co-doped ceria ceramics with an optimized average atomic number of dopants for electrolytes in IT-SOFCs. Ceram. Int. 44(6), 6300–6310 (2018)
J. Wright, A.V. Virkar, Conductivity of porous Sm2O3-doped CeO2 as a function of temperature and oxygen partial pressure. J Power Sources. 196(15), 6118–6124 (2011)
T.-N. Lin, M.-C. Lee, R.-J. Yang, J.-C. Chang, W.-X. Kao, L.-S. Lee, Chemical state identification of Ce3+/Ce4+ in the Sm0. 2Ce0. 8O2− δ electrolyte for an anode-supported solid oxide fuel cell after long-term operation. Mater. Lett. 81, 185–188 (2012)
A. Pesaran, A. Jaiswal, E.D. Wachsman, CHAPTER 1: Bilayer electrolytes for low temperature and intermediate temperature solid oxide fuel cells–a review. Energy Storage and Conversion Mater. pp 1–41 (2019)
D.W. Joh, J.H. Park, D.Y. Kim, B.-H. Yun, K.T. Lee, High performance zirconia-bismuth oxide nanocomposite electrolytes for lower temperature solid oxide fuel cells. J. Power Sources 320, 267–273 (2016)
B.-H. Yun, C.-W. Lee, I. Jeong, K.T. Lee, Dramatic enhancement of long-term stability of erbia-stabilized bismuth oxides via quadrivalent Hf doping. Chem. Mater. 29(24), 10289–10293 (2017)
B.-H. Yun, K.J. Kim, D.W. Joh, M.S. Chae, J.J. Lee, D.-W. Kim et al., Highly active and durable double-doped bismuth oxide-based oxygen electrodes for reversible solid oxide cells at reduced temperatures. J. Mater. Chem. A. 7(36), 20558–20566 (2019)
S. Boyapati, E.D. Wachsman, N. Jiang, Effect of oxygen sublattice ordering on interstitial transport mechanism and conductivity activation energies in phase-stabilized cubic bismuth oxides. Solid State Ion. 140(1–2), 149–160 (2001)
K.T. Lee, A.A. Lidie, S.Y. Jeon, G.T. Hitz, S.J. Song, E.D. Wachsman, Highly functional nano-scale stabilized bismuth oxides via reverse strike co-precipitation for solid oxide fuel cells. J. Mater. Chem. A. 1(20), 6199–6207 (2013)
H. Harwig, A. Gerards, Electrical properties of the α, β, γ, and δ phases of bismuth sesquioxide. J. Solid State Chem. 26(3), 265–274 (1978)
M. Verkerk, K. Keizer, A. Burggraaf, High oxygen ion conduction in sintered oxides of the Bi 2 O 3-Er 2 O 3 system. J. Appl. Electrochem. 10(1), 81–90 (1980)
T. Takahashi, H. Iwahara, T. Arao, High oxide ion conduction in sintered oxides of the system Bi 2 O 3-Y 2 O 3. J. Appl. Electrochem. 5(3), 187–195 (1975)
M. Verkerk, A. Burggraaf, High oxygen ion conduction in sintered oxides of the Bi2 O 3-Dy2 O 3 system. J. Electrochem. Soc. 128(1), 75 (1981)
N. Sammes, G. Tompsett, H. Näfe, F. Aldinger, Bismuth based oxide electrolytes—structure and ionic conductivity. J. Eur. Ceram. Soc. 19(10), 1801–1826 (1999)
D.W. Joh, J.H. Park, D. Kim, E.D. Wachsman, K.T. Lee, Functionally graded bismuth oxide/zirconia bilayer electrolytes for high-performance intermediate-temperature solid oxide fuel cells (IT-SOFCs). ACS Appl. Mater. Interfaces. 9(10), 8443–8449 (2017)
V.C.J. Ferreira, P. Tavares, J.L. Figueiredo, J.L. Faria, Effect of Mg, Ca, and Sr on CeO2 based catalysts for the oxidative coupling of methane: investigation on the oxygen species responsible for catalytic performance. Ind. Eng. Chem. Res. 51(32), 10535–10541 (2012)
Z. Jie, L. Er-Jun, S. Qiang, J. Yu, Oxygen vacancy formation and migration in Sr-and Mg-doped LaGaO3: a density functional theory study. Chin. Phys. B 21(4), 047201 (2012)
K.-N. Kim, J. Moon, J.-W. Son, J. Kim, H.-W. Lee, J.-H. Lee et al., Introduction of a Buffering Layer for the Interfacial Stability of LSGM-Based SOFCs. J. Korean Ceram. Soc. 42(9), 637–644 (2005)
H. Iwahara, Oxide-ionic and protonic conductors based on perovskite-type oxides and their possible applications. Solid State Ionics 52(1–3), 99–104 (1992)
E. Fabbri, L. Bi, D. Pergolesi, E. Traversa, Towards the next generation of solid oxide fuel cells operating below 600° C with chemically stable proton-conducting electrolytes. Adv. Mater. 24(2), 195–208 (2012)
D. Medvedev, A. Murashkina, E. Pikalova, A. Demin, A. Podias, P. Tsiakaras, BaCeO3: materials development, properties and application. Prog. Mater Sci. 60, 72–129 (2014)
R. Lan, S. Tao, Proton‐conducting materials as electrolytes for solid oxide fuel cells. Mater. High‐Temp. Fuel Cells, pp 133–58 (2013)
W. Jamsak, S. Assabumrungrat, P. Douglas, N. Laosiripojana, R. Suwanwarangkul, S. Charojrochkul et al., Performance of ethanol-fuelled solid oxide fuel cells: proton and oxygen ion conductors. Chem. Eng. J. 133(1–3), 187–194 (2007)
E.M. Sabolsky, M. Seabaugh, K. Sabolsky, S.A. Ibanez, Z. Zhong, SOFC cells and stacks for complex fuels. ECS Trans. 7(1), 503 (2007)
D. Medvedev, J. Lyagaeva, E. Gorbova, A. Demin, P. Tsiakaras, Advanced materials for SOFC application: strategies for the development of highly conductive and stable solid oxide proton electrolytes. Prog. Mater Sci. 75, 38–79 (2016)
H.H.M. Thi, B. Saubat, N. Sergent, T. Pagnier, In situ Raman and optical characterization of H2S reaction with Ni-based anodes for SOFCs. Solid State Ionics 272, 84–90 (2015)
K. Eguchi, K. Tanaka, T. Matsui, R. Kikuchi, Reforming activity and carbon deposition on cermet catalysts for fuel electrodes of solid oxide fuel cells. Catal. Today 146(1–2), 154–159 (2009)
S. Tao, J.T. Irvine, A stable, easily sintered proton-conducting oxide electrolyte for moderate-temperature fuel cells and electrolyzers. Adv. Mater. 18(12), 1581–1584 (2006)
C.W. Tanner, A.V. Virkar, Instability of BaCeO3 in H 2 O-Containing atmospheres. J. Electrochem. Soc. 143(4), 1386 (1996)
S. Yamanaka, M. Fujikane, T. Hamaguchi, H. Muta, T. Oyama, T. Matsuda et al., Thermophysical properties of BaZrO3 and BaCeO3. J. Alloy. Compd. 359(1–2), 109–113 (2003)
K. Kurosaki, J. Adachi, T. Maekawa, S. Yamanaka, Thermal conductivity analysis of BaUO3 and BaZrO3 by semiempirical molecular dynamics simulation. J. Alloy. Compd. 407(1–2), 49–52 (2006)
L. Gui, Y. Ling, G. Li, Z. Wang, Y. Wan, R. Wang et al., Enhanced sinterability and conductivity of BaZr0. 3Ce0. 5Y0. 2O3− δ by addition of bismuth oxide for proton conducting solid oxide fuel cells. J. Power Sources. 301, 369–375 (2016)
K. Katahira, Y. Kohchi, T. Shimura, H. Iwahara, Protonic conduction in Zr-substituted BaCeO3. Solid State Ion. 138(1–2), 91–98 (2000)
K.H. Ryu, S.M. Haile, Chemical stability and proton conductivity of doped BaCeO3–BaZrO3 solid solutions. Solid State Ion. 125(1–4), 355–367 (1999)
C. Zamfirescu, I. Dincer, Ammonia as a green fuel and hydrogen source for vehicular applications. Fuel Process. Technol. 90(5), 729–737 (2009)
Y. Shen, H. Zhao, X. Liu, N. Xu, Preparation and electrical properties of Ca-doped La 2 NiO 4+ δ cathode materials for IT-SOFC. Phys. Chem. Chem. Phys. 12(45), 15124–15131 (2010)
Y.-J. Gu, Z.-G. Liu, J.-H. Ouyang, F.-Y. Yan, Y. Zhou, Structure and electrical conductivity of BaCe0. 85Ln0. 15O3− δ (Ln= Gd, Y, Yb) ceramics. Electrochim. Acta 105, 547–553 (2013)
J. Bu, P.G. Jönsson, Z. Zhao, Ionic conductivity of dense BaZr0. 5Ce0. 3Ln0. 2O3− δ (Ln= Y, Sm, Gd, Dy) electrolytes. J. Power Sources. 272, 786–793 (2014)
P. Sawant, S. Varma, B. Wani, S. Bharadwaj, Synthesis, stability and conductivity of BaCe0. 8− xZrxY0. 2O3− δ as electrolyte for proton conducting SOFC. Int. J. Hydrogen Energy. 37(4), 3848–3856 (2012)
X. Wang, J. Yin, J. Xu, H. Wang, G. Ma, Chemical stability, ionic conductivity of BaCe0. 9− xZrxSm0. 10O3− α and its application to ammonia synthesis at atmospheric pressure. Chin. J. Chem. 29(6), 1114–1118 (2011)
Y. Li, R. Guo, C. Wang, Y. Liu, Z. Shao, J. An et al., Stable and easily sintered BaCe0. 5Zr0. 3Y0. 2O3− δ electrolytes using ZnO and Na2CO3 additives for protonic oxide fuel cells. Electrochim. Acta 95, 95–101 (2013)
E. Fabbri, A. D’Epifanio, E. Di Bartolomeo, S. Licoccia, E. Traversa, Tailoring the chemical stability of Ba (Ce0. 8− xZrx) Y0. 2O3− δ protonic conductors for intermediate temperature solid oxide fuel cells (IT-SOFCs). Solid State Ionics 179(15–16), 558–564 (2008)
G.C. Mather, M.S. Islam, Defect and dopant properties of the SrCeO3-based proton conductor. Chem. Mater. 17(7), 1736–1744 (2005)
L. Yang, S. Wang, K. Blinn, M. Liu, Z. Liu, Z. Cheng et al., Enhanced sulfur and coking tolerance of a mixed ion conductor for SOFCs: BaZr0. 1Ce0. 7Y0. 2–xYbxO3–δ. Science 326(5949), 126–129 (2009)
S. Choi, C.J. Kucharczyk, Y. Liang, X. Zhang, I. Takeuchi, H.-I. Ji et al., Exceptional power density and stability at intermediate temperatures in protonic ceramic fuel cells. Nat. Energy 3(3), 202–210 (2018)
L. Yang, C. Zuo, S. Wang, Z. Cheng, M. Liu, A Novel Composite cathode for low-temperature SOFCs based on oxide proton conductors. Adv. Mater. 20(17), 3280–3283 (2008)
M. Shiono, K. Kobayashi, T. Lannguyen, K. Hosoda, T. Kato, K. Ota et al., Effect of CeO2 interlayer on ZrO2 electrolyte/La(Sr)CoO3 cathode for low-temperature SOFCs. Solid State Ionics 170(1–2), 1–7 (2004)
O. Yamamoto, Y. Takeda, R. Kanno, M. Noda, Perovskite-type oxides as oxygen electrodes for high temperature oxide fuel cells. Solid State Ionics 22(2–3), 241–246 (1987)
F. Marques, L. Navarro, Performance of double layer electrolyte cells Part II: GCO/YSZ, a case study. Solid State Ionics 100(1–2), 29–38 (1997)
A.V. Virkar, Theoretical analysis of solid oxide fuel cells with two-layer, composite electrolytes: electrolyte stability. J. Electrochem. Soc. 138(5), 1481 (1991)
T.-H. Kwon, T. Lee, H.-I. Yoo, Partial electronic conductivity and electrolytic domain of bilayer electrolyte Zr0.84Y0.16O1.92/Ce0.9Gd0.1O1.95. Solid State Ionics 195(1), 25–35 (2011)
D.-H. Myung, J. Hong, K. Yoon, B.-K. Kim, H.-W. Lee, J.-H. Lee et al., The effect of an ultra-thin zirconia blocking layer on the performance of a 1-μm-thick gadolinia-doped ceria electrolyte solid-oxide fuel cell. J. Power Sources 206, 91–96 (2012)
J. Van Roosmalen, E. Cordfunke, Chemical reactivity and interdiffusion of (La, Sr) MnO3 and (Zr, Y) O2, solid oxide fuel cell cathode and electrolyte materials. Solid State Ionics 52(4), 303–312 (1992)
F. Wang, M.E. Brito, K. Yamaji, D.-H. Cho, M. Nishi, H. Kishimoto et al., Effect of polarization on Sr and Zr diffusion behavior in LSCF/GDC/YSZ system. Solid State Ionics 262, 454–459 (2014)
F. Tietz, D. Sebold, A. Brisse, J. Schefold, Degradation phenomena in a solid oxide electrolysis cell after 9000 h of operation. J. Power Sources 223, 129–135 (2013)
Z. Gao, V.Y. Zenou, D. Kennouche, L. Marks, S.A. Barnett, Solid oxide cells with zirconia/ceria Bi-Layer electrolytes fabricated by reduced temperature firing. J. Mater. Chem. A. 3(18), 9955–9964 (2015)
A. Infortuna, A.S. Harvey, L.J. Gauckler, Microstructures of CGO and YSZ thin films by pulsed laser deposition. Adv. Func. Mater. 18(1), 127–135 (2008)
E.O. Oh, C.M. Whang, Y.R. Lee, S.Y. Park, D.H. Prasad, K.J. Yoon et al., Extremely thin bilayer electrolyte for solid oxide fuel cells (SOFCs) fabricated by chemical solution deposition (CSD). Adv Mater. 24(25), 3373–3377 (2012)
I. Jang, S. Kim, C. Kim, H. Lee, H. Yoon, T. Song et al., Interface engineering of yttrium stabilized zirconia/gadolinium doped ceria bi-layer electrolyte solid oxide fuel cell for boosting electrochemical performance. J. Power Sources. 435, 226776 (2019)
Z. Fan, J. An, A. Iancu, F.B. Prinz, Thickness effects of yttria-doped ceria interlayers on solid oxide fuel cells. J. Power Sources 218, 187–191 (2012)
J.H. Park, C.H. Jung, K.J. Kim, D. Kim, H.R. Shin, J.-E. Hong et al., Enhancing bifunctional electrocatalytic activities of oxygen electrodes via incorporating highly conductive Sm3+ and Nd3+ double-doped ceria for reversible solid oxide cells. ACS Appl. Mater. Interfaces. 13(2), 2496–2506 (2021)
C. Nicollet, J. Waxin, T. Dupeyron, A. Flura, J.-M. Heintz, J.P. Ouweltjes et al., Gadolinium doped ceria interlayers for Solid Oxide Fuel Cells cathodes: enhanced reactivity with sintering aids (Li, Cu, Zn), and improved densification by infiltration. J. Power Sources 372, 157–165 (2017)
S. Lee, S. Lee, H.-J. Kim, S.M. Choi, H. An, M.Y. Park et al., Highly durable solid oxide fuel cells: suppressing chemical degradationviarational design of a diffusion-blocking layer. J. Mater. Chem. A. 6(31), 15083–15094 (2018)
C. Kim, S. Kim, I. Jang, H. Yoon, T. Song, U. Paik, Facile fabrication strategy of highly dense gadolinium-doped ceria/yttria-stabilized zirconia bilayer electrolyte via cold isostatic pressing for low temperature solid oxide fuel cells. J. Power Sources 415, 112–118 (2019)
R.D. Bayliss, S.N. Cook, S. Kotsantonis, R.J. Chater, J.A. Kilner, Oxygen ion diffusion and surface exchange properties of the α-and δ-phases of Bi2O3. Adv. Energy Mater. 4(10), 1301575 (2014)
C.-Y. Yoo, B.A. Boukamp, H.J. Bouwmeester, Oxygen surface exchange kinetics of erbia-stabilized bismuth oxide. J. Solid State Electrochem. 15(2), 231–236 (2011)
K.T. Lee, D.W. Jung, M.A. Camaratta, H.S. Yoon, J.S. Ahn, E.D. Wachsman, Gd0. 1Ce0. 9O1. 95/Er0. 4Bi1. 6O3 bilayered electrolytes fabricated by a simple colloidal route using nano-sized Er0. 4Bi1. 6O3 powders for high performance low temperature solid oxide fuel cells. J. Power Sources. 205, 122–128 (2012)
J. Hou, L. Bi, J. Qian, Z. Zhu, J. Zhang, W. Liu, High performance ceria–bismuth bilayer electrolyte low temperature solid oxide fuel cells (LT-SOFCs) fabricated by combining co-pressing with drop-coating. J. Mater. Chem. A. 3(19), 10219–10224 (2015)
A. Pesaran, A. Jaiswal, Y. Ren, E.D. Wachsman, Development of a new ceria/yttria-ceria double-doped bismuth oxide bilayer electrolyte low-temperature SOFC with higher stability. Ionics 25(7), 3153–3164 (2019)
K. Kim, B. Kim, J. Son, J. Kim, H.-W. Lee, J.-H. Lee et al., Characterization of the electrode and electrolyte interfaces of LSGM-based SOFCs. Solid State Ionics 177(19–25), 2155–2158 (2006)
K.B. Yoo, G.M. Choi, Performance of La-doped strontium titanate (LST) anode on LaGaO3-based SOFC. Solid State Ionics 11(180), 867–871 (2009)
E.-H. Song, T.-J. Chung, H.-R. Kim, J.-W. Son, B.-K. Kim, J.-H. Lee et al., Effect of the LDC Buffer Layer in LSGM-based Anode-supported SOFCs. J. Korean Ceram. Soc. 44(12), 710–714 (2007)
Z. Bi, B. Yi, Z. Wang, Y. Dong, H. Wu, Y. She et al., A high-performance anode-supported SOFC with LDC-LSGM bilayer electrolytes. Electrochem. Solid State Lett. 7(5), A105 (2004)
Z. Bi, Y. Dong, M. Cheng, B. Yi, Behavior of lanthanum-doped ceria and Sr-, Mg-doped LaGaO3 electrolytes in an anode-supported solid oxide fuel cell with a La0. 6Sr0. 4CoO3 cathode. J. Power Sources. 161(1), 34–39 (2006)
B. Zhu, X. Liu, P. Zhou, X. Yang, Z. Zhu, W. Zhu, Innovative solid carbonate–ceria composite electrolyte fuel cells. Electrochem. Commun. 3(10), 566–571 (2001)
M. Benamira, V. Albin, A. Ringuedé, R.-N. Vannier, A. Bodén, C. Lagergren et al., Structural and electrical properties of gadolinia-doped ceria mixed with alkali earth carbonates for SOFC applications. ECS Trans. 7(1), 2261 (2007)
R. Raza, X. Wang, Y. Ma, X. Liu, B. Zhu, Improved ceria–carbonate composite electrolytes. Int. J. Hydrogen Energy 35(7), 2684–2688 (2010)
L. Fan, C. He, B. Zhu, Role of carbonate phase in ceria–carbonate composite for low temperature solid oxide fuel cells: a review. Int. J. Energy Res. 41(4), 465–481 (2017)
X. Wang, Y. Ma, B. Zhu, State of the art ceria-carbonate composites (3C) electrolyte for advanced low temperature ceramic fuel cells (LTCFCs). Int. J. Hydrogen Energy 37(24), 19417–19425 (2012)
L. Fan, C. Wang, O. Osamudiamen, R. Raza, M. Singh, B. Zhu, Mixed ion and electron conductive composites for single component fuel cells: I. Effects of composition and pellet thickness. J. Power Sources. 217, 164–169 (2012)
S. Li, J. Sun, X. Sun, B. Zhu, A high functional cathode material: for low-temperature solid oxide fuel cells. Electrochem. Solid State Lett. 9(2), A86 (2005)
L. Fan, C. Wang, M. Chen, J. Di, J. Zheng, B. Zhu, Potential low-temperature application and hybrid-ionic conducting property of ceria-carbonate composite electrolytes for solid oxide fuel cells. Int. J. Hydrogen Energy 36(16), 9987–9993 (2011)
C. Xia, Y. Li, Y. Tian, Q. Liu, Y. Zhao, L. Jia et al., A high performance composite ionic conducting electrolyte for intermediate temperature fuel cell and evidence for ternary ionic conduction. J. Power Sources 188(1), 156–162 (2009)
J. Huang, L. Yang, R. Gao, Z. Mao, C. Wang, A high-performance ceramic fuel cell with samarium doped ceria–carbonate composite electrolyte at low temperatures. Electrochem. Commun. 8(5), 785–789 (2006)
X. Wang, Y. Ma, R. Raza, M. Muhammed, B. Zhu, Novel core–shell SDC/amorphous Na2CO3 nanocomposite electrolyte for low-temperature SOFCs. Electrochem. Commun. 10(10), 1617–1620 (2008)
L. Zhang, R. Lan, A. Kraft, S. Tao, A stable intermediate temperature fuel cell based on doped-ceria–carbonate composite electrolyte and perovskite cathode. Electrochem. Commun. 13(6), 582–585 (2011)
C. Xia, Y. Li, Y. Tian, Q. Liu, Z. Wang, L. Jia et al., Intermediate temperature fuel cell with a doped ceria–carbonate composite electrolyte. J. Power Sources 195(10), 3149–3154 (2010)
R. Raza, X. Wang, Y. Ma, B. Zhu, Study on calcium and samarium co-doped ceria based nanocomposite electrolytes. J. Power Sources 195(19), 6491–6495 (2010)
J. Huang, Z. Gao, Z. Mao, Effects of salt composition on the electrical properties of samaria-doped ceria/carbonate composite electrolytes for low-temperature SOFCs. Int. J. Hydrogen Energy 35(9), 4270–4275 (2010)
B. Zhu, S. Li, B.-E. Mellander, Theoretical approach on ceria-based two-phase electrolytes for low temperature (300–600° C) solid oxide fuel cells. Electrochem. Commun. 10(2), 302–305 (2008)
Z. Gao, J. Huang, Z. Mao, C. Wang, Z. Liu, Preparation and characterization of nanocrystalline Ce0. 8Sm0. 2O1. 9 for low temperature solid oxide fuel cells based on composite electrolyte. Int. J. Hydrogen Energy. 35(2), 731–737 (2010)
A. Ali, A. Rafique, M. Kaleemullah, G. Abbas, M. Ajmal Khan, M.A. Ahmad et al., Effect of alkali carbonates (single, binary, and ternary) on doped ceria: a composite electrolyte for low-temperature solid oxide fuel cells. ACS Appl. Mater. Interfaces. 10(1), 806–818 (2018)
Y. Jing, P. Lund, M.I. Asghar, F. Li, B. Zhu, B. Wang et al., Non-doped CeO2-carbonate nanocomposite electrolyte for low temperature solid oxide fuel cells. Ceram. Int. 46(18), 29290–29296 (2020)
S.M. Choi, J.H. Lee, H. An, J. Hong, H. Kim, K.J. Yoon et al., Fabrication of anode-supported protonic ceramic fuel cell with Ba(Zr0.85Y0.15)O3-delta-Ba(Ce0.9Y0.1)O3-delta dual-layer electrolyte. Int. J. Hydrogen Energy. 39(24), 12812–12818 (2014)
Y. Ma, B. He, J.Q. Wang, M. Cheng, X.Z. Zhong, J.B. Huang, Porous/dense bilayer BaZr0.8Y0.2O3-delta electrolyte matrix fabricated by tape casting combined with solid-state reactive sintering for protonic ceramic fuel cells. Int. J. Hydrogen Energy. 46(15), 9918–9926 (2021)
J. Basbus, M. Arce, H. Troiani, Q. Su, H. Wang, A. Caneiro et al., Study of BaCe0.4Zr0.4Y0.2O3-delta/BaCe0.8Pr0.2O3-delta (BCZY/BCP) bilayer membrane for Protonic Conductor Solid Oxide Fuel Cells (PC-SOFC). Int. J. Hydrogen Energy. 45(8), 5481–5490 (2020)
S. Lee, I. Park, H. Lee, D. Shin, Continuously gradient anode functional layer for BCZY based proton-conducting fuel cells. Int. J. Hydrogen Energy 39(26), 14342–14348 (2014)
Y. Zhang, R. Knibbe, J. Sunarso, Y. Zhong, W. Zhou, Z. Shao et al., Recent progress on advanced materials for solid-oxide fuel cells operating below 500° C. Adv Mater. 29(48), 1700132 (2017)
P.N. Huang, A. Petric, Superior oxygen ion conductivity of lanthanum gallate doped with strontium and magnesium. J Electrochem Soc. 143(5), 1644 (1996)
S.S. Shin, J.H. Kim, K.T. Bae, K.-T. Lee, S.M. Kim, J.-W. Son et al., Multiscale structured low-temperature solid oxide fuel cells with 13 W power at 500° C. Energy Environ. Sci. 13(10), 3459–3468 (2020)
Acknowledgements
This research was funded and conducted under the ‘Competency Development Program for Industry Specialists’ of Korean Ministry of Trade, Industry and Energy (MOTIE), operated by Korea Institute for Advancement of Technology (KIAT). (No. P0017120, HRD program for Foster R&D specialist of parts for eco-friendly vehicle(xEV)). This research was also supported by “Human Resources Program in Energy Technology” of the Korea Institute of Energy Technology Evaluation and Planning (KETEP), granted financial resource from the Ministry of Trade, Industry & Energy, Republic of Korea (20194030202360).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, D., Jeong, I., Kim, K.J. et al. A brief review of heterostructure electrolytes for high-performance solid oxide fuel cells at reduced temperatures. J. Korean Ceram. Soc. 59, 131–152 (2022). https://doi.org/10.1007/s43207-021-00175-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43207-021-00175-9