Abstract
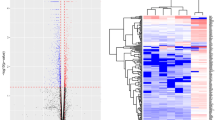
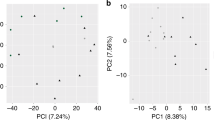
This study aims to investigate the effect of maternal nicotine exposure on the gene expression profiles in the liver of offspring mice. Pregnant mice were subcutaneously injected with either saline vehicle or nicotine twice a day on gestational days 11–21. Total RNA from the liver samples which collected from the offspring mice of postnatal day 7 and 21 was subjected to RNA sequencing. Gene Ontology (GO) functional enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) signaling pathway enrichment analysis were conducted to identify the functions of differentially expressed genes (DEGs). Four genes were selected for further validation by quantitative reverse transcription polymerase chain reaction (qRT-PCR). A total of 448 DEGs and 186 DEGs were identified on postnatal day 7 and 21, respectively. GO analysis revealed that the DEGs on postnatal day 7 mainly participated in the biological functions of cell growth and proliferation, and the DEGs on postnatal day 21 mainly participated in ion transport/activity. KEGG enrichment analysis showed that the DEGs on postnatal day 7 were mainly enriched in the cell cycle, cytokine–cytokine receptor interactions, hypertrophic cardiomyopathy, and the p53 signaling pathway, while the DEGs on postnatal day 21 were mainly enriched in neuroactive ligand-receptor interactions, the calcium signaling pathway, retinol metabolism, and axon guidance. The qRT-PCR results were consistent with the RNA sequencing data. The DEGs may affect the growth of liver in early postnatal period while may affect ion transport/activity in late postnatal period.





Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
References
Jamshed L, Perono GA, Jamshed S, et al. Early life exposure to nicotine: postnatal metabolic, neurobehavioral and respiratory outcomes and the development of childhood cancers. Toxicol Sci. 2020;178(1):3–15.
Gould GS, Havard A, Lim LL, et al. Exposure to tobacco, environmental tobacco smoke and nicotine in pregnancy: a pragmatic overview of reviews of maternal and child outcomes, effectiveness of interventions and barriers and facilitators to quitting. Int J Environ Res Public Health. 2020;17(6):2034.
McGrath-Morrow SA, Gorzkowski J, Groner JA, et al. The effects of nicotine on development. Pediatrics. 2020;145(3):e20191346.
Kuniyoshi KM, Rehan VK. The impact of perinatal nicotine exposure on fetal lung development and subsequent respiratory morbidity. Birth Defects Res. 2019;111(17):1270–83.
Bednarczuk N, Williams EE, Dassios T, et al. Nicotine replacement therapy and e-cigarettes in pregnancy and infant respiratory outcomes. Early Hum Dev. 2022;164:105509.
Chen HJ, Li GL, Zhang WX, et al. Maternal nicotine exposure during pregnancy and lactation induces brown adipose tissue whitening in female offspring. Toxicol Appl Pharmacol. 2020;409:115298.
Li GL, Ping J, Chen HJ, et al. Maternal nicotine exposure impairs brown adipose tissue via AMPK-SIRT1-PGC-1α signals in male offspring. Life Sci. 2021;264:118695.
Paccola CC, Souza GS, Freitas IMM, et al. Does maternal exposure to nicotine affect the oocyte quality and reproductive capacity in adult offspring? Toxicol Appl Pharmacol. 2021;426:115638.
Zhou L, Tao X, Pang G, et al. Maternal nicotine exposure alters hippocampal microglia polarization and promotes anti-inflammatory signaling in juvenile offspring in mice. Front Pharmacol. 2021;12:661304.
Peixoto TC, Gaspar de Moura E, Quitete FT, et al. Early life nicotine exposure alters mRNA and microRNA expressions related to thyroid function and lipid metabolism in liver and BAT of adult wistar rats. Mol Cell Endocrinol. 2021;523:111141.
Huang SJ, Chen SQ, Lin Y, et al. Maternal nicotine exposure aggravates metabolic associated fatty liver disease via PI3K/Akt signaling in adult offspring mice. Liver Int. 2021;41(8):1867–78.
Wongtrakool C, Wang N, Hyde DM, et al. Prenatal nicotine exposure alters lung function and airway geometry through α7 nicotinic receptors. Am J Respir Cell Mol Biol. 2012;46(5):695–702.
Tiesler CM, Heinrich J. Prenatal nicotine exposure and child behavioural problems. Eur Child Adolesc Psychiatry. 2014;23(10):913–29.
Dong T, Hu W, Zhou X, et al. Prenatal exposure to maternal smoking during pregnancy and attention-deficit/hyperactivity disorder in offspring: a meta-analysis. Reprod Toxicol. 2018;76:63–70.
Raez-Villanueva S, Debnath A, Hardy DB, et al. Prenatal nicotine exposure leads to decreased histone H3 lysine 9 (H3K9) methylation and increased p66shc expression in the neonatal pancreas. J Dev Orig Health Dis. 2022;13(2):156–60.
Shorey-Kendrick LE, McEvoy CT, O'Sullivan SM, et al. Impact of vitamin C supplementation on placental DNA methylation changes related to maternal smoking: association with gene expression and respiratory outcomes. Clin Epigenetics. 2021;13(1):177.
Duarte Azevedo M, Sander S, Tenenbaum L. GDNF, a neuron-derived factor upregulated in glial cells during disease. J Clin Med. 2020;9(2):456.
Dudanova I, Gatto G, Klein R. GDNF acts as a chemoattractant to support ephrinA-induced repulsion of limb motor axons. Curr Biol. 2010;20(23):2150–6.
Ibáñez CF, Andressoo JO. Biology of GDNF and its receptors - relevance for disorders of the central nervous system. Neurobiol Dis. 2017;97(Pt B):80–9.
Wang Q, Liu Y, Chen Y, et al. CD300LG improves the cytotoxic activity of CIK. Cent Eur J Immunol. 2017;42(2):117–22.
Umemoto E, Tanaka T, Kanda H, et al. Nepmucin, a novel HEV sialomucin, mediates L-selectin-dependent lymphocyte rolling and promotes lymphocyte adhesion under flow. J Exp Med. 2006;203(6):1603–14.
Shankar R, Leimanis ML, Newbury PA, et al. Gene expression signatures identify paediatric patients with multiple organ dysfunction who require advanced life support in the intensive care unit. EBioMedicine. 2020;62:103122.
Zhou M, Ye Z, Gu Y, et al. Genomic analysis of drug resistant pancreatic cancer cell line by combining long non-coding RNA and mRNA expression profling. Int J Clin Exp Pathol. 2015;8(1):38–52.
Jeung HC, Rha SY, Kim HK, et al. Multi-institutional phase II study of S-1 monotherapy in advanced gastric cancer with pharmacokinetic and pharmacogenomic evaluations. Oncologist. 2007;12(5):543–54.
Gao Q, Khan R, Yu C, et al. The testis-specific LINC component SUN3 is essential for sperm head shaping during mouse spermiogenesis. J Biol Chem. 2020;295(19):6289–98.
Acknowledgements
The authors extend thanks to the laboratory assistants for providing assistance in the laboratory of the First Affiliated Hospital of Xiamen University, Xiamen. The authors also give thanks to funding from the National Natural Science Foundation of China (NO.81600669), the Natural Science Foundation of Fujian Province, China (NO.2016J01646, 2021J05273), the Science and Technology Foundation of Xiamen (NO.3502Z20164004, 3502Z20199017), and Collaborative Innovation Center for Maternal and Infant Health Service Application Technology, Quanzhou Medical College (NO.XJM1803). A special thanks is given to Yi-Ming Su for valuable suggestions for this experiment design and timely help in analyzing the data and English spelling corrections.
Code Availability
Not applicable.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Natural Science Foundation of China (grant number 81600669); the Natural Science Foundation of Fujian Province, China (grant number 2016J01646, 2021J05273); the Science and Technology Foundation of Xiamen, China (grant number 3502Z20164004, 3502Z20199017); and Collaborative Innovation Center for Maternal and Infant Health Service Application Technology, Quanzhou Medical College (grant number XJM1803).
Author information
Authors and Affiliations
Contributions
All the authors participated in the design, interpretation of the studies and analysis of the data, and review of the manuscript. Yi-Ming Su and Mei Huang designed the study; investigation and data curation were done by Shu-Jing Huang and Yu-Qing Su; writing—original draft was by Yan-Ting Lin and Yan Lin; methodology was by Jing Ran and Fang-Fang Yan; validation was by Xian-Lan Liu and Long-Cheng Hong; supervision was by Xiao-Dong Zhang; and writing–review and editing was by Xian-Lan Liu and Huan-Zhong Su.
Corresponding author
Ethics declarations
Ethics Approval
The animal use protocol “The gene expression profiles associated with maternal nicotine exposure in the liver of offspring mice” (Animal Ethics Review Number (XMYY-2020KYSB040)) has been reviewed and approved by the Animal Care and Ethics Committee of Xiamen University. All the animals were acclimated under standard laboratory conditions (ventilated room, 25 ± 1 °C, 60 ± 5% humility, 12-h light/dark cycle) and had free access to standard water and food. All procedures were conducted in accordance with the “Guiding Principles in the Care and Use of Animal” and were approved by the Laboratory Animal Ethics Committee of Xiamen University.
Consent to Participate
Not applicable.
Consent for Publication
The authors consented for the publication.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yan-Ting Lin, Yan Lin, and Xian-Lan Liu contributed to this work equally.
Supplementary information
ESM 1
(XLSX 7131 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, YT., Lin, Y., Huang, SJ. et al. The Gene Expression Profiles Associated with Maternal Nicotine Exposure in the Liver of Offspring Mice. Reprod. Sci. 31, 212–221 (2024). https://doi.org/10.1007/s43032-023-01328-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-023-01328-3