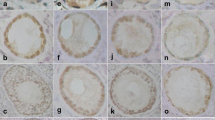
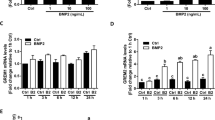
Abstract
Bone morphogenetic proteins (BMPs) are the largest subfamily of the transforming growth factor-β (TGF-β) superfamily. BMP4 is a secreted protein that was originally identified due to its role in bone and cartilage development. Over the past decades, extensive literature has indicated that BMP4 and its receptors are widely expressed in the ovary. Dysregulation of BMP4 expression may play a vital role in follicular development, polycystic ovary syndrome (PCOS), and ovarian cancer. In this review, we summarized the expression pattern of BMP4 in the ovary, focused on the role of BMP4 in follicular development and steroidogenesis, and discussed the role of BMP4 in ovarian diseases such as polycystic ovary syndrome and ovarian cancer. Some studies have shown that the expression of BMP4 in the ovary is spatiotemporal and species specific, but the effects of BMP4 seem to be similar in follicular development of different species. In addition, BMP4 is involved in the development of hyperandrogenemia in PCOS and drug resistance in ovarian cancer, but further research is still needed to clarify the specific mechanisms.

Similar content being viewed by others
References
Uemura MT, Ihara M, Maki T, Nakagomi T, Kaji S, Uemura K, et al. Pericyte-derived bone morphogenetic protein 4 underlies white matter damage after chronic hypoperfusion. Brain Pathol. 2018;28(4):521–35. https://doi.org/10.1111/bpa.12523.
Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, et al. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242(4885):1528–34. https://doi.org/10.1126/science.3201241.
Yanai I, Benjamin H, Shmoish M, Chalifa-Caspi V, Shklar M, Ophir R, et al. Genome-wide midrange transcription profiles reveal expression level relationships in human tissue specification. Bioinformatics. 2005;21(5):650–9. https://doi.org/10.1093/bioinformatics/bti042.
Persani L, Rossetti R, Di Pasquale E, Cacciatore C, Fabre S. The fundamental role of bone morphogenetic protein 15 in ovarian function and its involvement in female fertility disorders. Hum Reprod Update. 2014;20(6):869–83. https://doi.org/10.1093/humupd/dmu036.
Wu X, Yao J, Wang L, Zhang D, Zhang L, Reynolds EX, et al. Crosstalk between BMP and Notch Induces Sox2 in Cerebral Endothelial Cells. Cells. 2019;8(6). https://doi.org/10.3390/cells8060549.
Gunne-Braden A, Sullivan A, Gharibi B, Sheriff RSM, Maity A, Wang YF, et al. GATA3 mediates a fast, irreversible commitment to BMP4-driven differentiation in human embryonic stem cells. Cell Stem Cell. 2020;26:693–706.e9. https://doi.org/10.1016/j.stem.2020.03.005.
Oichi T, Taniguchi Y, Soma K, Oshima Y, Yano F, Mori Y, et al. Adamts17 is involved in skeletogenesis through modulation of BMP-Smad1/5/8 pathway. Cell Mol Life Sci. 2019;76(23):4795–809. https://doi.org/10.1007/s00018-019-03188-0.
Deng G, Zeng S, Qu Y, Luo Q, Guo C, Yin L, et al. BMP4 promotes hepatocellular carcinoma proliferation by autophagy activation through JNK1-mediated Bcl-2 phosphorylation. J Exp Clin Cancer Res. 2018;37(1):156. https://doi.org/10.1186/s13046-018-0828-x.
Rossetti R, Ferrari I, Bestetti I, Moleri S, Brancati F, Petrone L, et al. Fundamental role of BMP15 in human ovarian folliculogenesis revealed by null and missense mutations associated with primary ovarian insufficiency. Hum Mutat. 2020;41(5):983–97. https://doi.org/10.1002/humu.23988.
Tabas JA, Hahn GV, Cohen RB, Seaunez HN, Modi WS, Wozney JM, et al. Chromosomal assignment of the human gene for bone morphogenetic protein 4. Clin Orthop Relat Res. 1993;293:310–6.
Liem KF Jr, Tremml G, Roelink H, Jessell TM. Dorsal differentiation of neural plate cells induced by BMP-mediated signals from epidermal ectoderm. Cell. 1995;82(6):969–79. https://doi.org/10.1016/0092-8674(95)90276-7.
Bellusci S, Henderson R, Winnier G, Oikawa T, Hogan BL. Evidence from normal expression and targeted misexpression that bone morphogenetic protein (Bmp-4) plays a role in mouse embryonic lung morphogenesis. Development. 1996;122(6):1693–702.
Cossu G, Tajbakhsh S, Buckingham M. How is myogenesis initiated in the embryo? Trends Genet. 1996;12(6):218–23. https://doi.org/10.1016/0168-9525(96)10025-1.
Modica S, Straub LG, Balaz M, Sun W, Varga L, Stefanicka P, et al. Bmp4 promotes a brown to white-like adipocyte shift. Cell Rep. 2016;16(8):2243–58. https://doi.org/10.1016/j.celrep.2016.07.048.
Gustafson B, Hammarstedt A, Hedjazifar S, Hoffmann JM, Svensson PA, Grimsby J, et al. BMP4 and BMP antagonists regulate human white and beige adipogenesis. Diabetes. 2015;64(5):1670–81. https://doi.org/10.2337/db14-1127.
Ou M, Zhao Y, Zhang F, Huang X. Bmp2 and Bmp4 accelerate alveolar bone development. Connect Tissue Res. 2015;56(3):204–11. https://doi.org/10.3109/03008207.2014.996701.
Chen S, Jia L, Zhang S, Zheng Y, Zhou Y. DEPTOR regulates osteogenic differentiation via inhibiting MEG3-mediated activation of BMP4 signaling and is involved in osteoporosis. Stem Cell Res Ther. 2018;9(1):185. https://doi.org/10.1186/s13287-018-0935-9.
Jin X, Nie E, Zhou X, Zeng A, Yu T, Zhi T, et al. Fstl1 Promotes glioma growth through the BMP4/Smad1/5/8 signaling pathway. Cell Physiol Biochem. 2017;44(4):1616–28. https://doi.org/10.1159/000485759.
Shee K, Jiang A, Varn FS, Liu S, Traphagen NA, Owens P, et al. Cytokine sensitivity screening highlights BMP4 pathway signaling as a therapeutic opportunity in ER(+) breast cancer. FASEB J. 2019;33(2):1644–57. https://doi.org/10.1096/fj.201801241R.
Nemashkalo A, Ruzo A, Heemskerk I, Warmflash A. Morphogen and community effects determine cell fates in response to BMP4 signaling in human embryonic stem cells. Development. 2017;144(17):3042–53. https://doi.org/10.1242/dev.153239.
Costa J, de Souza GB, Bernardo JMP, Ribeiro RP, Passos JRS, Bezerra FTG, et al. Expression of markers for germ cells and oocytes in cow dermal fibroblast treated with 5-azacytidine and cultured in differentiation medium containing BMP2, BMP4 or follicular fluid. Zygote. 2017;25(3):341–57. https://doi.org/10.1017/S0967199417000211.
Al-Samerria S, Al-Ali I, McFarlane JR, Almahbobi G. The impact of passive immunisation against BMPRIB and BMP4 on follicle development and ovulation in mice. Reproduction. 2015;149(5):403–11. https://doi.org/10.1530/REP-14-0451.
Chang HM, Cheng JC, Fang L, Qiu X, Klausen C, Taylor EL, et al. Recombinant BMP4 and BMP7 downregulate pentraxin 3 in human granulosa cells. J Clin Endocrinol Metab. 2015;100(3):E365–74. https://doi.org/10.1210/jc.2014-2496.
Zhang H, Klausen C, Zhu H, Chang HM, Leung PC. BMP4 and BMP7 suppress StAR and progesterone production via ALK3 and SMAD1/5/8-SMAD4 in human granulosa-lutein cells. Endocrinology. 2015;156(11):4269–80. https://doi.org/10.1210/en.2015-1494.
Yang L, Chang CC, Sun Z, Madsen D, Zhu H, Padkjaer SB, et al. GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand. Nat Med. 2017;23(10):1158–66. https://doi.org/10.1038/nm.4394.
Baraban E, Chavakis T, Hamilton BS, Sales S, Wabitsch M, Bornstein SR, et al. Anti-inflammatory properties of bone morphogenetic protein 4 in human adipocytes. Int J Obes. 2016;40(2):319–27. https://doi.org/10.1038/ijo.2015.141.
Walton KL, Kelly EK, Johnson KE, Robertson DM, Stanton PG, Harrison CA. A novel, more efficient approach to generate bioactive inhibins. Endocrinology. 2016;157(7):2799–809. https://doi.org/10.1210/en.2015-1963.
Pickup MW, Owens P, Moses HL. TGF-beta, bone morphogenetic protein, and activin signaling and the tumor microenvironment. Cold Spring Harb Perspect Biol. 2017;9(5). https://doi.org/10.1101/cshperspect.a022285.
van den Wijngaard A, Weghuis DO, Boersma CJ, van Zoelen EJ, Geurts van Kessel A, Olijve W. Fine mapping of the human bone morphogenetic protein-4 gene (BMP4) to chromosome 14q22-q23 by in situ hybridization. Genomics. 1995;27(3):559–60. https://doi.org/10.1006/geno.1995.1096.
Li M, Chen Q, Sun G, Shi X, Zhao Q, Zhang C, et al. Characterization and expression of bone morphogenetic protein 4 gene in postnatal pigs. Mol Biol Rep. 2010;37(5):2369–77. https://doi.org/10.1007/s11033-009-9743-8.
Cui Y, Jean F, Thomas G, Christian JL. BMP-4 is proteolytically activated by furin and/or PC6 during vertebrate embryonic development. EMBO J. 1998;17(16):4735–43. https://doi.org/10.1093/emboj/17.16.4735.
Bragdon B, Moseychuk O, Saldanha S, King D, Julian J, Nohe A. Bone morphogenetic proteins: a critical review. Cell Signal. 2011;23(4):609–20. https://doi.org/10.1016/j.cellsig.2010.10.003.
Kim HS, Neugebauer J, McKnite A, Tilak A, Christian JL. BMP7 functions predominantly as a heterodimer with BMP2 or BMP4 during mammalian embryogenesis. Elife. 2019;8. https://doi.org/10.7554/eLife.48872.
Goldman DC, Hackenmiller R, Nakayama T, Sopory S, Wong C, Kulessa H, et al. Mutation of an upstream cleavage site in the BMP4 prodomain leads to tissue-specific loss of activity. Development. 2006;133(10):1933–42. https://doi.org/10.1242/dev.02368.
Qian SW, Wu MY, Wang YN, Zhao YX, Zou Y, Pan JB, et al. BMP4 facilitates beige fat biogenesis via regulating adipose tissue macrophages. J Mol Cell Biol. 2019;11(1):14–25. https://doi.org/10.1093/jmcb/mjy011.
Wilkinson L, Kolle G, Wen D, Piper M, Scott J, Little M. CRIM1 regulates the rate of processing and delivery of bone morphogenetic proteins to the cell surface. J Biol Chem. 2003;278(36):34181–8. https://doi.org/10.1074/jbc.M301247200.
Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425(6958):577–84. https://doi.org/10.1038/nature02006.
Gomez-Puerto MC, Iyengar PV, Garcia de Vinuesa A, Ten Dijke P, Sanchez-Duffhues G. Bone morphogenetic protein receptor signal transduction in human disease. J Pathol. 2019;247(1):9–20. https://doi.org/10.1002/path.5170.
Tielemans B, Delcroix M, Belge C, Quarck R. TGFbeta and BMPRII signalling pathways in the pathogenesis of pulmonary arterial hypertension. Drug Discov Today. 2019;24(3):703–16. https://doi.org/10.1016/j.drudis.2018.12.001.
Chowdhury HM, Sharmin N, Yuzbasioglu Baran M, Long L, Morrell NW, Trembath RC, et al. BMPRII deficiency impairs apoptosis via the BMPRII-ALK1-BclX-mediated pathway in pulmonary arterial hypertension. Hum Mol Genet. 2019;28(13):2161–73. https://doi.org/10.1093/hmg/ddz047.
Rezzola S, Di Somma M, Corsini M, Leali D, Ravelli C, Polli VAB, et al. VEGFR2 activation mediates the pro-angiogenic activity of BMP4. Angiogenesis. 2019;22(4):521–33. https://doi.org/10.1007/s10456-019-09676-y.
Luo B, Chen K, Feng Q, Xiao W, Ma D, Yang H, et al. The interplay of BMP4 and IL7 regulates the apoptosis of intestinal intraepithelial lymphocytes under conditions of ischemiareperfusion. Int J Mol Med. 2018;41(5):2640–50. https://doi.org/10.3892/ijmm.2018.3480.
Di Giovanni V, Alday A, Chi L, Mishina Y, Rosenblum ND. Alk3 controls nephron number and androgen production via lineage-specific effects in intermediate mesoderm. Development. 2011;138(13):2717–27. https://doi.org/10.1242/dev.059030.
Leibovich A, Steinbeisser H, Fainsod A. Expression of the ALK1 family of type I BMP/ADMP receptors during gastrula stages in Xenopus embryos. Int J Dev Biol. 2017;61(6-7):465–70. https://doi.org/10.1387/ijdb.170037af.
Modica S, Wolfrum C. The dual role of BMP4 in adipogenesis and metabolism. Adipocyte. 2017;6(2):141–6. https://doi.org/10.1080/21623945.2017.1287637.
Li SN, Wu JF. TGF-beta/SMAD signaling regulation of mesenchymal stem cells in adipocyte commitment. Stem Cell Res Ther. 2020;11(1):41. https://doi.org/10.1186/s13287-020-1552-y.
Eckhardt BL, Cao Y, Redfern AD, Chi LH, Burrows AD, Roslan S, et al. Activation of canonical BMP4-SMAD7 signaling suppresses breast cancer metastasis. Cancer Res. 2020;80:1304–15. https://doi.org/10.1158/0008-5472.CAN-19-0743.
Tsukamoto S, Mizuta T, Fujimoto M, Ohte S, Osawa K, Miyamoto A, et al. Smad9 is a new type of transcriptional regulator in bone morphogenetic protein signaling. Sci Rep. 2014;4:7596. https://doi.org/10.1038/srep07596.
Massague J. TGFbeta signalling in context. Nat Rev Mol Cell Biol. 2012;13(10):616–30. https://doi.org/10.1038/nrm3434.
Zi Z, Chapnick DA, Liu X. Dynamics of TGF-beta/Smad signaling. FEBS Lett. 2012;586(14):1921–8. https://doi.org/10.1016/j.febslet.2012.03.063.
David CJ, Massague J. Contextual determinants of TGFbeta action in development, immunity and cancer. Nat Rev Mol Cell Biol. 2018;19(7):419–35. https://doi.org/10.1038/s41580-018-0007-0.
Miyazono KI, Ohno Y, Wada H, Ito T, Fukatsu Y, Kurisaki A, et al. Structural basis for receptor-regulated SMAD recognition by MAN1. Nucleic Acids Res. 2018;46(22):12139–53. https://doi.org/10.1093/nar/gky925.
Zhao M, Mishra L, Deng CX. The role of TGF-beta/SMAD4 signaling in cancer. Int J Biol Sci. 2018;14(2):111–23. https://doi.org/10.7150/ijbs.23230.
Wu FJ, Lin TY, Sung LY, Chang WF, Wu PC, Luo CW. BMP8A sustains spermatogenesis by activating both SMAD1/5/8 and SMAD2/3 in spermatogonia. Sci Signal. 2017;10(477). https://doi.org/10.1126/scisignal.aal1910.
Caddy JC, Luoma LM, Berry FB. FOXC1 negatively regulates BMP-SMAD activity and Id1 expression during osteoblast differentiation. J Cell Biochem. 2020;121:3266–77. https://doi.org/10.1002/jcb.29595.
Miyazawa K, Miyazono K. Regulation of TGF-beta family signaling by inhibitory Smads. Cold Spring Harb Perspect Biol. 2017;9(3). https://doi.org/10.1101/cshperspect.a022095.
Yuan M, Ge M, Yin J, Dai Z, Xie L, Li Y, et al. Isoflurane post-conditioning down-regulates expression of aquaporin 4 in rats with cerebral ischemia/reperfusion injury and is possibly related to bone morphogenetic protein 4/Smad1/5/8 signaling pathway. Biomed Pharmacother. 2018;97:429–38. https://doi.org/10.1016/j.biopha.2017.10.082.
Zhou F, Xie F, Jin K, Zhang Z, Clerici M, Gao R, et al. USP4 inhibits SMAD4 monoubiquitination and promotes activin and BMP signaling. EMBO J. 2017;36(11):1623–39. https://doi.org/10.15252/embj.201695372.
Lawrence DA. Deficient R-smad/smad4 complex formation in fibroblasts growth-stimulated by TGF-beta 1. Int J Oncol. 2002;20(4):803–6.
Zhang H, Du L, Zhong Y, Flanders KC, Roberts JD Jr. Transforming growth factor-beta stimulates Smad1/5 signaling in pulmonary artery smooth muscle cells and fibroblasts of the newborn mouse through ALK1. Am J Phys Lung Cell Mol Phys. 2017;313(3):L615–L27. https://doi.org/10.1152/ajplung.00079.2017.
Kaminska B, Cyranowski S. Recent advances in understanding mechanisms of TGF beta signaling and its role in glioma pathogenesis. Adv Exp Med Biol. 2020;1202:179–201. https://doi.org/10.1007/978-3-030-30651-9_9.
Shen S, Wang S, He Y, Hu H, Yao B, Zhang Y. Regulation of bone morphogenetic protein 4 on epithelial tissue. J Cell Commun Signal. 2020;14:283–92. https://doi.org/10.1007/s12079-019-00537-3.
Li W, Li W, Zou L, Ji S, Li C, Liu K, et al. Membrane targeting of inhibitory Smads through palmitoylation controls TGF-beta/BMP signaling. Proc Natl Acad Sci U S A. 2017;114(50):13206–11. https://doi.org/10.1073/pnas.1710540114.
Yan X, Liao H, Cheng M, Shi X, Lin X, Feng XH, et al. Smad7 protein interacts with receptor-regulated Smads (R-Smads) to inhibit transforming growth factor-beta (TGF-beta)/Smad signaling. J Biol Chem. 2016;291(1):382–92. https://doi.org/10.1074/jbc.M115.694281.
Han P, Cui Q, Yang S, Wang H, Gao P, Li Z. Tumor necrosis factor-alpha and transforming growth factor-beta1 facilitate differentiation and proliferation of tendon-derived stem cells in vitro. Biotechnol Lett. 2017;39(5):711–9. https://doi.org/10.1007/s10529-017-2296-3.
Chiu CY, Kuo KK, Kuo TL, Lee KT, Cheng KH. The activation of MEK/ERK signaling pathway by bone morphogenetic protein 4 to increase hepatocellular carcinoma cell proliferation and migration. Mol Cancer Res. 2012;10(3):415–27. https://doi.org/10.1158/1541-7786.MCR-11-0293.
Wang L, Li JY, Zhang XZ, Liu L, Wan ZM, Li RX, et al. Involvement of p38MAPK/NF-kappaB signaling pathways in osteoblasts differentiation in response to mechanical stretch. Ann Biomed Eng. 2012;40(9):1884–94. https://doi.org/10.1007/s10439-012-0548-x.
Zhang YE. Non-Smad signaling pathways of the TGF-beta family. Cold Spring Harb Perspect Biol. 2017;9(2). https://doi.org/10.1101/cshperspect.a022129.
Tian XY, Yung LH, Wong WT, Liu J, Leung FP, Liu L, et al. Bone morphogenic protein-4 induces endothelial cell apoptosis through oxidative stress-dependent p38MAPK and JNK pathway. J Mol Cell Cardiol. 2012;52(1):237–44. https://doi.org/10.1016/j.yjmcc.2011.10.013.
Jeffery TK, Upton PD, Trembath RC, Morrell NW. BMP4 inhibits proliferation and promotes myocyte differentiation of lung fibroblasts via Smad1 and JNK pathways. Am J Phys Lung Cell Mol Phys. 2005;288(2):L370–8. https://doi.org/10.1152/ajplung.00242.2004.
Imran KM, Rahman N, Yoon D, Jeon M, Lee BT, Kim YS. Cryptotanshinone promotes commitment to the brown adipocyte lineage and mitochondrial biogenesis in C3H10T1/2 mesenchymal stem cells via AMPK and p38-MAPK signaling. Biochim Biophys Acta Mol Cell Biol Lipids. 2017;1862(10 Pt A):1110–20. https://doi.org/10.1016/j.bbalip.2017.08.001.
Li X, Lu W, Fu X, Zhang Y, Yang K, Zhong N, et al. BMP4 increases canonical transient receptor potential protein expression by activating p38 MAPK and ERK1/2 signaling pathways in pulmonary arterial smooth muscle cells. Am J Respir Cell Mol Biol. 2013;49(2):212–20. https://doi.org/10.1165/rcmb.2012-0051OC.
Lee MY, Lim HW, Lee SH, Han HJ. Smad, PI3K/Akt, and Wnt-dependent signaling pathways are involved in BMP-4-induced ESC self-renewal. Stem Cells. 2009;27(8):1858–68. https://doi.org/10.1002/stem.124.
Cui Y, Zhang F, Jia Y, Sun L, Chen M, Wu S, et al. The BMP antagonist, SOSTDC1, restrains gastric cancer progression via inactivation of c-Jun signaling. Am J Cancer Res. 2019;9(11):2331–48.
Yang X, Lee PJ, Long L, Trembath RC, Morrell NW. BMP4 induces HO-1 via a Smad-independent, p38MAPK-dependent pathway in pulmonary artery myocytes. Am J Respir Cell Mol Biol. 2007;37(5):598–605. https://doi.org/10.1165/rcmb.2006-0360OC.
Abir R, Ben-Haroush A, Melamed N, Felz C, Krissi H, Fisch B. Expression of bone morphogenetic proteins 4 and 7 and their receptors IA, IB, and II in human ovaries from fetuses and adults. Fertil Steril. 2008;89(5 Suppl):1430–40. https://doi.org/10.1016/j.fertnstert.2007.04.064.
Brazert M, Izycki D, Kranc W, Borowiec B, Popis M, Ozegowska K, et al. Genes involved in hormone metabolism and cellular response in human ovarian granulosa cells. J Biol Regul Homeost Agents. 2019;33(2):461–8.
Erickson GF, Shimasaki S. The spatiotemporal expression pattern of the bone morphogenetic protein family in rat ovary cell types during the estrous cycle. Reprod Biol Endocrinol. 2003;1:9. https://doi.org/10.1186/1477-7827-1-9.
Shimasaki S, Zachow RJ, Li D, Kim H, Iemura S, Ueno N, et al. A functional bone morphogenetic protein system in the ovary. Proc Natl Acad Sci U S A. 1999;96(13):7282–7. https://doi.org/10.1073/pnas.96.13.7282.
Tanwar PS, McFarlane JR. Dynamic expression of bone morphogenetic protein 4 in reproductive organs of female mice. Reproduction. 2011;142(4):573–9. https://doi.org/10.1530/REP-10-0299.
Li CW, Ge W. Spatiotemporal expression of bone morphogenetic protein family ligands and receptors in the zebrafish ovary: a potential paracrine-signaling mechanism for oocyte-follicle cell communication. Biol Reprod. 2011;85(5):977–86. https://doi.org/10.1095/biolreprod.111.092239.
Li CW, Zhou R, Ge W. Differential regulation of gonadotropin receptors by bone morphogenetic proteins in the zebrafish ovary. Gen Comp Endocrinol. 2012;176(3):420–5. https://doi.org/10.1016/j.ygcen.2011.12.032.
La Rosa I, Camargo L, Pereira MM, Fernandez-Martin R, Paz DA, Salamone DF. Effects of bone morphogenic protein 4 (BMP4) and its inhibitor, Noggin, on in vitro maturation and culture of bovine preimplantation embryos. Reprod Biol Endocrinol. 2011;9:18. https://doi.org/10.1186/1477-7827-9-18.
Diaz PU, Hein GJ, Belotti EM, Rodriguez FM, Rey F, Amweg AN, et al. BMP2, 4 and 6 and BMPR1B are altered from early stages of bovine cystic ovarian disease development. Reproduction. 2016;152(4):333–50. https://doi.org/10.1530/REP-15-0315.
Fatehi AN, van den Hurk R, Colenbrander B, Daemen AJ, van Tol HT, Monteiro RM, et al. Expression of bone morphogenetic protein2 (BMP2), BMP4 and BMP receptors in the bovine ovary but absence of effects of BMP2 and BMP4 during IVM on bovine oocyte nuclear maturation and subsequent embryo development. Theriogenology. 2005;63(3):872–89. https://doi.org/10.1016/j.theriogenology.2004.05.013.
Kayani AR, Glister C, Knight PG. Evidence for an inhibitory role of bone morphogenetic protein(s) in the follicular-luteal transition in cattle. Reproduction. 2009;137(1):67–78. https://doi.org/10.1530/REP-08-0198.
Kim D, Ocon-Grove O, Johnson AL. Bone morphogenetic protein 4 supports the initial differentiation of hen (Gallus gallus) granulosa cells. Biol Reprod. 2013;88(6):161. https://doi.org/10.1095/biolreprod.113.109694.
Nio-Kobayashi J, Trendell J, Giakoumelou S, Boswell L, Nicol L, Kudo M, et al. Bone morphogenetic proteins are mediators of luteolysis in the human corpus luteum. Endocrinology. 2015;156(4):1494–503. https://doi.org/10.1210/en.2014-1704.
Rajesh G, Paul A, Mishra SR, Bharati J, Thakur N, Mondal T, et al. Expression and functional role of Bone Morphogenetic Proteins (BMPs) in cyclical corpus luteum in buffalo (Bubalus bubalis). Gen Comp Endocrinol. 2017;240:198–213. https://doi.org/10.1016/j.ygcen.2016.10.016.
Rajesh G, Mishra SR, Paul A, Punetha M, Vidyalakshmi GM, Narayanan K, et al. Transcriptional and translational abundance of Bone morphogenetic protein (BMP) 2, 4, 6, 7 and their receptors BMPR1A, 1B and BMPR2 in buffalo ovarian follicle and the role of BMP4 and BMP7 on estrogen production and survival of cultured granulosa cells. Res Vet Sci. 2018;118:371–88. https://doi.org/10.1016/j.rvsc.2018.04.002.
Yuan J, Deng Y, Zhang Y, Gan X, Gao S, Hu H, et al. Bmp4 inhibits goose granulosa cell apoptosis via PI3K/AKT/Caspase-9 signaling pathway. Anim Reprod Sci. 2019;200:86–95. https://doi.org/10.1016/j.anireprosci.2018.11.014.
Glister C, Satchell L, Knight PG. Changes in expression of bone morphogenetic proteins (BMPs), their receptors and inhibin co-receptor betaglycan during bovine antral follicle development: inhibin can antagonize the suppressive effect of BMPs on thecal androgen production. Reproduction. 2010;140(5):699–712. https://doi.org/10.1530/REP-10-0216.
Glister C, Satchell L, Knight PG. Granulosal and thecal expression of bone morphogenetic protein- and activin-binding protein mRNA transcripts during bovine follicle development and factors modulating their expression in vitro. Reproduction. 2011;142(4):581–91. https://doi.org/10.1530/REP-11-0150.
Estienne A, Pierre A, di Clemente N, Picard JY, Jarrier P, Mansanet C, et al. Anti-Mullerian hormone regulation by the bone morphogenetic proteins in the sheep ovary: deciphering a direct regulatory pathway. Endocrinology. 2015;156(1):301–13. https://doi.org/10.1210/en.2014-1551.
Pierre A, Estienne A, Racine C, Picard JY, Fanchin R, Lahoz B, et al. The bone morphogenetic protein 15 up-regulates the anti-mullerian hormone receptor expression in granulosa cells. J Clin Endocrinol Metab. 2016;101(6):2602–11. https://doi.org/10.1210/jc.2015-4066.
Sudo S, Avsian-Kretchmer O, Wang LS, Hsueh AJ. Protein related to DAN and cerberus is a bone morphogenetic protein antagonist that participates in ovarian paracrine regulation. J Biol Chem. 2004;279(22):23134–41. https://doi.org/10.1074/jbc.M402376200.
Pierre A, Pisselet C, Dupont J, Mandon-Pepin B, Monniaux D, Monget P, et al. Molecular basis of bone morphogenetic protein-4 inhibitory action on progesterone secretion by ovine granulosa cells. J Mol Endocrinol. 2004;33(3):805–17. https://doi.org/10.1677/jme.1.01545.
Juengel JL, Reader KL, Bibby AH, Lun S, Ross I, Haydon LJ, et al. The role of bone morphogenetic proteins 2, 4, 6 and 7 during ovarian follicular development in sheep: contrast to rat. Reproduction. 2006;131(3):501–13. https://doi.org/10.1530/rep.1.00958.
Glister C, Regan SL, Samir M, Knight P. Gremlin, Noggin, Chordin and follistatin differentially modulate BMP induced suppression of androgen secretion by bovine ovarian theca cells. J Mol Endocrinol. 2018. https://doi.org/10.1530/JME-18-0198.
Yamashita H, Murayama C, Takasugi R, Miyamoto A, Shimizu T. BMP-4 suppresses progesterone production by inhibiting histone H3 acetylation of StAR in bovine granulosa cells in vitro. Mol Cell Biochem. 2011;348(1-2):183–90. https://doi.org/10.1007/s11010-010-0653-9.
Roy S, Gandra D, Seger C, Biswas A, Kushnir VA, Gleicher N, et al. Oocyte-derived factors (GDF9 and BMP15) and FSH regulate AMH expression via modulation of H3K27AC in granulosa cells. Endocrinology. 2018;159(9):3433–45. https://doi.org/10.1210/en.2018-00609.
Chang HM, Cheng JC, Leung PC. Theca-derived BMP4 and BMP7 down-regulate connexin43 expression and decrease gap junction intercellular communication activity in immortalized human granulosa cells. J Clin Endocrinol Metab. 2013;98(3):E437–45. https://doi.org/10.1210/jc.2012-3851.
Shimizu T, Kayamori T, Murayama C, Miyamoto A. Bone morphogenetic protein (BMP)-4 and BMP-7 suppress granulosa cell apoptosis via different pathways: BMP-4 via PI3K/PDK-1/Akt and BMP-7 via PI3K/PDK-1/PKC. Biochem Biophys Res Commun. 2012;417(2):869–73. https://doi.org/10.1016/j.bbrc.2011.12.064.
Ding X, Zhang X, Mu Y, Li Y, Hao J. Effects of BMP4/SMAD signaling pathway on mouse primordial follicle growth and survival via up-regulation of Sohlh2 and c-kit. Mol Reprod Dev. 2013;80(1):70–8. https://doi.org/10.1002/mrd.22138.
Zhang X, Zhang H, Gao Q, Ji S, Bing L, Hao J. Sohlh2 inhibits the apoptosis of mouse primordial follicle oocytes via C-kit/PI3K/Akt/Foxo3a signalling pathway. Reprod BioMed Online. 2015;30(5):514–21. https://doi.org/10.1016/j.rbmo.2015.01.015.
Bayne RA, Donnachie DJ, Kinnell HL, Childs AJ, Anderson RA. BMP signalling in human fetal ovary somatic cells is modulated in a gene-specific fashion by GREM1 and GREM2. Mol Hum Reprod. 2016;22(9):622–33. https://doi.org/10.1093/molehr/gaw044.
Nilsson EE, Larsen G, Skinner MK. Roles of Gremlin 1 and Gremlin 2 in regulating ovarian primordial to primary follicle transition. Reproduction. 2014;147(6):865–74. https://doi.org/10.1530/REP-14-0005.
Nilsson EE, Skinner MK. Bone morphogenetic protein-4 acts as an ovarian follicle survival factor and promotes primordial follicle development. Biol Reprod. 2003;69(4):1265–72. https://doi.org/10.1095/biolreprod.103.018671.
Tanwar PS, O'Shea T, McFarlane JR. In vivo evidence of role of bone morphogenetic protein-4 in the mouse ovary. Anim Reprod Sci. 2008;106(3-4):232–40. https://doi.org/10.1016/j.anireprosci.2007.04.015.
Brito AB, Santos RR, van den Hurk R, Lima JS, Miranda MS, Ohashi OM, et al. Short-term culture of ovarian cortical strips from capuchin monkeys (Sapajus apella): a morphological, viability, and molecular study of preantral follicular development in vitro. Reprod Sci. 2013;20(8):990–7. https://doi.org/10.1177/1933719112472737.
da Cunha EV, Melo LRF, Sousa GB, Araujo VR, Vasconcelos GL, Silva AWB, et al. Effect of bone morphogenetic proteins 2 and 4 on survival and development of bovine secondary follicles cultured in vitro. Theriogenology. 2018;110:44–51. https://doi.org/10.1016/j.theriogenology.2017.12.032.
da Cunha EV, de Souza GB, Passos JRS, Silva AWB, Dau AM, Saraiva MVA, et al. Effects of bone morphogenetic protein 4 (BMP4) on in vitro development and survival of bovine preantral follicles enclosed in fragments ovarian tissue. Zygote. 2017;25(3):256–64. https://doi.org/10.1017/S0967199417000089.
Bertoldo MJ, Duffard N, Bernard J, Frapsauce C, Calais L, Rico C, et al. Effects of bone morphogenetic protein 4 (BMP4) supplementation during culture of the sheep ovarian cortex. Anim Reprod Sci. 2014;149(3-4):124–34. https://doi.org/10.1016/j.anireprosci.2014.07.010.
Zhang H, Tian S, Klausen C, Zhu H, Liu R, Leung PC. Differential activation of noncanonical SMAD2/SMAD3 signaling by bone morphogenetic proteins causes disproportionate induction of hyaluronan production in immortalized human granulosa cells. Mol Cell Endocrinol. 2016;428:17–27. https://doi.org/10.1016/j.mce.2016.03.016.
Childs AJ, Kinnell HL, Collins CS, Hogg K, Bayne RA, Green SJ, et al. BMP signaling in the human fetal ovary is developmentally regulated and promotes primordial germ cell apoptosis. Stem Cells. 2010;28(8):1368–78. https://doi.org/10.1002/stem.440.
Shirazi R, Zarnani AH, Soleimani M, Abdolvahabi MA, Nayernia K, Ragerdi KI. BMP4 can generate primordial germ cells from bone-marrow-derived pluripotent stem cells. Cell Biol Int. 2012;36(12):1185–93. https://doi.org/10.1042/CBI20110651.
Bahmanpour S, Zarei Fard N, Talaei-Khozani T, Hosseini A, Esmaeilpour T. Effect of BMP4 preceded by retinoic acid and co-culturing ovarian somatic cells on differentiation of mouse embryonic stem cells into oocyte-like cells. Develop Growth Differ. 2015;57(5):378–88. https://doi.org/10.1111/dgd.12217.
Park ES, Woods DC, Tilly JL. Bone morphogenetic protein 4 promotes mammalian oogonial stem cell differentiation via Smad1/5/8 signaling. Fertil Steril. 2013;100(5):1468–75. https://doi.org/10.1016/j.fertnstert.2013.07.1978.
de Souza GB, Costa J, da Cunha EV, Passos J, Ribeiro RP, Saraiva M, et al. Bovine ovarian stem cells differentiate into germ cells and oocyte-like structures after culture in vitro. Reprod Domest Anim. 2017;52(2):243–50. https://doi.org/10.1111/rda.12886.
Chuang CY, Lin KI, Hsiao M, Stone L, Chen HF, Huang YH, et al. Meiotic competent human germ cell-like cells derived from human embryonic stem cells induced by BMP4/WNT3A signaling and OCT4/EpCAM (epithelial cell adhesion molecule) selection. J Biol Chem. 2012;287(18):14389–401. https://doi.org/10.1074/jbc.M111.338434.
Ying Y, Zhao GQ. Cooperation of endoderm-derived BMP2 and extraembryonic ectoderm-derived BMP4 in primordial germ cell generation in the mouse. Dev Biol. 2001;232(2):484–92. https://doi.org/10.1006/dbio.2001.0173.
Lawson KA, Dunn NR, Roelen BA, Zeinstra LM, Davis AM, Wright CV, et al. Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev. 1999;13(4):424–36. https://doi.org/10.1101/gad.13.4.424.
Pesce M, Klinger FG, De Felici M. Derivation in culture of primordial germ cells from cells of the mouse epiblast: phenotypic induction and growth control by Bmp4 signalling. Mech Dev. 2002;112(1-2):15–24. https://doi.org/10.1016/s0925-4773(01)00624-4.
Dudley BM, Runyan C, Takeuchi Y, Schaible K, Molyneaux K. BMP signaling regulates PGC numbers and motility in organ culture. Mech Dev. 2007;124(1):68–77. https://doi.org/10.1016/j.mod.2006.09.005.
Ross A, Munger S, Capel B. Bmp7 regulates germ cell proliferation in mouse fetal gonads. Sex Dev. 2007;1(2):127–37. https://doi.org/10.1159/000100034.
Gu Y, Runyan C, Shoemaker A, Surani A, Wylie C. Steel factor controls primordial germ cell survival and motility from the time of their specification in the allantois, and provides a continuous niche throughout their migration. Development. 2009;136(8):1295–303. https://doi.org/10.1242/dev.030619.
Findlay JK, Hutt KJ, Hickey M, Anderson RA. How Is the Number of Primordial Follicles in the Ovarian Reserve Established? Biol Reprod. 2015;93(5):111. https://doi.org/10.1095/biolreprod.115.133652.
Delcour C, Amazit L, Patino LC, Magnin F, Fagart J, Delemer B, et al. ATG7 and ATG9A loss-of-function variants trigger autophagy impairment and ovarian failure. Genet Med. 2019;21(4):930–8. https://doi.org/10.1038/s41436-018-0287-y.
Tan S, Feng B, Yin M, Zhou HJ, Lou G, Ji W, et al. Stromal Senp1 promotes mouse early folliculogenesis by regulating BMP4 expression. Cell Biosci. 2017;7:36. https://doi.org/10.1186/s13578-017-0163-5.
Xu Y, Li E, Han Y, Chen L, Xie Z. Differential expression of mRNAs encoding BMP/Smad pathway molecules in antral follicles of high- and low-fecundity Hu sheep. Anim Reprod Sci. 2010;120(1-4):47–55. https://doi.org/10.1016/j.anireprosci.2010.02.009.
Zhang Y, Xu Y, Kuai Y, Wang S, Xue Q, Shang J. Effect of testosterone on the Connexin37 of sexual mature mouse cumulus oocyte complex. J Ovarian Res. 2016;9(1):82. https://doi.org/10.1186/s13048-016-0290-3.
Simon AM, Goodenough DA, Li E, Paul DL. Female infertility in mice lacking connexin 37. Nature. 1997;385(6616):525–9. https://doi.org/10.1038/385525a0.
Ackert CL, Gittens JE, O'Brien MJ, Eppig JJ, Kidder GM. Intercellular communication via connexin43 gap junctions is required for ovarian folliculogenesis in the mouse. Dev Biol. 2001;233(2):258–70. https://doi.org/10.1006/dbio.2001.0216.
Wiesen JF, Midgley AR Jr. Changes in expression of connexin 43 gap junction messenger ribonucleic acid and protein during ovarian follicular growth. Endocrinology. 1993;133(2):741–6. https://doi.org/10.1210/endo.133.2.8393773.
Salustri A, Campagnolo L, Klinger FG, Camaioni A. Molecular organization and mechanical properties of the hyaluronan matrix surrounding the mammalian oocyte. Matrix Biol. 2019;78-79:11–23. https://doi.org/10.1016/j.matbio.2018.02.002.
Varani S, Elvin JA, Yan C, DeMayo J, DeMayo FJ, Horton HF, et al. Knockout of pentraxin 3, a downstream target of growth differentiation factor-9, causes female subfertility. Mol Endocrinol. 2002;16(6):1154–67. https://doi.org/10.1210/mend.16.6.0859.
Salustri A, Garlanda C, Hirsch E, De Acetis M, Maccagno A, Bottazzi B, et al. PTX3 plays a key role in the organization of the cumulus oophorus extracellular matrix and in in vivo fertilization. Development. 2004;131(7):1577–86. https://doi.org/10.1242/dev.01056.
Liu ZH, Yue KZ, Ma SF, Sun XS, Tan JH. Effects of pregnant mare serum gonadotropin (eCG) on follicle development and granulosa-cell apoptosis in the pig. Theriogenology. 2003;59(3-4):775–85. https://doi.org/10.1016/s0093-691x(02)01122-6.
Murdoch WJ. Programmed cell death in preovulatory ovine follicles. Biol Reprod. 1995;53(1):8–12. https://doi.org/10.1095/biolreprod53.1.8.
Shimasaki S, Moore RK, Otsuka F, Erickson GF. The bone morphogenetic protein system in mammalian reproduction. Endocr Rev. 2004;25(1):72–101. https://doi.org/10.1210/er.2003-0007.
Oktem O, Urman B. Understanding follicle growth in vivo. Hum Reprod. 2010;25(12):2944–54. https://doi.org/10.1093/humrep/deq275.
Feldberg D, Goldman GA, Ashkenazi J, Dicker D, Shelef M, Goldman JA. The impact of high progesterone levels in the follicular phase of in vitro fertilization (IVF) cycles: a comparative study. J In Vitro Fert Embryo Transf. 1989;6(1):11–4. https://doi.org/10.1007/BF01134575.
Khalaf M, Morera J, Bourret A, Reznik Y, Denoual C, Herlicoviez M, et al. BMP system expression in GCs from polycystic ovary syndrome women and the in vitro effects of BMP4, BMP6, and BMP7 on GC steroidogenesis. Eur J Endocrinol. 2013;168(3):437–44. https://doi.org/10.1530/EJE-12-0891.
Cheng L, Lu W, Kulkarni B, Pejovic T, Yan X, Chiang JH, et al. Analysis of chemotherapy response programs in ovarian cancers by the next-generation sequencing technologies. Gynecol Oncol. 2010;117(2):159–69. https://doi.org/10.1016/j.ygyno.2010.01.041.
Laatio L, Myllynen P, Serpi R, Rysa J, Ilves M, Lappi-Blanco E, et al. BMP-4 expression has prognostic significance in advanced serous ovarian carcinoma and is affected by cisplatin in OVCAR-3 cells. Tumour Biol. 2011;32(5):985–95. https://doi.org/10.1007/s13277-011-0200-7.
Liu Y, Du SY, Ding M, Dou X, Zhang FF, Wu ZY, et al. The BMP4-Smad signaling pathway regulates hyperandrogenism development in a female mouse model. J Biol Chem. 2017;292(28):11740–50. https://doi.org/10.1074/jbc.M117.781369.
Hansen SL, Svendsen PF, Jeppesen JF, Hoeg LD, Andersen NR, Kristensen JM, et al. Molecular mechanisms in skeletal muscle underlying insulin resistance in women who are lean with polycystic ovary syndrome. J Clin Endocrinol Metab. 2019;104(5):1841–54. https://doi.org/10.1210/jc.2018-01771.
Li R, Zhang Q, Yang D, Li S, Lu S, Wu X, et al. Prevalence of polycystic ovary syndrome in women in China: a large community-based study. Hum Reprod. 2013;28(9):2562–9. https://doi.org/10.1093/humrep/det262.
van Houten EL, Laven JS, Louwers YV, McLuskey A, Themmen AP, Visser JA. Bone morphogenetic proteins and the polycystic ovary syndrome. J Ovarian Res. 2013;6(1):32. https://doi.org/10.1186/1757-2215-6-32.
Galloway SM, McNatty KP, Cambridge LM, Laitinen MP, Juengel JL, Jokiranta TS, et al. Mutations in an oocyte-derived growth factor gene (BMP15) cause increased ovulation rate and infertility in a dosage-sensitive manner. Nat Genet. 2000;25(3):279–83. https://doi.org/10.1038/77033.
Bodensteiner KJ, Clay CM, Moeller CL, Sawyer HR. Molecular cloning of the ovine Growth/Differentiation factor-9 gene and expression of growth/differentiation factor-9 in ovine and bovine ovaries. Biol Reprod. 1999;60(2):381–6. https://doi.org/10.1095/biolreprod60.2.381.
Amsterdam EA-SrPCWG. Consensus on women's health aspects of polycystic ovary syndrome (PCOS). Hum Reprod. 2012;27(1):14–24. https://doi.org/10.1093/humrep/der396.
Vendola KA, Zhou J, Adesanya OO, Weil SJ, Bondy CA. Androgens stimulate early stages of follicular growth in the primate ovary. J Clin Invest. 1998;101(12):2622–9. https://doi.org/10.1172/JCI2081.
Jonard S, Dewailly D. The follicular excess in polycystic ovaries, due to intra-ovarian hyperandrogenism, may be the main culprit for the follicular arrest. Hum Reprod Update. 2004;10(2):107–17. https://doi.org/10.1093/humupd/dmh010.
Thackray VG. Sex, microbes, and polycystic ovary syndrome. Trends Endocrinol Metab. 2019;30(1):54–65. https://doi.org/10.1016/j.tem.2018.11.001.
Suenaga M, Kurosawa N, Asano H, Kanamori Y, Umemoto T, Yoshida H, et al. Bmp4 expressed in preadipocytes is required for the onset of adipocyte differentiation. Cytokine. 2013;64(1):138–45. https://doi.org/10.1016/j.cyto.2013.07.011.
Gustafson B, Hedjazifar S, Gogg S, Hammarstedt A, Smith U. Insulin resistance and impaired adipogenesis. Trends Endocrinol Metab. 2015;26(4):193–200. https://doi.org/10.1016/j.tem.2015.01.006.
Lheureux S, Gourley C, Vergote I, Oza AM. Epithelial ovarian cancer. Lancet. 2019;393(10177):1240–53. https://doi.org/10.1016/S0140-6736(18)32552-2.
Shepherd TG, Nachtigal MW. Identification of a putative autocrine bone morphogenetic protein-signaling pathway in human ovarian surface epithelium and ovarian cancer cells. Endocrinology. 2003;144(8):3306–14. https://doi.org/10.1210/en.2003-0185.
Theriault BL, Nachtigal MW. Human ovarian cancer cell morphology, motility, and proliferation are differentially influenced by autocrine TGFbeta superfamily signalling. Cancer Lett. 2011;313(1):108–21. https://doi.org/10.1016/j.canlet.2011.08.033.
Theriault BL, Shepherd TG, Mujoomdar ML, Nachtigal MW. BMP4 induces EMT and Rho GTPase activation in human ovarian cancer cells. Carcinogenesis. 2007;28(6):1153–62. https://doi.org/10.1093/carcin/bgm015.
Shepherd TG, Theriault BL, Nachtigal MW. Autocrine BMP4 signalling regulates ID3 proto-oncogene expression in human ovarian cancer cells. Gene. 2008;414(1-2):95–105. https://doi.org/10.1016/j.gene.2008.02.015.
Ma W, Ma J, Xu J, Qiao C, Branscum A, Cardenas A, et al. Lin28 regulates BMP4 and functions with Oct4 to affect ovarian tumor microenvironment. Cell Cycle. 2013;12(1):88–97. https://doi.org/10.4161/cc.23028.
Wu T, Dai Y. Tumor microenvironment and therapeutic response. Cancer Lett. 2017;387:61–8. https://doi.org/10.1016/j.canlet.2016.01.043.
Ho CM, Shih DT, Hsiao CC, Huang SH, Chang SF, Cheng WF. Gene methylation of human ovarian carcinoma stromal progenitor cells promotes tumorigenesis. J Transl Med. 2015;13:367. https://doi.org/10.1186/s12967-015-0722-7.
Ho CM, Chang SF, Hsiao CC, Chien TY, Shih DT. Isolation and characterization of stromal progenitor cells from ascites of patients with epithelial ovarian adenocarcinoma. J Biomed Sci. 2012;19:23. https://doi.org/10.1186/1423-0127-19-23.
McLean K, Gong Y, Choi Y, Deng N, Yang K, Bai S, et al. Human ovarian carcinoma-associated mesenchymal stem cells regulate cancer stem cells and tumorigenesis via altered BMP production. J Clin Invest. 2011;121(8):3206–19. https://doi.org/10.1172/JCI45273.
Coffman LG, Choi YJ, McLean K, Allen BL, di Magliano MP, Buckanovich RJ. Human carcinoma-associated mesenchymal stem cells promote ovarian cancer chemotherapy resistance via a BMP4/HH signaling loop. Oncotarget. 2016;7(6):6916–32. https://doi.org/10.18632/oncotarget.6870.
Availability of Data and Materials
Not applicable.
Code Availability
Not applicable.
Funding
This research was funded by the National Key R&D Program of China (2018YFC1003200), the National Natural Science Foundation of China (81860276), the National Natural Science Foundation of China (11774274), and the Special Fund of Fundamental Scientific Research Business Expense for Higher School of Central Government (2042020kf0088).
Author information
Authors and Affiliations
Contributions
Dongyong Yang, Yi Yang, Min Hu, and Yanxiang Cheng performed the PubMed search. Dongyong Yang and Xiao Yang prepared the sections on the BMP4 and BMP4 signaling pathways, the expression pattern of BMP4 in the ovary, and its role in ovarian steroidogenesis. Fangfang Dai, Yanqing Wang, and Min Hu prepared the sections on the role of BMP4 in follicular development. Yi Yang and Yanxiang Cheng prepared the sections on the role of BMP4 in reproductive diseases. Dongyong Yang and Xiao Yang organized and edited the manuscript. Dongyong Yang prepared the figures, and all authors thoroughly revised and approved the manuscript before submission.
Corresponding authors
Ethics declarations
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dongyong Yang and Xiao Yang are joint first authors.
Rights and permissions
About this article
Cite this article
Yang, D., Yang, X., Dai, F. et al. The Role of Bone Morphogenetic Protein 4 in Ovarian Function and Diseases. Reprod. Sci. 28, 3316–3330 (2021). https://doi.org/10.1007/s43032-021-00600-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-021-00600-8