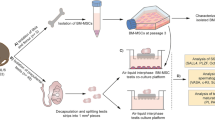
Abstract
Non-obstructive azoospermia is a severe form of male infertility, with limited effective treatments. Bone marrow mesenchymal stem cells (BMSCs) can differentiate to different cell lines; therefore, transplantation of these cells is used for treatment of several diseases. Since these cells require induction factors to differentiate into germ cells, we co-transplanted bone marrow stem cells (BMSCs) with Sertoli cell–conditioned medium (SCCM) into the testis of azoospermic mice. This study was carried out in two sections, in vitro and in vivo. For in vitro study, differentiating factors (c-kit and ID4) were examined after 15 days of co-culture of bone marrow cells with Sertoli cell–conditioned medium, while for in vivo study, the azoospermia model was first created by intraperitoneal administration of a single-dose busulfan (40 mg/kg) followed by single-dose CdCl2 (2 mg/kg) after 4 weeks. Mice were divided into 4 groups including control (azoospermia), BMSC, SCCM, and BMSC + SCCM. Eight weeks after transplantation, samples were assessed for proliferation and differentiation via the expression level of MVH, ID4, SCP3, Tp1, Tp2, and Prm1 differentiation markers. The results showed that BMSC co-cultured with SCCM in vitro differentiated BMSC to germ-like cells. Similarly, in vivo studies revealed a higher level of BMSC differentiation into germ-like cells with significant higher expression of differentiation markers in transplanted groups compared to the control. This study confirmed the role of SCCM as an inductive factor for BMSC differentiation to germ cells both in vivo and in vitro conditions.












Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code Availability
In this study, we used ImageJ (ImageJ, RRID:SCR_003070) and GraphPad Prism (GraphPad Prism, RRID:SCR_002798) software. We also used anti-MVH (Abcam Cat# ab13840, RRID: AB_443012), anti-ID4 polyclonal (Geno Tex Cat# GTX89728, RRID: AB_ 10,726,681), anti-SCP3 (Abcam Cat# ab15093, RRID: AB_301639), anti-c-kit (Fitzgerald Industries International Cat# 10R-3024, RRID: AB_11193325), Goat anti-Rabbit IgG-H&L polyclonal, HRP conjugated (Abcam Cat# ab721, RRID: AB_955447), goat anti-Mouse IgG (H&L)-HRP conjugate (Bio-Rad Cat# 170–6516, RRID: AB_11125547), goat anti-Rabbit IgG-H&L Polyclonal (FITC conjugated) (Abcam Cat# ab6717, RRID: AB_955238), and hamster anti-rat CD29 (β1-integrin) (FITC) (BD Biosciences Cat# 555,005, RRID: AB_395639) antibodies.
References
Duca Y, et al. Current and emerging medical therapeutic agents for idiopathic male infertility. Expert Opin Pharmacother. 2019;20(1):55–67.
Punab M, et al. Causes of male infertility: a 9-year prospective monocentre study on 1737 patients with reduced total sperm counts. Hum Reprod. 2017;32(1):18–31.
Omotosho Dhulqarnain A, Takzaree N, Hassanzadeh G, Solhjo S, Tooli H, Yaaghoobi Nejad M, Shafaat P, Rastegar T. Osteocacin favours the expression of synaptonemal complex protein 3 in azoospermic mouse model. J Contemp Med Sci. 2019;5(1):28–34.
Jarvi K, et al. CUA Guideline: The workup of azoospermic males. Can Urol Assoc J. 2010;4(3):163–7.
Tharakan T, et al. The role of hormone stimulation in men with nonobstructive azoospermia undergoing surgical sperm retrieval. J Clin Endocr Metab. 2020;105(12):e4896–906.
Esteves SC. Clinical management of infertile men with nonobstructive azoospermia. Asian J Androl. 2015;17(3):459–70.
Vij SC, Sabanegh E Jr, Agarwal A. Biological therapy for non-obstructive azoospermia. Expert Opin Biol Ther. 2018;18(1):19–23.
Shirazi R, et al. Differentiation of bone marrow-derived stage-specific embryonic antigen 1 positive pluripotent stem cells into male germ cells. Microsc Res Tech. 2017;80(4):430–40.
Luo Y, et al. Efficient generation of male germ-like cells derived during co-culturing of adipose-derived mesenchymal stem cells with Sertoli cells under retinoic acid and testosterone induction. Stem Cell Res Ther. 2019;10(1):1–18.
Ghorbanlou M, et al. Indirect co-culture of testicular cells with bone marrow mesenchymal stem cells leads to male germ cell-specific gene expressions. Cell J (Yakhteh). 2019;20(4):505.
Karimaghai N, et al. Spermatogenesis after transplantation of adipose tissue-derived mesenchymal stem cells in busulfan-induced azoospermic hamster. Iran J Basic Med Sci. 2018;21(7):660.
Zhankina R, Afshar A, Farrar Z, Khoradmehr A et al. Restoration of spermatogenesis in azoospermic mice by bone marrow mesenchymal stromal/stem cells conditioned medium. Research Square. 2021. https://doi.org/10.21203/rs.3.rs-169243/v1
Martin-Inaraja M, et al. Improving in vitro culture of human male fetal germ cells. Cells. 2021;10(8):2033.
Nayernia K, et al. Derivation of male germ cells from bone marrow stem cells. Lab Invest. 2006;86(7):654–63.
ArefNezhad R, Motedayyen H, Mohammadi A. Therapeutic aspects of mesenchymal stem cell-based cell therapy with a focus on human amniotic epithelial cells in multiple sclerosis: a mechanistic review. Int J Stem Cells. 2021;14(3):241–51.
Monsefi M, et al. Mesenchymal stem cells repair germinal cells of seminiferous tubules of sterile rats. Iran J Reprod Med. 2013;11(7):537–44.
Hajihoseini M, et al. Induction of spermatogenesis after stem cell therapy of azoospermic guinea pigs. Vet Arh. 2017;87(3):333–50.
Bellve A, Zheng W. Growth factors as autocrine and paracrine modulators of male gonadal functions. J Reprod Fertil. 1989;85(2):771–93.
Scarpino S, et al. A rapid method of Sertoli cell isolation by DSA lectin, allowing mitotic analyses. Mol Cell Endocrinol. 1998;146(1–2):121–7.
Kadam P, et al. Co-transplantation of mesenchymal stem cells improves spermatogonial stem cell transplantation efficiency in mice. Stem Cell Res Ther. 2018;9(1):1–11.
Mohaqiq M, et al. In vitro transplantation of spermatogonial stem cells isolated from human frozen–thawed testis tissue can induce spermatogenesis under 3-dimensional tissue culture conditions. Biol Res. 2019;52:1–9.
Babaei H, et al. Increased circulation mobilization of endothelial progenitor cells in preterm infants with retinopathy of prematurity. Mol Cell Biochem. 2018;119(8):6575–83.
Jabarpour M, et al. Hyperbilirubinemia-induced pro-angiogenic activity of infantile endothelial progenitor cells. Microvasc Res. 2018;118:49–56.
Stukenborg J-B, et al. Cancer treatment in childhood and testicular function: the importance of the somatic environment. Endocr Connect. 2018;7(2):R69–87.
Anand S, et al. Underlying mechanisms that restore spermatogenesis on transplanting healthy niche cells in busulphan treated mouse testis. Stem Cell Rev Rep. 2016;12(6):682–97.
Bhartiya D, Anand S. Effects of oncotherapy on testicular stem cells and niche. MHR: Basic Sci Reprod Med. 2017;23(9):654–5.
Shinohara T, et al. Restoration of spermatogenesis in infertile mice by Sertoli cell transplantation. Biol Reprod. 2003;68(3):1064–71.
Marettová E, Maretta M, Legáth J. Toxic effects of cadmium on testis of birds and mammals: a review. Anim Reprod Sci. 2015;155:1–10.
Tokas J, Mukhopadhyay CS, Verma A. Self renewal of spermatogonial stem cells: the most promising multipotent cells-A review. Vet World. 2011;4:234–40.
Dym M, Cavicchia JK. Functional morphology of the testis. Biol Reprod. 1978;18:1–15.
Dominici M, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7.
Wang HS, et al. Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem Cells. 2004;22(7):1330–7.
Toyooka Y, et al. Embryonic stem cells can form germ cells in vitro. Proc Natl Acad Sci U S A. 2003;100(20):11457–62.
Easley CAT, et al. Direct differentiation of human pluripotent stem cells into haploid spermatogenic cells. Cell Rep. 2012;2(3):440–6.
Mehrabani D, et al. Adipose tissue-derived mesenchymal stem cells repair germinal cells of seminiferous tubules of busulfan-induced azoospermic rats. J Hum Reprod Sci. 2015;8(2):103–10.
West FD, et al. In vitro-derived gametes from stem cells. Semin Reprod Med. 2013;31(1):33–8.
Nayernia K, et al. In vitro-differentiated embryonic stem cells give rise to male gametes that can generate offspring mice. Dev Cell. 2006;11(1):125–32.
Aflatoonian B, et al. In vitro post-meiotic germ cell development from human embryonic stem cells. Hum Reprod. 2009;24(12):3150–9.
Oatley JM, Brinster RL. The germline stem cell niche unit in mammalian testes. Physiol Rev. 2012;92(2):577–95.
Kadam P, et al. Co-transplantation of mesenchymal stem cells improves spermatogonial stem cell transplantation efficiency in mice. Stem Cell Res Ther. 2018;9(1):317.
Monfared MH, et al. Sertoli cell condition medium can induce germ like cells from bone marrow derived mesenchymal stem cells. Iran J Basic Med Sci. 2016;19(11):1186.
Oatley MJ, et al. Inhibitor of DNA binding 4 is expressed selectively by single spermatogonia in the male germline and regulates the self-renewal of spermatogonial stem cells in mice. Biol Reprod. 2011;85(2):347–56.
Rathke C, et al. Chromatin dynamics during spermiogenesis. Biochim Biophys Acta (BBA)-Gene Regul Mech. 2014;1839(3):155–68.
Ghasemzadeh-Hasankolaei M, Eslaminejad MB, Sedighi-Gilani M. Derivation of male germ cells from ram bone marrow mesenchymal stem cells by three different methods and evaluation of their fate after transplantation into the testis. Vitro Cell Dev Biol Anim. 2016;52(1):49–61.
Dissanayake D. In vitro spermatogenesis; past, present, and future. In Meccariello R, Chianese R (eds) Spermatozoa facts and perspectives. 2018; IntechOpen. https://doi.org/10.5772/INTECHOPEN.73505
Lee JH, et al. In vitro spermatogenesis by three-dimensional culture of rat testicular cells in collagen gel matrix. Biomaterials. 2006;27(14):2845–53.
Deng C, et al. Urine-derived stem cells facilitate endogenous spermatogenesis restoration of busulfan-induced nonobstructive azoospermic mice by paracrine exosomes. Stem Cells Dev. 2019;28(19):1322–33.
Sun R, et al. The crucial role of Activin A on the formation of primordial germ cell-like cells from skin-derived stem cells in vitro. Cell Cycle. 2015;14(19):3016–29.
Acknowledgements
The authors thank the Tehran University of Medical Sciences and Health Services, Tehran, Iran.
Funding
This study was supported in part by a grant received from the Tehran University of Medical Sciences and Health Services, Tehran, Iran (grant number:48758).
Author information
Authors and Affiliations
Contributions
All authors participated in the design, interpretation of the studies, analysis of the data, and review of the manuscript. Nasrin Khanmohammadi and Tayebeh Rastegar designed the study, Fatemeh Malek supervised the histological studies, Nasrin Takzaree, Mehrnoush Malekzadeh and Maryam Khanehzad supervised the data collection and analyzed the data, Omotosho Dhulqarnain Akanji prepared the manuscript for publication. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This article does not contain any studies with human participants performed by any of the authors. All experiments involving the use of animals were approved by the Institutional Animal Care and Use Committee (IACUC) of the Tehran University of Medical Science. All applicable international, national, and institutional guidelines for the care and use of animals were followed.
Consent to Participate
All authors had final approval of the submitted versions.
Consent for Publication
This is not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khanmohammadi, N., Malek, F., Takzaree, N. et al. Sertoli Cell–Conditioned Medium Induces Differentiation of Bone Marrow–Derived Mesenchymal Stem Cells to Male Germ-Like Cells in Busulfan-Induced Azoospermic Mouse Model. Reprod. Sci. 31, 375–392 (2024). https://doi.org/10.1007/s43032-023-01332-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-023-01332-7