Abstract
Tubulin beta 8 class VIII (TUBB8) is a β-tubulin isotype that is specifically expressed in human oocytes and early embryos. It has been identified as a disease-causing gene in primary female infertility by affecting oocyte maturation arrest. This study investigated the genetic cause of female infertility in five patients from four families. Five women with primary infertility were recruited. Medical-exome sequencing and Sanger sequencing were performed on the patients, and their family members to identify candidate genes that explained infertility. Additionally, the morphology of oocytes and zygotes from the patients and controls were assessed. We observed recurrent oocytes MI arrest, oocytes abnormal fertilization, uncleaved embryos, and embryo transfer failure in the patients. Heterozygous missense variants in TUBB8, c.538G > A (p.V180M), c.527C > G (p.S176W), c.124C > G (p.L42V), and c.628A > C (p.I210L), were verified in four unrelated families. This study expanded the mutational spectrum of TUBB8 by identifying three novel heterozygous missense variants. Screening for TUBB8 mutation demonstrated the diagnostic utility of female infertility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infertility affects approximately 10–15% of couples worldwide, and female infertility accounts for nearly two-thirds of them [1]. Assisted reproductive technologies (ART) such as in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) successfully help many infertile couples to have offspring. However, there are still women experiencing recurrent failures of IVF/ICSI due to oocyte maturation arrest, embryo transfer failure, or embryonic arrest.
Successful human reproduction requires proper oocyte maturation and embryonic development. The meiotic cell cycle of oocyte starts in the neonatal ovary and pauses at prophase I of meiosis until the luteinizing hormone restores meiosis during puberty. Oocyte maturation takes place in the sequence of germinal vesicle (GV) breakdown, spindle assembly, and asymmetric division. This progression completes after extruding the first polar body (PB) and again arrests at the MII stage until fertilization [2]. Any disturbance during these periods can lead to oocyte maturation arresting and result in female infertility [3, 4]. Although several studies have investigated the mechanism of oocyte maturation defects in mice models, the genetic etiology in human remains largely unknown.
Tubulin beta eight class VIII (TUBB8) is a highly conserved primate-specific β-tubulin isotype that is uniquely expressed in human oocytes and early embryos. Spindle assembly plays an important role in both mitosis and meiosis. The spindles consist of dynamic microtubules, which are polymerization by the α/β-tubulin heterodimer [5]. Feng et al. [6] first identified that disease in TUBB8 gene compromise spindle assembly and then led to oocyte arrest at MI. In the subsequent studies, a phenotype spectrum was discovered, including oocyte maturation arrest at the MI or MII stages, inability for fertilization, recurrent failures of embryo transfer, and embryos that were uncleaved or in early arrest. The inheritance pattern of TUBB8 and pathogenic mechanisms are also variable. The autosomal dominant form is caused by dominant-negative effects, while autosomal recessive is through haploinsufficiency effects [6, 7]. Previous studies found that mutations in the TUBB8 gene can explain approximately 30% of women with oocyte maturation arrest [8, 9].
In this study, we reported five female patients from four unrelated families with primary infertility and IVF failure, characterized by a range of oocyte and embryo developmental arrest phenotypes. Medical exome sequencing revealed four heterozygous missense variants in TUBB8, of which three variants were novel and one variant was recurrent.
Materials and Methods
Human Subjects
A total of five infertility women due to oocyte maturation arrest, abnormal fertilization, embryonic development disorder, and large polar body oocyte from four families were recruited from the Reproductive Medicine Center, Henan Provincial People Hospital (Fig. 1A; Table 1). Informed consent forms were signed by all patients and their family members. Peripheral blood samples were collected from all members for DNA extraction and subsequent sequencing.
Evaluation of Oocyte and Embryo Phenotype
The morphology and maturity of oocytes or embryos were examined by light microscopy. Both patients followed the controlled ovarian hyperstimulation (COH) protocols, and transvaginal ultrasound was used to monitor the diameters of follicles. After 2–3 dominant follicles reached a diameter of 18 mm, human HCG was injected 36–38 h before oocytes retrieval. Oocytes were retrieved from the patients and underwent IVF/ICSI. At 16–18 h after the insemination, the oocytes were checked for fertilization. The presence of two pronuclei (PN) was considered normal fertilization. The fertilized zygotes were cultured, and morphology was assessed on days 2 and 3 by optical microscope.
DNA Extraction and Medical Exome Sequencing
A total of 3–5 mL peripheral blood was drawn from the proband and her six family members. The blood samples were sent to the independent laboratory (AmCare Genomics Laboratory, Guangzhou, China) for medical exome sequencing (MES) and data analysis. Genomic DNA was extracted utilizing the Solpure Blood DNA Kit (Magen) following the manufacturer’s instructions. The genomic DNA was fragmented by the Q800R Sonicator (Qsonica) to generate 300–500 bp insert fragments. Custom-designed NimbleGen SeqCap probes (Roche NimbleGen, Madison, WI, USA) were used for in-solution hybridization to enrich target sequences, which included coding exonic regions of approximately 5000 OMIM recorded genes and known pathogenic variants from deep intronic or other non-coding regions. Enriched DNA samples were then sequenced by the Illumina NovaSeq 6000 platform. The average coverage depth was 200 × with coverage greater than 20 × accounting for more than 99% of the exome. Sequenced reads were mapped to the reference human genome version (GRCh37/hg19) by NextGENe. Sequence variants were annotated and included population (gnomAD, 1000 Genomes, dbSNP) and variant databases (Clinvar, HGMD). Variant interpretation followed the American College of Medical Genetics (ACMG) guidelines [10]. Sanger sequencing was performed to confirm the variants identified by MES.
Results
Clinical Presentation
A total of 5 infertility women from 4 unrelated families who underwent IVF/ICSI attempts in our center were recruited. They had normal menstrual cycles, basal sex hormone levels, thyroid function, ovary function, and karyotype. All of their husbands were healthy with normal karyotype and normal sperm concentration, motility, and morphology. None of the couples had a family history of infertility (except for Case 2) and no history of exposure to reproductive toxicities. Ultrasound and hysterosalpingography examinations showed that Cases 1, 2, and 3 had obstructed left fallopian tubes, and all patients showed normal development of the uteruses and ovaries.
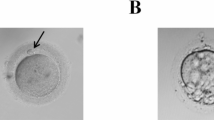
Detailed patients’ clinical information including age, the duration of infertility, previous COH protocols, history of IVF/ICSI circles, oocyte maturity and morphology, fertilization, and embryo development outcomes are summarized in Table 1. The five patients showed rather different features of oocytes and embryos. Case 1 (Family 1, II-2) and Case 3 (Family 2, I-1) both showed oocyte maturation arrest; all of their retrieved oocytes were arrested at the MI stage after removing the granulosa cells, and no MII oocyte was found after in vitro maturation cultures (Fig. 2). Both of the patients had no successfully fertilized embryo for transfer; therefore, their IVF/ICSI cycles were canceled. Case 3 also showed oocytes with abnormal morphology, all oocytes without granulosa cells (6/29, 20.7%). Despite Case 2 (Family 1, II-4) coming from the same family with Case 1, her oocyte phenotype was different from her sister. She was able to obtain a small number of MII oocytes (6/35, 17.1%), and two 2PN zygotes and two 0PN2PB zygotes were seen after ICSI. Only the 2PN zygotes were considered normal fertilization, but the embryos were uncleaved after culture, so the circles were canceled. Similarly, Case 4 (Family 3, I-1) was able to obtain mature MII oocytes and had normal fertilized embryos by ICSI, but she failed to be pregnant after embryo transfer. Twenty percent (2/10, 20%) of her oocytes showed abnormal fertilization. Case 5 (Family 4, I-1) received 6 COH circles, and a total of 99 oocytes were obtained. She had a rather high proportion of MII oocytes (52/99, 52.5%), and oocytes with abnormal morphology (21/99, 21.1%). The MII oocytes could be fertilized with a large proportion of abnormal fertilized oocytes (33/52, 63.5%). The abnormal morphologies of oocyte presented the absence of GV, large polar body, multiple polar bodies, and huge oocyte. The abnormal fertilized oocytes mainly showed multiple PN. She tried 4 times of frozen embryo transfer (FET) but failed to conceive. Overall, the 5 patients suffered from infertility lasted range 2–7 years, and they all at least received 2 IVF/ICSI cycles but failed to be pregnant.
Mutation Analysis
We analyzed the medical exome sequencing data focusing on the known genes related to female infertility. The variant spectra of TUBB8 in 5 patients are summarized in Table 2. In family 1, a novel heterozygous missense mutation in TUBB8, c.538G > A (p.V180M), was found in the proband (II-2), her elderly fertile brother (II-1), and her younger sister (II-4) who also suffered from infertility. The mutation in the three siblings was inherited from their father (I-1). None of the proband’s mother (I-2) and her other two fertile sisters (II-3, II-5) carried the same mutation. In families 2–4, heterozygous mutations c.527C > G (p.S176W), c.124C > G (p.L42V), and c.628A > C (p.I210L) were found in Cases 3, 4, and 5, respectively. Because of a lack of parental information, the three variants were considered as an unknown inheritance pattern (Fig. 1A). We performed in silico prediction for the variants by SIFT and PolyPhen-2; all variants were predicted to be damaging, except p.L42V had a controversial result between two methods (SIFT: damaging; Polyphen-2: benign). We performed additional predictions for p.L42V through PROVEAN (Tolerable) and MutationTaster (Diseasing-causing). As TUBB8 protein is a primate-specific β-tubulin isotype, we also performed multiple alignments across various species, and the result showed that all variants were highly conserved among primates, except for p.L42V (Fig. 1B).
Discussion
Female infertility is a multicausal reproductive disease, which affects 10% of women worldwide [11]. In this study, we reported five patients from four unrelated families with primary infertility, representing oocyte maturation arrest, abnormal fertilization, uncleaved embryos, and embryo transfer failure. Four different missense variants (3 of which are novel) in TUBB8, c.538G > A, c.527C > G, c.124C > G, and c.628A > C were identified in each family, respectively.
There were more than 100 unique variants in TUBB8 that have been reported, most of them were missense, and other mutation types include frameshift, splice site, and small indels [8]. Heterozygous TUBB8 missense variants that caused oocyte maturation arrest were mostly paternally inherited or de novo through dominant-negative effects, while homozygous variants in consanguineous families and heterozygous variants maternally inherited with incomplete penetrance were also reported [12]. In this study, the variant p.V180M found in Family 1 was identified in Cases 1 and 2, inherited from their father and their fertile brother also carried the variant, while it was not found in the female family members with normal fertility. In this pedigree, the segregation pattern suggested that p.V180M was autosomal dominant inherited. The variant p.V180M was not reported in infertility cases before, while a variant, p.V179M, only one residue apart, was reported in two patients which both showed oocytes arrest at the MI stage [13]. In addition, p.S176L, a variant at the same position with p.S176W but different amino acid change, was reported in two unrelated families and was de novo. The patients who carried p.S176L had no morphologically identifiable MII oocytes, whose phenotypes were consistent with Case 3 who carried p.S176W [6, 14]. The variant p.L42V in Case 4 was previously reported in a patient with embryonic arrest [9]. Case 4 and a former patient who carried p.L42V both could obtain MII oocytes and had normal fertilized embryos, but they either had no usable embryos due to embryonic arrest or suffered embryo transfer failure. Similarly, the variant p.I210L in Case 5 was novel, while a variant at the same position, p.I210V, was reported in a patient with normal fertilization but embryonic arrest [14]. Case 5 had identifiable MII oocytes and normal fertilized embryos, but experienced recurrent embryo transfer failures, which was similar to patient who carried p. I210V. In vivo experiments revealed that p.S176L and p.I210V caused complete loss of the microtubule network and impaired spindle assembly [6, 14]. Moreover, the functional change of p.L42V and p.V180M still require further investigation.
According to the current data, TUBB8 variants can explain 30% of patients with oocyte maturation arrest [15]. The phenotypic spectrum of oocytes with TUBB8 deficiency is associated with the defect of spindle assembly. Phenotypes can be variable, even with the same variant in one family. New phenotypes of TUBB8 variants were successively discovered in recent years, including (1) oocytes arrested at the MI stage, (2) MII oocytes that cannot be fertilized, (3) MII oocytes that can be fertilized but uncleaved, (4) embryos with early arrest, (5) viable embryos but recurrent transfer failure, (6) large polar bodies, and (7) multiple pronuclei in zygotes [6, 12, 14, 15, 16].
The tubulin family has 8 β-tubulin isotypes in human, and different isotypes are distinguished by different coding genes or posttranslational modifications [5]. Beta-tubulin forms heterodimers with α-tubulin and polymerizes into microtubules to carry out cellular functions, including intracellular transport and cell division. Carboxy-terminal tail of β-tubulin isotypes is variable, and these isotypes of β-tubulin are enriched in certain cells or tissue. Variants in TUBB1, TUBB2A, TUBB2B, TUBB3, TUBB4A, and TUBB4B affected neuronal migration, differentiation, and axon maintenance [17]. These variants have been reported concerning tubulinopathies in neurons, resulting in nervous system malformations [18]. On the other hand, TUBB8 function still remains largely unclear, possibly due to its expression being limited to primate oocytes [19]. Several in vivo studies showed that the missense variants in TUBB8 caused microtubule disruption and severely or completely impaired spindle assembly [6, 14, 15]. In addition, in vitro studies that expressed mutant TUBB8 in culture cells showed that missense variants did not influence translational efficiency but reduced the yield of de novo assembled heterodimers that could result in tubulin haploinsufficiency [14]. In short, most missense variants in TUBB8 are likely to influence microtubules by impacting either β-tubulin stability or lateral contacts between protofilaments. Structure analysis of β-tubulin [6, 13] confirmed that p.V179, and p.S176 are located at a longitudinal interface between assembled alpha/beta-tubulin heterodimer and the variants presumably disrupt heterodimer longitudinal interactions and affect microtubule assembly. We speculated that p.V180M, p.S176W, p.L42V, and p.I210L possibly present similar effects.
TUBB8-deficiency primary female infertility had a poor prognosis in assisted reproductive treatment. Scientists are attempting to explore therapy for TUBB8 deficiency in mouse models. Jia et al. [20] found that co-injection of wild-type TUBB8 cRNA into oocytes that expressed mutant TUBB8 can successfully improve spindle assembly and produce live offspring. However, centrioles do not participate in mouse fertilization, and the pattern of early embryo cleavage in mice is different from that in human. Therefore, the safety and reliability of gene therapy in human still require further experimental evidence and verification. However, women with infertility caused by TUBB8 variants were able to conceive through egg donation [21]. TUBB8 variants have a high proportion of infertile females, and males who carried TUBB8 variants are fertile. Exome and genome sequencing has not been widely used in infertility clinics. It is possible that the prevalence of TUBB8 variants may be underestimated. Thus, we suggested that TUBB8 screening should be performed for women with primary infertility to avoid recurrent IVF failure and to reduce the financial and psychological burden of the patients.
Conclusion
In summary, we reported 5 women with infertility in 4 families characterized by oocyte maturation arrest, abnormal fertilization, uncleaved embryos, and embryo transfer failure. Three novel variants and one recurrent missense variant were identified in TUBB8. Our findings emphasize the importance of screening for TUBB8 variant in infertile patients with oocyte maturation and or embryo development arrest.
Data Availability
The data in this article are not publicly available because of concerns regarding patient anonymity. Requests to access the data should be directed to the corresponding author.
Code Availability
Not applicable.
References
Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod. 2007;22:1506–12.
Mehlmann LM. Stops and starts in mammalian oocytes: recent advances in understanding the regulation of meiotic arrest and oocyte maturation. Reproduction. 2005;130:791–9.
Dean J. Exacting Requirements for Development of the Egg. N Engl J Med. 2016;374:279–80.
Eppig JJ. Coordination of nuclear and cytoplasmic oocyte maturation in eutherian mammals. Reprod Fertil Dev. 1996;8:485–9.
Sirajuddin M, Rice LM, Vale RD. Regulation of microtubule motors by tubulin isotypes and post-translational modifications. Nat Cell Biol. 2014;16:335–44.
Feng R, Sang Q, Kuang Y, Sun X, Yan Z, Zhang S, Shi J, Tian G, Luchniak A, Fukuda Y, et al. Mutations in TUBB8 and human oocyte meiotic arrest. N Engl J Med. 2016;374:223–32.
Xing Q, Wang R, Chen B, Li L, Pan H, Li T, Ma X, Cao Y, Wang B. Rare homozygous mutation in TUBB8 associated with oocyte maturation defect-2 in a consanguineous mating family. J Ovarian Res. 2020;13:42.
Yao Z, Zeng J, Zhu H, Zhao J, Wang X, Xia Q, Li Y, Wu L. Mutation analysis of the TUBB8 gene in primary infertile women with oocyte maturation arrest. J Ovarian Res. 2022;15:38.
Zheng W, Hu H, Zhang S, Xu X, Gao Y, Gong F, Lu G, Lin G. The comprehensive variant and phenotypic spectrum of TUBB8 in female infertility. J Assist Reprod Genet. 2021;38:2261–72.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–24.
Maddirevula S, Awartani K, Coskun S, AlNaim LF, Ibrahim N, Abdulwahab F, Hashem M, Alhassan S, Alkuraya FS. A genomics approach to females with infertility and recurrent pregnancy loss. Hum Genet. 2020;139:605–13.
Chen B, Wang W, Peng X, Jiang H, Zhang S, Li D, Li B, Fu J, Kuang Y, Sun X, et al. The comprehensive mutational and phenotypic spectrum of TUBB8 in female infertility. Eur J Hum Genet. 2019;27:300–7.
Huang L, Tong X, Luo L, Zheng S, Jin R, Fu Y, Zhou G, Li D, Liu Y. Mutation analysis of the TUBB8 gene in nine infertile women with oocyte maturation arrest. Reprod Biomed Online. 2017;35:305–10.
Feng R, Yan Z, Li B, Yu M, Sang Q, Tian G, Xu Y, Chen B, Qu R, Sun Z, et al. Mutations in TUBB8 cause a multiplicity of phenotypes in human oocytes and early embryos. J Med Genet. 2016;53:662–71.
Zhao L, Guan Y, Wang W, Chen B, Xu S, Wu L, Yan Z, Li B, Fu J, Shi R, et al. Identification novel mutations in TUBB8 in female infertility and a novel phenotype of large polar body in oocytes with TUBB8 mutations. J Assist Reprod Genet. 2020;37:1837–47.
Sha Q, Zheng W, Feng X, Yuan R, Hu H, Gong F, Hu L, Lin G, Ou X. Novel mutations in TUBB8 expand the mutational and phenotypic spectrum of patients with zygotes containing multiple pronuclei. Gene. 2021;769: 145227.
Bahi-Buisson N, Poirier K, Fourniol F, Saillour Y, Valence S, Lebrun N, Hully M, Bianco CF, Boddaert N, Elie C, et al. The wide spectrum of tubulinopathies: what are the key features for the diagnosis? Brain. 2014;137:1676–700.
Binarova P, Tuszynski J. Tubulin: structure, functions and roles in disease. Cells. 2019;8(10):1294.
Biswas L, Tyc K, El Yakoubi W, Morgan K, Xing J, Schindler K. Meiosis interrupted: the genetics of female infertility via meiotic failure. Reproduction. 2021;161:R13–35.
Jia Y, Li K, Zheng C, Tang Y, Bai D, Yin J, Chi F, Zhang Y, Li Y, Tu Z, et al. Identification and rescue of a novel TUBB8 mutation that causes the first mitotic division defects and infertility. J Assist Reprod Genet. 2020;37:2713–22.
Lanuza-Lopez MC, Martinez-Garza SG, Solorzano-Vazquez JF, Paz-Cervantes D, Gonzalez-Ortega C, Maldonado-Rosas I, Villegas-Moreno G, Villar-Munoz LG, Arroyo-Mendez FA, Gutierrez-Gutierrez AM, Pina-Aguilar RE. Oocyte maturation arrest produced by TUBB8 mutations: impact of genetic disorders in infertility treatment. Gynecol Endocrinol. 2020;36:829–34.
Acknowledgements
We thank the patients and their family members for their participation in this study.
Funding
This study was funded by the Major Project in Provincial and National Union Construction of Henan Medical Science Research Plan: SBGJ202001002.
Author information
Authors and Affiliations
Contributions
Cuilian Zhang created the research design. Shaodi Zhang selects the patients. Wenzhu Yu wrote the manuscript and formulated and submitted the paper. Chang Dong and Baoli Yin performed the data analysis. Zhang VW performed the genetic analysis and reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
The study protocol was approved by the Reproductive Medicine Ethics Committee of Henan Provincial People’s Hospital.
Consent to Participate
Informed consents were signed by all participants in this study.
Consent for Publication
All authors consent to the publication of the manuscript.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yu, W., Zhang, S., Yin, B. et al. Identification of TUBB8 Variants in 5 Primary Infertile Women with Multiple Phenotypes in Oocytes and Early Embryos. Reprod. Sci. 30, 1376–1382 (2023). https://doi.org/10.1007/s43032-022-01079-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-022-01079-7