Abstract
Endometriosis, characterized by endometrial-like mucosal tissue outside the uterine cavity, is a reproductive disorder afflicting about 10% of women within the reproductive age. The pathogenesis of endometriosis has been attributed to factors like genetics, environmental particles, and hormones. A comprehensive review of studies from July 2010 to July 2023 across multiple databases was done to aid in a better understanding of the same. The investigation focused on studies delineating the correlation between endocrine disruptors, microRNAs, and endometriosis. To optimize the search scope, keywords and subject headings were used as search terms. Then, two authors rigorously assessed studies using criteria, selecting 27 studies from various databases. Notably, dioxins, organochlorine pesticides, and polychlorinated biphenyls exhibited a solid connection for endometriosis, while bisphenol A and phthalates yielded conflicting results. The heightened presence of bisphenol A, polychlorinated biphenyls, and phthalates was linked to altered gene expression, including genes like AKR1B10, AKR1C3, and FAM49B. MicroRNAs like miRNA-31, miRNA-144, and miRNA-145 emerged as vital factors in the onset of endometriosis and progression. Furthermore, elevated expression of miR-1304-3p, miR-544, and miR-3684 and reduced expression of miR-3935 and miR-4427 exert substantial influence on signaling pathways like NF-κB, MAPK, and Wnt/β-catenin. Currently, literature shows an independent link between endocrine disruptor exposure and endometriosis and between microRNA dysregulation and endometriosis. However, research lacks the combination of all three factors. The review delves into the effects of endocrine disruptors and microRNAs on the pathogenesis of endometriosis to improve our understanding of the disorder and in finding therapies.
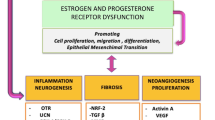
Graphical Abstract


Similar content being viewed by others
Data Availability
All data supporting the findings of this study are available within the paper and its Supplementary Information.
Code Availability
NA.
Abbreviations
- EDC:
-
Endocrine-disrupting chemicals
- BPA:
-
Bisphenol A
- PCB:
-
Polychlorinated biphenyl
- DEHP:
-
Di-(2-ethylhexyl)-phthalate
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- GPX:
-
Glutathione peroxidase
- HO:
-
Heme oxygenase
- CAT:
-
Catalase
- miRNA:
-
MicroRNA
- F1:
-
Filial 1
- OCP:
-
Organochlorine pesticide
- PAE:
-
Phthalate ester
- PDBE:
-
Polybrominated diphenyl ethers
- DIE:
-
Deep infiltrating endometriosis
- 2,3,7,8-TCDD:
-
2,3,7,8-Tetrachlorodibenzo-p-dioxin
- 1,2,3,7,8-PeCDD:
-
1,2,3,7,8-Pentachlorodibenzo-p-dioxin
- MnBP:
-
Mono-n-butyl phthalate
- BIRC3:
-
Baculoviral IAP repeat containing 3
- BUB1B:
-
Mitotic checkpoint serine/threonine-protein kinase BUB1 beta
- CDC20:
-
Cell division cycle protein 2
- IL-1β:
-
Interleukin-1 beta
- TNF-α:
-
Tumor necrosis factor-alpha
- DBP:
-
Dibutyl phthalate
- GTP:
-
Guanine triphosphate
- E2F:
-
E2 transcription factor
- MMP:
-
Metalloproteinase
- MiBP:
-
Mono-isobutyl phthalate
- MOP:
-
Mono-octyl phthalate
- MBP:
-
Mono-butyl phthalate
- MCHP:
-
Mono-cyclohexyl phthalate
- MEHP:
-
Mono-(ethylhexyl) phthalate
- MiNP:
-
Mono-isononyl phthalate
- MBzP:
-
Methylbenzylpiperazine
- POP:
-
Persistent organic pollutants
- WQS:
-
Weighted quantile sum
- BKMR:
-
Bayesian kernel machine regression
- OF:
-
Omental fat
- PBDE:
-
Polybrominated diphenyl ether
- γ-HCH:
-
Gamma-hexachlorocyclohexane
- PF:
-
Peritoneal fluid
- MEHHP:
-
Mono(2-ethyl-5-hydroxyhexyl) phthalate
- MEOHP:
-
Mono-(2-ethyl-5-oxohexyl) phthalate
- MEP:
-
Monoethyl phthalate
- AKR:
-
Aldo-keto reductase
- qPCR:
-
Quantitative polymerase chain reaction
- mTOR:
-
Mammalian target of rapamycin
- VEGF:
-
Vascular endothelial growth factor
- NK:
-
Natural killer
- PI3K/Akt:
-
Phosphoinositide-3-kinase-protein kinase B/Ak strain transforming
- MAPK:
-
Mitogen-activated protein kinase
- mRNA:
-
Messenger RNA
- HOXA10:
-
Homeobox
- EuE:
-
Ectopic endometrial
- EcE:
-
Eutopic endometrial
- IGFBP3:
-
Insulin-like growth factor binding protein 3
- COL8A1:
-
Collagen type VIII alpha 1 chain
- Crk:
-
Protein-proto-oncogene
- AFS:
-
American Fertility Society
- NTN4:
-
Netrin 4
- CD14:
-
Cluster of differentiation 14
- NF-κB:
-
Nuclear factor-kappa B
- M1:
-
Macrophage markers
- IRF5:
-
Interferon regulatory factor 5
- SF -1:
-
Steroidogenic factor 1
- ECSCs:
-
Ectopic endometrial stroma cells
- StAR:
-
Steroidogenic acute regulatory protein
- CYP19A1:
-
Cytochrome P450 family 19 subfamily A member 1
- ELNE:
-
Neutrophil elastase
- AMH:
-
Anti-Müllerian hormone
- JAK/STAT:
-
Janus kinase/signal transducers and activators of transcription
- YAP/TAZ:
-
Yes-associated protein and the transcriptional coactivator with PDZ-binding motif
- Wnt:
-
Wingless-related integration site
- FOXO:
-
Forkhead box O
- p53:
-
Tumor protein
- TGF-ß:
-
Transforming growth factor beta
- ECM:
-
Extracellular matrix
- BMP7:
-
Bone morphogenetic protein-7
References
Haydardedeoglu B, Zeyneloglu HB. The impact of endometriosis on fertility. Womens Health. 2015;11(5):619–23.
Vercellini P, Viganò P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014;10(5):261–75.
Smarr MM, Kannan K, Louis GM. Endocrine disrupting chemicals and endometriosis. Fertil Steril. 2016;106(4):959–66.
Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30(4):293–342.
Sirohi D, Al Ramadhani R, Knibbs LD. Environmental exposures to endocrine disrupting chemicals (EDCs) and their role in endometriosis: a systematic literature review. Rev Environ Health. 2021;36(1):101–15.
Upson K, De Roos AJ, Thompson ML, Sathyanarayana S, Scholes D, Barr DB, Holt VL. Organochlorine pesticides and risk of endometriosis: findings from a population-based case–control study. Environ Health Perspect. 2013;121(11–12):1319–24.
Upson K, Sathyanarayana S, De Roos AJ, Thompson ML, Scholes D, Dills R, Holt VL. Phthalates and risk of endometriosis. Environ Res. 2013;126:91–7.
Bruner-Tran KL, Osteen KG. Dioxin-like PCBs and endometriosis. Syst Biol Reprod Med. 2010;56(2):132–46.
Louis GM, Peterson CM, Chen Z, Croughan M, Sundaram R, Stanford J, Varner MW, Kennedy A, Giudice L, Fujimoto VY, Sun L. Bisphenol A and phthalates and endometriosis: the endometriosis: natural history, diagnosis and outcomes study. Fertil Steril. 2013;100(1):162–9.
Hiroi H, Tsutsumi O, Takeuchi T, Momoeda M, Ikezuki Y, Okamura A, Yokota H, Taketani Y. Differences in serum bisphenol a concentrations in premenopausal normal women and women with endometrial hyperplasia. Endocr J. 2004;51(6):595–600.
Nazir S, Usman Z, Imran M, Lone KP, Ahmad G. Women diagnosed with endometriosis show high serum levels of diethyl hexyl phthalate. J Hum Reprod Sci. 2018;11(2):131.
Fernandez MA, Cardeal ZL, Carneiro MM, André LC. Study of possible association between endometriosis and phthalate and bisphenol A by biomarkers analysis. J Pharm Biomed Anal. 2019;172:238–42.
Cho YJ, Park SB, Han M. Di-(2-ethylhexyl)-phthalate induces oxidative stress in human endometrial stromal cells in vitro. Mol Cell Endocrinol. 2015;407:9–17.
Mallozzi M, Leone C, Manurita F, Bellati F, Caserta D. Endocrine disrupting chemicals and endometrial cancer: an overview of recent laboratory evidence and epidemiological studies. Int J Environ Res Public Health. 2017;14(3):334.
Sabry R, Yamate J, Favetta L, LaMarre J. MicroRNAs: potential targets and agents of endocrine disruption in female reproduction. J Toxicol Pathol. 2019;32(4):213–21.
Lite C, Ahmed SS, Santosh W, Seetharaman B. Prenatal exposure to bisphenol-A altered miRNA-224 and protein expression of aromatase in ovarian granulosa cells concomitant with elevated serum estradiol levels in F1 adult offspring. J Biochem Mol Toxicol. 2019;33(6):e22317.
Ohlsson Teague EM, Print CG, Hull ML. The role of microRNAs in endometriosis and associated reproductive conditions. Hum Reprod Update. 2010;16(2):142–65.
Ohlsson Teague EM, Van der Hoek KH, Van der Hoek MB, Perry N, Wagaarachchi P, Robertson SA, Print CG, Hull LM. MicroRNA-regulated pathways associated with endometriosis. Mol Endocrinol. 2009;23(2):265–75.
Zheng B, Xue X, Zhao Y, Chen J, Xu CY, Duan P. The differential expression of microRNA-143,145 in endometriosis. Iran J Reprod Med. 2014;12(8):555.
Bashti O, Noruzinia M, Garshasbi M, Abtahi M. miR-31 and miR-145 as potential non-invasive regulatory biomarkers in patients with endometriosis. Cell J. 2018;20(1):84.
Hu T, Yao M, Fu X, Chen C, Wu R. Polychlorinated biphenyl 104 promotes migration of endometrial stromal cells in endometriosis. Toxicol Lett. 2018;290:19–28.
Chou YC, Chen YC, Chen MJ, Chang CW, Lai GL, Tzeng CR. Exposure to mono-n-butyl phthalate in women with endometriosis and its association with the biological effects on human granulosa cells. Int J Mol Sci. 2020;21(5):1794.
Adir M, Salmon-Divon M, Combelles CM, Mansur A, Cohen Y, Machtinger R. In vitro exposure of human luteinized mural granulosa cells to dibutyl phthalate affects global gene expression. Toxicol Sci. 2017;160(1):180–8.
Matta K, Lefebvre T, Vigneau E, Cariou V, Marchand P, Guitton Y, Royer AL, Ploteau S, Le Bizec B, Antignac JP, Cano-Sancho G. Associations between persistent organic pollutants and endometriosis: a multiblock approach integrating metabolic and cytokine profiling. Environ Int. 2022;158:106926.
Zhang Y, Lu Y, Ma H, Xu Q, Wu X. Combined exposure to multiple endocrine disruptors and uterine leiomyomata and endometriosis in US women. Front Endocrinol. 2021;20(12):726876.
Williams KE, Miroshnychenko O, Johansen EB, Niles RK, Sundaram R, Kannan K, Albertolle M, Zhou Y, Prasad N, Drake PM, Giudice LC. Urine, peritoneal fluid and omental fat proteomes of reproductive age women: endometriosis-related changes and associations with endocrine disrupting chemicals. J Proteomics. 2015;113:194–205.
Simonelli A, Guadagni R, De Franciscis P, Colacurci N, Pieri M, Basilicata P, Pedata P, Lamberti M, Sannolo N, Miraglia N. Environmental and occupational exposure to bisphenol A and endometriosis: urinary and peritoneal fluid concentration levels. Int Arch Occup Environ Health. 2017;90:49–61.
Upson K, Sathyanarayana S, De Roos AJ, Koch HM, Scholes D, Holt VL. A population-based case–control study of urinary bisphenol A concentrations and risk of endometriosis. Hum Reprod. 2014;29(11):2457–64.
Kim SH, Chun S, Jang JY, Chae HD, Kim CH, Kang BM. Increased plasma levels of phthalate esters in women with advanced-stage endometriosis: a prospective case-control study. Fertil Steril. 2011;95(1):357–9.
Kim MR, Kim JH, Cho HH. Aldo-keto reductase activity after diethylhexyl phthalate exposure in eutopic and ectopic endometrial cells. Eur J Obstet Gynecol Reprod Biol. 2017;215:215–9.
Xu X, Li Z, Liu J, Yu S, Wei Z. MicroRNA expression profiling in endometriosis-associated infertility and its relationship with endometrial receptivity evaluated by ultrasound. J Xray Sci Technol. 2017;25(3):523–32.
Laudanski P, Charkiewicz R, Kuzmicki M, Szamatowicz J, Charkiewicz A, Niklinski J. MicroRNAs expression profiling of eutopic proliferative endometrium in women with ovarian endometriosis. Reprod Biol Endocrinol. 2013;11(1):1–7.
Laudanski P, Charkiewicz R, Tolwinska A, Szamatowicz J, Charkiewicz A, Niklinski J. Profiling of selected microRNAs in proliferative eutopic endometrium of women with ovarian endometriosis. Biomed Res Int. 2015;2015:760698.
Yang WW, Hong L, Xu XX, Wang Q, Huang JL, Jiang L. Regulation of miR-33b on endometriosis and expression of related factors. Eur Rev Med Pharmacol Sci. 2017;21(9):2027-2033.
Petracco R, Grechukhina O, Popkhadze S, Massasa E, Zhou Y, Taylor HS. MicroRNA 135 regulates HOXA10 expression in endometriosis. J Clin Endocrinol Metab. 2011;96(12):E1925–33.
Kai K, Joshi NR, Burns GW, Hrbek SM, Vegter EL, Ochoa-Bernal MA, Song Y, Moldovan GE, Sempere LF, Miyadahira EH, Serafini PC, Fazleabas AT. MicroRNA-210–3p regulates endometriotic lesion development by targeting IGFBP3 in baboons and women with endometriosis. Reprod Sci. 2023;30(10):2932–44.
Liu S, Gao S, Wang XY, Wang DB. Expression of miR-126 and Crk in endometriosis: miR-126 may affect the progression of endometriosis by regulating Crk expression. Arch Gynecol Obstet. 2012;285:1065–72.
Zhao M, Tang Q, Wu W, Xia Y, Chen D, Wang X. miR-20a contributes to endometriosis by regulating NTN4 expression. Mol Biol Rep. 2014;41:5793–7.
Zhang Z, Li H, Zhao Z, Gao B, Meng L, Feng X. miR-146b level and variants is associated with endometriosis related macrophages phenotype and plays a pivotal role in the endometriotic pain symptom. Taiwan J Obstet Gynecol. 2019;58(3):401–8.
Hu Z, Mamillapalli R, Taylor HS. Increased circulating miR-370-3p regulates steroidogenic factor 1 in endometriosis. Am J Physiol Endocrinol Metab. 2019;316(3):E373–82.
Martínez-Zamora MA, Mattioli L, Parera J, Abad E, Coloma JL, Van Babel B, Galceran MT, Balasch J, Carmona F. Increased levels of dioxin-like substances in adipose tissue in patients with deep infiltrating endometriosis. Hum Reprod. 2015;30(5):1059–68.
Upson K, Harmon QE, Baird DD. Soy-based infant formula feeding and ultrasound-detected uterine fibroids among young African-American women with no prior clinical diagnosis of fibroids. Environ Health Perspect. 2016;124(6):769–75.
Gao M, Wang H. Frequent milk and soybean consumption are high risks for uterine leiomyoma: a prospective cohort study. Medicine. 2018;97(41):e12009.
Zhang Y, Lu Y, Ma H, Xu Q, Wu X. Combined exposure to multiple endocrine disruptors and uterine leiomyomata and endometriosis in US women. Front Endocrinol. 2021;12:726876.
Bendifallah S, Dabi Y, Suisse S, Jornea L, Bouteiller D, Touboul C, Puchar A, Daraï E. MicroRNome analysis generates a blood-based signature for endometriosis. Sci Rep. 2022;12(1):4051.
Stejskalová A, Fincke V, Nowak M, Schmidt Y, Borrmann K, von Wahlde MK, Schäfer SD, Kiesel L, Greve B, Götte M. Collagen I triggers directional migration, invasion and matrix remodeling of stroma cells in a 3D spheroid model of endometriosis. Sci Rep. 2021;11(1):4115.
Zubrzycka A, Migdalska-Sęk M, Jędrzejczyk S, Brzeziańska-Lasota E. Assessment of BMP7, SMAD4, and CDH1 expression profile and regulatory miRNA-542-3p in eutopic and ectopic endometrium of women with endometriosis. Int J Mol Sci. 2023;24(7):6637.
Acknowledgements
The collection of data has been contributed by all the students working in the laboratory and supported by the Department of Biotechnology, SRM Institute of Science and Technology. We sincerely feel grateful towards Usharani Balu, Deva Sureshbabu, S. Murali Krishnan, Sweta Thakkar, Sooriyakumar S., Sandhya Ramanan, and Uma K. Arun.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
All the studies quoted in this review article have studies involving human participants that were in accordance with the ethical standards of the institutional and/or national research committee.
Consent to Participate
NA for systematic review.
Consent for Publication
The authors affirm that human samples were not collected for this study.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chandrakanth, A., Firdous, S., Vasantharekha, R. et al. Exploring the Effects of Endocrine-Disrupting Chemicals and miRNA Expression in the Pathogenesis of Endometriosis by Unveiling the Pathways: a Systematic Review. Reprod. Sci. 31, 932–941 (2024). https://doi.org/10.1007/s43032-023-01412-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-023-01412-8