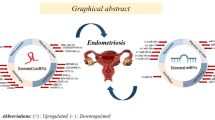
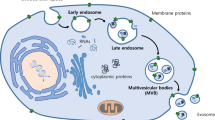
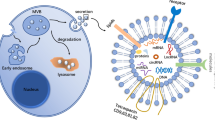
Abstract
Exosomes are small bilayer-lipid membrane vesicles secreted by living cells that are able to transfer regulatory molecules and genetic information from one cell to another. These vesicles are enriched with several nucleic acids including mRNAs, microRNAs (miRNAs), other non-coding RNAs, as well as proteins and lipids. Alterations in the exosomal content and functions are observed in numerous reproductive diseases in both animals and human cases. MicroRNAs, a class of small endogenous RNA molecules, can negatively regulate gene expression at the post-transcription level. Aberrant microRNA expression has been reported in multiple human reproductive diseases such as polycystic ovary syndrome, preeclampsia, uterine leiomyomata, ovarian cancer, endometriosis, and Asherman’s syndrome. This study focuses to review recent research on alterations of microRNA expression and the role of exosomes in female reproductive diseases. It has been demonstrated that exosomes may be a potential therapeutic approach in various female reproductive diseases. In addition, changes in expression of microRNAs act as molecular biomarkers for diagnosis of several reproductive diseases in women, and regulation of their expression can potentially reduce infertility.




Similar content being viewed by others
References
Johnstone RM. Revisiting the road to the discovery of exosomes. Blood Cells Mol Dis. 2005;34(3):214–9.
Sun X, Ma X, Yang X, Zhang X. Exosomes and female infertility. Curr Drug Metab. 2019;20(10):773–80.
Simons M, Raposo G. Exosomes–vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21(4):575–81.
Minciacchi VR, Freeman MR, Di Vizio D, editors. Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes. Semin in Cell & developmental Biol; 2015: Elsevier.
Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, et al. Syndecan–syntenin–ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012;14(7):677–85.
Sluijter JP, Verhage V, Deddens JC, van den Akker F, Doevendans PA. Microvesicles and exosomes for intracardiac communication. Cardiovasc Res. 2014;102(2):302–11.
Kumar MS, Erkeland SJ, Pester RE, Chen CY, Ebert MS, Sharp PA, et al. Suppression of non-small cell lung tumor development by the let-7 microRNA family. Proc Natl Acad Sci. 2008;105(10):3903–8.
Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim et Biophysica Acta (BBA)-General Subjects. 2012;1820(7):940–8.
Qin J, Xu Q. Functions and application of exosomes. Acta Pol Pharm. 2014;71(4):537–43.
Ha D, Yang N, Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm Sin B. 2016;6(4):287–96.
Cortez MA, Bueso-Ramos C, Ferdin J, Lopez-Berestein G, Sood AK, Calin GA. MicroRNAs in body fluids—the mix of hormones and biomarkers. Nat Rev Clin Oncol. 2011;8(8):467–77.
Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009;10(10):704–14.
Bang C, Thum T. Exosomes: new players in cell–cell communication. Int J Biochem Cell Biol. 2012;44(11):2060–4.
Milane L, Singh A, Mattheolabakis G, Suresh M, Amiji MM. Exosome mediated communication within the tumor microenvironment. J Control Release. 2015;219:278–94.
Console L, Scalise M, Indiveri C. Exosomes in inflammation and role as biomarkers. Clin Chim Acta. 2019;488:165–71.
Kahlert C, Kalluri R. Exosomes in tumor microenvironment influence cancer progression and metastasis. J Mol Med. 2013;91(4):431–7.
Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–9.
Di Pietro C. Exosome-mediated communication in the ovarian follicle. J Assist Reprod Genet. 2016;33(3):303–11.
Schmunk G, Gargus JJ. Channelopathy pathogenesis in autism spectrum disorders. Front Genet. 2013;4:222.
McGinnis LK, Luense LJ, Christenson LK. MicroRNA in ovarian biology and disease. Cold Spring Harb Perspect Med. 2015;5(9):a022962.
Li Y, Fang Y, Liu Y, Yang X. MicroRNAs in ovarian function and disorders. J Ovarian Res. 2015;8(1):51.
Duley L, editor. The global impact of pre-eclampsia and eclampsia. Semin in Perinatol; 2009: Elsevier.
Young BC, Levine RJ, Karumanchi SA. Pathogenesis of preeclampsia. Ann Rev Pathol: Mech Dis. 2010;5:173–92.
Payne BA, Hutcheon JA, Ansermino JM, Hall DR, Bhutta ZA, Bhutta SZ, et al. A risk prediction model for the assessment and triage of women with hypertensive disorders of pregnancy in low-resourced settings: the miniPIERS (Pre-eclampsia Integrated Estimate of RiSk) multi-country prospective cohort study. PLoS Med. 2014;11(1):e1001589.
Salomon C, Guanzon D, Scholz-Romero K, Longo S, Correa P, Illanes SE, et al. Placental exosomes as early biomarker of preeclampsia: potential role of exosomal microRNAs across gestation. J Clin Endocrinol Metab. 2017;102(9):3182–94.
Biró OB. The pathogenic role and expression profile of microRNAs in preeclampsia. 2018.
Rana S, Lemoine E, Granger JP, Karumanchi SA. Preeclampsia: pathophysiology, challenges, and perspectives. Circ Res. 2019;124(7):1094–112.
Biró O, Fóthi Á, Alasztics B, Nagy B, Orbán TI, Rigó J Jr. Circulating exosomal and Argonaute-bound microRNAs in preeclampsia. Gene. 2019;692:138–44.
Hu Y, Li P, Hao S, Liu L, Zhao J, Hou Y. Differential expression of microRNAs in the placentae of Chinese patients with severe pre-eclampsia. Clin Chem Lab Med (CCLM). 2009;47(8):923–9.
Jiang L, Long A, Tan L, Hong M, Wu J, Cai L, et al. Elevated microRNA-520g in pre-eclampsia inhibits migration and invasion of trophoblasts. Placenta. 2017;51:70–5.
Zhao Z, Moley KH, Gronowski AM. Diagnostic potential for miRNAs as biomarkers for pregnancy-specific diseases. Clin Biochem. 2013;46(10-11):953–60.
Devor EJ, Santillan DA, Santillan MK. Preeclampsia and microRNAs. Proc Obstet Gynecol. 2013;3(1):1–10.
Taylor DD, Bohler HC, Gercel-Taylor C. Pregnancy-linked suppression of TcR signaling pathways by a circulating factor absent in recurrent spontaneous pregnancy loss (RPL). Mol Immunol. 2006;43(11):1872–80.
Mincheva-Nilsson L, Baranov V. Placenta-derived exosomes and their role in the immune protection of the fetus. Recent Advances in Res on the Hum Placenta Chapter March pg. 2012:243-260.
Salomon C, Torres MJ, Kobayashi M, Scholz-Romero K, Sobrevia L, Dobierzewska A, et al. A gestational profile of placental exosomes in maternal plasma and their effects on endothelial cell migration. PLoS One. 2014;9(6):e98667.
Fu G, Brkić J, Hayder H, Peng C. MicroRNAs in human placental development and pregnancy complications. Int J Mol Sci. 2013;14(3):5519–44.
Rodrigues ML, Nimrichter L, Oliveira DL, Nosanchuk JD, Casadevall A. Vesicular trans-cell wall transport in fungi: a mechanism for the delivery of virulence-associated macromolecules? Lipid insights. 2008;2:LPI. S1000.
Pineles BL, Romero R, Montenegro D, Tarca AL, Han YM, Kim YM et al. Distinct subsets of microRNAs are expressed differentially in the human placentas of patients with preeclampsia. Am J Obstet Gynecol. 2007;196(3):261. e1-. e6.
Enquobahrie DA, Abetew DF, Sorensen TK, Willoughby D, Chidambaram K, Williams MA. Placental microRNA expression in pregnancies complicated by preeclampsia. Am J Obstet Gynecol 2011;204(2):178. e12-. e21.
Thway TM, Shlykov SG, Day M-C, Sanborn BM, Gilstrap LC III, Xia Y, et al. Antibodies from preeclamptic patients stimulate increased intracellular Ca2+ mobilization through angiotensin receptor activation. Circ. 2004;110(12):1612–9.
Suzuki Y, Lopez V, Lönnerdal B. Lactoferrin. Cell Mol Life Sci. 2005;62(22):2560–75.
Soleymanlou N, Jurisica I, Nevo O, Ietta F, Zhang X, Zamudio S, et al. Molecular evidence of placental hypoxia in preeclampsia. J Clin Endocrinol Metab. 2005;90(7):4299–308.
Vashukova ES, Glotov AS, Fedotov PV, Efimova OA, Pakin VS, Mozgovaya EV, et al. Placental microRNA expression in pregnancies complicated by superimposed pre-eclampsia on chronic hypertension. Mol Med Rep. 2016;14(1):22–32.
Niu Z-r, Han T, Sun X-l, Luan L-x, Gou W-l, Zhu X-m. MicroRNA-30a-3p is overexpressed in the placentas of patients with preeclampsia and affects trophoblast invasion and apoptosis by its effects on IGF-1. Am J Obstet Gynecol. 2018;218(2):249. e1-. e12.
Timofeeva AV, Gusar VA, Kan NE, Prozorovskaya KN, Karapetyan AO, Bayev OR, et al. Identification of potential early biomarkers of preeclampsia. Placenta. 2018;61:61–71.
Li H, Ge Q, Guo L, Lu Z. Maternal plasma miRNAs expression in preeclamptic pregnancies. Biomed Res Int. 2013;2013:1–9.
Wu L, Zhou H, Lin H, Qi J, Zhu C, Gao Z et al. Circulating microRNAs are elevated in plasma from severe preeclamptic pregnancies. 2012
Jairajpuri DS, Malalla ZH, Mahmood N, Almawi WY. Circulating microRNA expression as predictor of preeclampsia and its severity. Gene. 2017;627:543–8.
Ura B, Feriotto G, Monasta L, Bilel S, Zweyer M, Celeghini C. Potential role of circulating microRNAs as early markers of preeclampsia. Taiwan J Obstet Gynecol. 2014;53(2):232–4.
Li Q, Long A, Jiang L, Cai L, Xie L. Gu Ja et al. Quantification of preeclampsia-related microRNAs in maternal serum. Biomed Rep. 2015;3(6):792–6.
Stewart EA, Laughlin-Tommaso SK, Catherino WH, Lalitkumar S, Gupta D, Vollenhoven B. Uterine fibroids. Nat Rev Dis Primers. 2016;2(1):1–18.
Al-Hendy A, Myers ER, Stewart E, editors. Uterine fibroids: burden and unmet medical need. Seminars in reproductive medicine; 2017: Thieme Med Publ.
Stewart EA, Cookson C, Gandolfo RA, Schulze-Rath R. Epidemiology of uterine fibroids: a systematic review. BJOG: An Int J Obstet Gynaecol. 2017;124(10):1501–12.
Wise LA, Laughlin-Tommaso SK. Epidemiology of uterine fibroids–from menarche to menopause. Clin Obstet Gynecol. 2016;59(1):2–24.
Oliveto S, Mancino M, Manfrini N, Biffo S. Role of microRNAs in translation regulation and cancer. World J Biol Chem. 2017;8(1):45–56.
Karmon AE, Cardozo ER, Rueda BR, Styer AK. MicroRNAs in the development and pathobiology of uterine leiomyomata: does evidence support future strategies for clinical intervention? Hum Reprod Update. 2014;20(5):670–87.
Nothnick WB. Non-coding RNAs in uterine development, function and disease. Non-coding RNA and the Reproductive System. Springer; 2016. p. 171-189.
Wang T, Zhang X, Obijuru L, Laser J, Aris V, Lee P, et al. A micro-RNA signature associated with race, tumor size, and target gene activity in human uterine leiomyomas. Genes Chromosom Cancer. 2007;46(4):336–47.
Zavadil J, Ye H, Liu Z, Wu J, Lee P, Hernando E, et al. Profiling and functional analyses of microRNAs and their target gene products in human uterine leiomyomas. PLoS One. 2010;5(8):e12362.
Iorio MV, Ferracin M, Liu C-G, Veronese A, Spizzo R, Sabbioni S, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65(16):7065–70.
Davis B. Uterine leiomyoma longitudinal interventions studies: the fibroid growth study. NIH.: Bethesda MD; 2005.
Wei J-J, Soteropoulos P, editors. MicroRNA: a new tool for biomedical risk assessment and target identification in human uterine leiomyomas. Semin in Reprod Med; 2008: © Thieme Medical Publishers.
Shell S, Park S-M, Radjabi AR, Schickel R, Kistner EO, Jewell DA, et al. Let-7 expression defines two differentiation stages of cancer. Proc Nati Acad Sci. 2007;104(27):11400–5.
Hennig Y, Deichert U, Bonk U, Thode B, Bartnitzke S, Bullerdiek J. Chromosomal translocations affecting 12q14–15 but not deletions of the long arm of chromosome 7 associated with a growth advantage of uterine smooth muscle cells. MHR: Basic Sci Reprod Med. 1999;5(12):1150–4.
Chen R, Sheng L, Zhang HJ, Ji M, Qian WQ. miR-15b-5p facilitates the tumorigenicity by targeting RECK and predicts tumour recurrence in prostate cancer. J Cell Mol Med. 2018;22(3):1855–63.
Sun X, Jiao X, Pestell T, Fan C, Qin S, Mirabelli E, et al. MicroRNAs and cancer stem cells: the sword and the shield. Oncog. 2014;33(42):4967–77.
Islam MS, Ciavattini A, Petraglia F, Castellucci M, Ciarmela P. Extracellular matrix in uterine leiomyoma pathogenesis: a potential target for future therapeutics. Hum Reprod Update. 2018;24(1):59–85.
Marsh EE, Steinberg ML, Parker JB, Wu J, Chakravarti D, Bulun SE. Decreased expression of microRNA-29 family in leiomyoma contributes to increased major fibrillar collagen production. Fertil Steril. 2016;106(3):766–72.
Chuang T-D, Khorram O. Mechanisms underlying aberrant expression of miR-29c in uterine leiomyoma. Fertil Steril. 2016;105(1):236-45. e1.
Chuang T-D, Luo X, Panda H, Chegini N. miR-93/106b and their host gene, MCM7, are differentially expressed in leiomyomas and functionally target F3 and IL-8. Mol Endocrinol. 2012;26(6):1028–42.
Yang E, Xue L, Li Z, Yi T. Lnc-AL445665. 1–4 may be involved in the development of multiple uterine leiomyoma through interacting with miR-146b-5p. BMC Cancer. 2019;19(1):709.
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA: A Cancer J Clin. 2009;59(4):225–49.
Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014;384(9951):1376–88.
Bray F, Ferlay J, Soerjomataram I, Siegel R, Torre L, Jemal A. Erratum: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries.
Clarke BA, Gilks B. Ovarian carcinoma: recent developments in classification of tumour histological subtype. Can J Pathol. 2011;3:33–42.
Ling H, Fabbri M, Calin GA. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat Rev Drug Discov. 2013;12(11):847–65.
Alharbi M, Sharma S, Guanzon D, Lai A, Zuñiga F, Shiddiky MJ et al. miRNA signature in small extracellular vesicles and their association with platinum resistance and cancer recurrence in ovarian cancer. Nanomedicine. 2020:102207.
Shaffer J, Schlumpberger M, Lader E. miRNA profiling from blood—challenges and recommendations. Qiagen Sci Artic. 2012:1-10.
Sohel MH. Extracellular/circulating microRNAs: release mechanisms, functions and challenges. Achiev Life Sci. 2016;10(2):175–86.
Baran J, Baj-Krzyworzeka M, Weglarczyk K, Szatanek R, Zembala M, Barbasz J, et al. Circulating tumour-derived microvesicles in plasma of gastric cancer patients. Cancer Immunol Immunother. 2010;59(6):841–50.
Matsumoto Y, Kano M, Akutsu Y, Hanari N, Hoshino I, Murakami K, et al. Quantification of plasma exosome is a potential prognostic marker for esophageal squamous cell carcinoma. Oncol Rep. 2016;36(5):2535–43.
Peter ME. Let-7 and miR-200 microRNAs: guardians against pluripotency and cancer progression. Cell Cycle. 2009;8(6):843–52.
Park S-M, Shell S, Radjabi AR, Schickel R, Feig C, Boyerinas B, et al. Let-7 prevents early cancer progression by suppressing expression of the embryonic gene HMGA2. Cell Cycle. 2007;6(21):2585–90.
Nam EJ, Yoon H, Kim SW, Kim H, Kim YT, Kim JH, et al. MicroRNA expression profiles in serous ovarian carcinoma. Clin Cancer Res. 2008;14(9):2690–5.
Yang N, Kaur S, Volinia S, Greshock J, Lassus H, Hasegawa K, et al. MicroRNA microarray identifies Let-7i as a novel biomarker and therapeutic target in human epithelial ovarian cancer. Cancer Res. 2008;68(24):10307–14.
Hu X, Macdonald DM, Huettner PC, Feng Z, El Naqa IM, Schwarz JK, et al. A miR-200 microRNA cluster as prognostic marker in advanced ovarian cancer. Gynecol Oncol. 2009;114(3):457–64.
Raver-Shapira N, Marciano E, Meiri E, Spector Y, Rosenfeld N, Moskovits N, et al. Transcriptional activation of miR-34a contributes to p53-mediated apoptosis. Mol Cell. 2007;26(5):731–43.
Chang T-C, Wentzel EA, Kent OA, Ramachandran K, Mullendore M, Lee KH, et al. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell. 2007;26(5):745–52.
Bommer GT, Gerin I, Feng Y, Kaczorowski AJ, Kuick R, Love RE, et al. p53-mediated activation of miRNA34 candidate tumor-suppressor genes. Curr Biol. 2007;17(15):1298–307.
Ghafouri-Fard S, Shoorei H, Taheri M. miRNA profile in ovarian cancer. Exp Mol Pathol. 2020;113:104381.
Roth LW, McCallie B, Alvero R, Schoolcraft WB, Minjarez D, Katz-Jaffe MG. Altered microRNA and gene expression in the follicular fluid of women with polycystic ovary syndrome. J Assist Reprod Genet. 2014;31(3):355–62.
Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7(4):219–31.
Lindholm Å, Andersson L, Eliasson M, Bixo M, Sundström-Poromaa I. Prevalence of symptoms associated with polycystic ovary syndrome. Int J Gynecol Obstet. 2008;102(1):39–43.
Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2010;16(4):347–63.
Kalluri R. The biology and function of exosomes in cancer. J Clin Invest. 2016;126(4):1208–15.
Li H, Huang X, Chang X, Yao J, He Q, Shen Z, et al. S100-A9 protein in exosomes derived from follicular fluid promotes inflammation via activation of NF-κB pathway in polycystic ovary syndrome. J Cell Mol Med. 2020;24(1):114–25.
Liu S, Zhang X, Shi C, Lin J, Chen G, Wu B, et al. Altered microRNAs expression profiling in cumulus cells from patients with polycystic ovary syndrome. J Transl Med. 2015;13(1):238.
Sang Q, Yao Z, Wang H, Feng R, Wang H, Zhao X, et al. Identification of microRNAs in human follicular fluid: characterization of microRNAs that govern steroidogenesis in vitro and are associated with polycystic ovary syndrome in vivo. J Clin Endocrinol Metab. 2013;98(7):3068–79.
Long W, Zhao C, Ji C, Ding H, Cui Y, Guo X, et al. Characterization of serum microRNAs profile of PCOS and identification of novel non-invasive biomarkers. Cell Physiol Biochem. 2014;33(5):1304–15.
Hu J, Tang T, Zeng Z, Wu J, Tan X, Yan J. The expression of small RNAs in exosomes of follicular fluid altered in human polycystic ovarian syndrome. PeerJ. 2020;8:e8640.
Schauer S, Sontakke S, Watson E, Esteves C, Donadeu FX. Involvement of miRNAs in equine follicle development. Reprod. 2013;146(3):273–82.
Sirotkin AV, Ovcharenko D, Grossmann R, Lauková M, Mlynček M. Identification of MicroRNAs controlling human ovarian cell steroidogenesis via a genome-scale screen. J Cell Physiol. 2009;219(2):415–20.
Naji M, Nekoonam S, Aleyasin A, Arefian E, Mahdian R, Azizi E, et al. Expression of miR-15a, miR-145, and miR-182 in granulosa-lutein cells, follicular fluid, and serum of women with polycystic ovary syndrome (PCOS). Arch Gynecol Obstet. 2018;297(1):221–31.
Eisenberg I, Nahmias N, Persky MN, Greenfield C, Goldman-Wohl D, Hurwitz A, et al. Elevated circulating micro-ribonucleic acid (miRNA)-200b and miRNA-429 levels in anovulatory women. Fertil Steril. 2017;107(1):269–75.
Sørensen AE, Udesen PB, Maciag G, Geiger J, Saliani N, Januszewski A, et al. Hyperandrogenism and metabolic syndrome is associated with changes in serum-derived microRNAs in women with polycystic ovary syndrome. Front Med. 2019;6:242.
Li D, Xu D, Xu Y, Chen L, Li C, Dai X, et al. MicroRNA-141-3p targets DAPK1 and inhibits apoptosis in rat ovarian granulosa cells. Cell Biochem Funct. 2017;35(4):197–201.
Belgardt B-F, Ahmed K, Spranger M, Latreille M, Denzler R, Kondratiuk N, et al. The microRNA-200 family regulates pancreatic beta cell survival in type 2 diabetes. Nat Med. 2015;21(6):619–27.
Choi SK, Kim HS, Jin T, Hwang EH, Jung M, Moon WK. Overexpression of the miR-141/200c cluster promotes the migratory and invasive ability of triple-negative breast cancer cells through the activation of the FAK and PI3K/AKT signaling pathways by secreting VEGF-A. BMC Cancer. 2016;16(1):1–14.
Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7:219–31.
He T, Liu Y, Jia Y, Wang H, Yang X, Lu G, et al. MicroRNA-141 and MicroRNA-200c are overexpressed in granulosa cells of polycystic ovary syndrome patients. Front Med. 2018;5:299.
He T, Sun Y, Zhang Y, Zhao S, Zheng Y, Hao G, et al. MicroRNA-200b and microRNA-200c are up-regulated in PCOS granulosa cell and inhibit KGN cell proliferation via targeting PTEN. Reprod Biol Endocrinol. 2019;17(1):1–8.
Cui X, Jing X, Liu J, Yan M, Bi X, Wu X. miR-132 is up-regulated in polycystic ovarian syndrome and inhibits granulosa cells proliferation via targeting Foxa1. 2020.
Giudice LC. Endometriosis. New England J Med. 2010;362(25):2389–98.
Fassbender A, Burney RO, F O D, D’Hooghe T, Giudice L. Update on biomarkers for the detection of endometriosis. BioMed Res Int. 2015;2015.
Harp D, Driss A, Mehrabi S, Chowdhury I, Xu W, Liu D, et al. Exosomes derived from endometriotic stromal cells have enhanced angiogenic effects in vitro. Cell Tissue Res. 2016;365(1):187–96.
Zhao L, Gu C, Ye M, Zhang Z, Fan W, Meng Y. Integration analysis of microRNA and mRNA paired expression profiling identifies deregulated microRNA-transcription factor-gene regulatory networks in ovarian endometriosis. Reprod Biol Endocrinol. 2018;16(1):4.
Shi X-Y, Gu L, Chen J, Guo X-R, Shi Y-L. Downregulation of miR-183 inhibits apoptosis and enhances the invasive potential of endometrial stromal cells in endometriosis. Int J Mol Med. 2013;33(1):59–67.
Wu D, Lu P, Mi X, Miao J. Exosomal miR-214 from endometrial stromal cells inhibits endometriosis fibrosis. MHR: Basic Sci Reprod Med. 2018;24(7):357–65.
Sun H, Li D, Yuan M, Li Q, Li N, Wang G. Eutopic stromal cells of endometriosis promote neuroangiogenesis via exosome pathway. Biol Reprod. 2019;100(3):649–59.
Nothnick WB, editor. MicroRNAs and endometriosis: distinguishing drivers from passengers in disease pathogenesis. Seminars in reproductive medicine; 2017: Thieme Med Publ.
Zhang L, Li H, Yuan M, Li D, Sun C, Wang G. Serum exosomal MicroRNAs as potential circulating biomarkers for endometriosis. Dis Markers. 2020;2020:1–10.
Zhang A, Wang G, Jia L, Su T, Zhang L. Exosome-mediated microRNA-138 and vascular endothelial growth factor in endometriosis through inflammation and apoptosis via the nuclear factor-κB signaling pathway. Int J Mol Med. 2019;43(1):358–70.
Liang L, Wang L, Zhou S, Li J, Meng L, Zhang H, et al. Exosomes derived from human umbilical cord mesenchymal stem cells repair injured endometrial epithelial cells. J Assist Reprod Genet. 2020;37(2):395–403.
Moustafa S, Burn M, Mamillapalli R, Nematian S, Flores V, Taylor HS. Accurate diagnosis of endometriosis using serum microRNAs. Am J Obstet Gynecol. 2020;223:557.e1–557.e11.
Li X, Zhang W, Fu J, Xu Y, Gu R, Qu R, et al. MicroRNA-451 is downregulated in the follicular fluid of women with endometriosis and influences mouse and human embryonic potential. Reprod Biol Endocrinol. 2019;17(1):96.
Ma L, Li Z, Li W, Ai J, Chen X. MicroRNA-142-3p suppresses endometriosis by regulating KLF9-mediated autophagy in vitro and in vivo. RNA Biol. 2019;16(12):1733–48.
Schiffgens S, Wilkens L, Brandes AA, Meier T, Franceschi E, Ermani M, et al. Sex-specific clinicopathological significance of novel (Frizzled-7) and established (MGMT, IDH1) biomarkers in glioblastoma. Oncotarget. 2016;7(34):55169–80.
Qiu X, Jiao J, Li Y, Tian T. Overexpression of FZD7 promotes glioma cell proliferation by upregulating TAZ. Oncotarget. 2016;7(52):85987–99.
Zhu H, Cao XX, Liu J, Hua H. MicroRNA-488 inhibits endometrial glandular epithelial cell proliferation, migration, and invasion in endometriosis mice via Wnt by inhibiting FZD7. J Cell Mol Med. 2019;23(4):2419–30.
Yu H, Zhong Q, Xia Y, Li E, Wang S, Ren R. MicroRNA-2861 targets STAT3 and MMP2 to regulate the proliferation and apoptosis of ectopic endometrial cells in endometriosis. Die Pharmazie-An Int J Pharm Sci. 2019;74(4):243–9.
Meng X, Liu J, Wang H, Chen P, Wang D. MicroRNA-126-5p downregulates BCAR3 expression to promote cell migration and invasion in endometriosis. Mol Cell Endocrinol. 2019;494:110486.
Papari E, Noruzinia M, Kashani L, Foster WG. Identification of candidate microRNA markers of endometriosis with the use of next-generation sequencing and quantitative real-time polymerase chain reaction. Fertil Steril. 2020;113(6):1232–41.
Zhou W, Lian Y, Jiang J, Wang L, Ren L, Li Y, et al. Differential expression of microRNA in exosomes derived from endometrial stromal cells of women with endometriosis-associated infertility. Reprod BioMed Online. S1472-6483(20):30192–9.
Johary J, Xue M, Zhu X, Xu D, Velu PP. Efficacy of estrogen therapy in patients with intrauterine adhesions: systematic review. J Minim Invasive Gynecol. 2014;21(1):44–54.
Wang X, Ma N, Sun Q, Huang C, Liu Y, Luo X. Elevated NF-κB signaling in Asherman syndrome patients and animal models. Oncotarget. 2017;8(9):15399–406.
Saribas GS, Ozogul C, Tiryaki M, Pinarli FA, Kilic SH. Effects of uterus derived mesenchymal stem cells and their exosomes on Asherman’s syndrome. Acta Histochem. 2020;122(1):151465.
Zhao S, Qi W, Zheng J, Tian Y, Qi X, Kong D, et al. Exosomes derived from adipose mesenchymal stem cells restore functional endometrium in a rat model of intrauterine adhesions. Reprod Sci. 2020:1–10.
Ning J, Zhang H, Yang H. MicroRNA-326 inhibits endometrial fibrosis by regulating TGF-β1/Smad3 pathway in intrauterine adhesions. Mol Med Rep. 2018;18(2):2286–92.
Xu Q, Duan H, Gan L, Liu X, Chen F, Shen X, et al. MicroRNA-1291 promotes endometrial fibrosis by regulating the ArhGAP29-RhoA/ROCK1 signaling pathway in a murine model. Mol Med Rep. 2017;16(4):4501–10.
Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1(6):a001651. https://doi.org/10.1101/cshperspect.a001651.
Liu M, Zhao D, Wu X, Guo S, Yan L, Zhao S, et al. miR-466 and NUS1 regulate the AKT/nuclear factor kappa B (NFκB) signaling pathway in intrauterine adhesions in a rat model. Med Sci Monit: Int Med J Exp Clin Res. 2019;25:4094.
Acknowledgements
This article is in line with Mrs. Maryam Javadi’s dissertation about the effect of plasma-derived exosomes on female fertility to acquire a Ph.D. in anatomy from the Tabriz University of Medical University. This review was endorsed by the Stem Cell Research Center, Tabriz University of Medical Sciences, Tabriz, Iran (Grant Number: 63134).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Javadi, M., Rad, J.S., Farashah, M.S.G. et al. An Insight on the Role of Altered Function and Expression of Exosomes and MicroRNAs in Female Reproductive Diseases. Reprod. Sci. 29, 1395–1407 (2022). https://doi.org/10.1007/s43032-021-00556-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-021-00556-9