Abstract
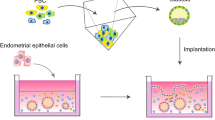
In the last decade, organoids have become emerging novel models for biomedical research. Organoids are small, self-organized three-dimensional (3D) tissue cultures derived from stem cells that mimic certain tissues or organs. In reproductive medicine, researchers have generated numerous organoids including blastoid (blastocyst organoid), endometrial organoid, and trophoblast organoid. These organdies provide useful models for studying the embryo implantation mechanism through observation of cell differentiation, gene expression, and epigenetic profiles at the implantation stage. As in vitro tissue models, organoids could be coupled with many other frontier technologies such as gene editing and genomic sequencing. However, the main drawback of organoids is that they do not fully mimic their counterparts in vivo tissues. Furthermore, there is a consensus of research ethics on organoids that may limit the types of studies that scientists perform with. Nevertheless, all discoveries and efforts surrounding organoids still greatly benefit therapy development for reproductive clinics.

Similar content being viewed by others
References
Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med. 2012;9(12):e1001356. https://doi.org/10.1371/journal.pmed.1001356.
Cha J, Sun X, Dey SK. Mechanisms of implantation: strategies for successful pregnancy. Nat Med. 2012;18(12):1754–67. https://doi.org/10.1038/nm.3012.
Haider S, Meinhardt G, Saleh L, Kunihs V, Gamperl M, Kaindl U, et al. Self-renewing trophoblast organoids recapitulate the developmental program of the early human placenta. Stem Cell Reports. 2018;11(2):537–51. https://doi.org/10.1016/j.stemcr.2018.07.004.
Turco MY, Gardner L, Kay RG, Hamilton RS, Prater M, Hollinshead MS, et al. Trophoblast organoids as a model for maternal-fetal interactions during human placentation. Nature. 2018;564(7735):263–7. https://doi.org/10.1038/s41586-018-0753-3.
Rivron NC, Frias-Aldeguer J, Vrij EJ, Boisset JC, Korving J, Vivie J, et al. Blastocyst-like structures generated solely from stem cells. Nature. 2018;557(7703):106–11. https://doi.org/10.1038/s41586-018-0051-0.
Sozen B, Cox AL, De Jonghe J, Bao M, Hollfelder F, Glover DM, et al. Self-organization of mouse stem cells into an extended potential blastoid. Dev Cell. 2019;51(6):698–712 e8. https://doi.org/10.1016/j.devcel.2019.11.014.
Boretto M, Maenhoudt N, Luo X, Hennes A, Boeckx B, Bui B, et al. Patient-derived organoids from endometrial disease capture clinical heterogeneity and are amenable to drug screening. Nat Cell Biol. 2019;21(8):1041–51. https://doi.org/10.1038/s41556-019-0360-z.
Fitzgerald HC, Dhakal P, Behura SK, Schust DJ, Spencer TE. Self-renewing endometrial epithelial organoids of the human uterus. Proc Natl Acad Sci U S A. 2019;116(46):23132–42. https://doi.org/10.1073/pnas.1915389116.
Ashok A, Choudhury D, Fang Y, Hunziker W. Towards manufacturing of human organoids. Biotechnol Adv. 2020;39:107460. https://doi.org/10.1016/j.biotechadv.2019.107460.
Wilson HV. A new method by which sponges may be artificially reared. Science. 1907;25(649):912–5. https://doi.org/10.1126/science.25.649.912.
Kessler M, Hoffmann K, Brinkmann V, Thieck O, Jackisch S, Toelle B, et al. The Notch and Wnt pathways regulate stemness and differentiation in human fallopian tube organoids. Nat Commun. 2015;6:8989. https://doi.org/10.1038/ncomms9989.
Kretzschmar K, Clevers H. Organoids: modeling development and the stem cell niche in a dish. Dev Cell. 2016;38(6):590–600. https://doi.org/10.1016/j.devcel.2016.08.014.
Boretto M, Cox B, Noben M, Hendriks N, Fassbender A, Roose H, et al. Development of organoids from mouse and human endometrium showing endometrial epithelium physiology and long-term expandability. Development. 2017;144(10):1775–86. https://doi.org/10.1242/dev.148478.
Turco MY, Gardner L, Hughes J, Cindrova-Davies T, Gomez MJ, Farrell L, et al. Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat Cell Biol. 2017;19(5):568–77. https://doi.org/10.1038/ncb3516.
Akbari S, Sevinc GG, Ersoy N, Basak O, Kaplan K, Sevinc K, et al. Robust, long-term culture of endoderm-derived hepatic organoids for disease modeling. Stem Cell Reports. 2019;13(4):627–41. https://doi.org/10.1016/j.stemcr.2019.08.007.
Lee J, Bscke R, Tang PC, Hartman BH, Heller S, Koehler KR. Hair follicle development in mouse pluripotent stem cell-derived skin organoids. Cell Rep. 2018;22(1):242–54. https://doi.org/10.1016/j.celrep.2017.12.007.
Fukui Y, Hirota Y, Matsuo M, Gebril M, Akaeda S, Hiraoka T, et al. Uterine receptivity, embryo attachment, and embryo invasion: multistep processes in embryo implantation. Reprod Med Biol. 2019;18(3):234–40. https://doi.org/10.1002/rmb2.12280.
Melford SE, Taylor AH, Konje JC. Of mice and (wo)men: factors influencing successful implantation including endocannabinoids. Hum Reprod Update. 2014;20(3):415–28. https://doi.org/10.1093/humupd/dmt060.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. https://doi.org/10.1016/j.cell.2006.07.024.
Kojima J, Fukuda A, Taira H, Kawasaki T, Ito H, Kuji N, et al. Efficient production of trophoblast lineage cells from human induced pluripotent stem cells. Lab Investig. 2017;97(10):1188–200. https://doi.org/10.1038/labinvest.2016.159.
Renaud SJ. Strategies for investigating hemochorial placentation. Reprod Dev Toxicol. 2017:1259–73. https://doi.org/10.1016/B978-0-12-804239-7.00066-4.
Oda M, Tanaka S, Yamazaki Y, Ohta H, Iwatani M, Suzuki M, et al. Establishment of trophoblast stem cell lines from somatic cell nuclear-transferred embryos. Proc Natl Acad Sci U S A. 2009;106(38):16293–7. https://doi.org/10.1073/pnas.0908009106.
Lee CQ, Gardner L, Turco M, Zhao N, Murray MJ, Coleman N, et al. What is trophoblast? A combination of criteria define human first-trimester trophoblast. Stem Cell Reports. 2016;6(2):257–72. https://doi.org/10.1016/j.stemcr.2016.01.006.
Cox B, Kotlyar M, Evangelou AI, Ignatchenko V, Ignatchenko A, Whiteley K, et al. Comparative systems biology of human and mouse as a tool to guide the modeling of human placental pathology. Mol Syst Biol. 2009;5:279. https://doi.org/10.1038/msb.2009.37.
Wildman DE. Review: Toward an integrated evolutionary understanding of the mammalian placenta. Placenta. 2011;32(Suppl 2):S142–5. https://doi.org/10.1016/j.placenta.2011.01.005.
Hou Z, Romero R, Uddin M, Than NG, Wildman DE. Adaptive history of single copy genes highly expressed in the term human placenta. Genomics. 2009;93(1):33–41. https://doi.org/10.1016/j.ygeno.2008.09.005.
Maltepe E, Fisher SJ. Placenta: the forgotten organ. Annu Rev Cell Dev Biol. 2015;31:523–52. https://doi.org/10.1146/annurev-cellbio-100814-125620.
Aplin JD, Ruane PT. Embryo-epithelium interactions during implantation at a glance. J Cell Sci. 2017;130(1):15–22. https://doi.org/10.1242/jcs.175943.
James JL, Carter AM, Chamley LW. Human placentation from nidation to 5 weeks of gestation. Part I: What do we know about formative placental development following implantation? Placenta. 2012;33(5):327–34. https://doi.org/10.1016/j.placenta.2012.01.020.
Enders AC, Schlafke S. Cytological aspects of trophoblast-uterine interaction in early implantation. Am J Anat. 1969;125(1):1–29. https://doi.org/10.1002/aja.1001250102.
Pollard RM, Finn CA. Influence of the trophoblast upon differentiation of the uterine epithelium during implantation in the mouse. J Endocrinol. 1974;62(3):669–74. https://doi.org/10.1677/joe.0.0620669.
Li R, Zhong C, Yu Y, Liu H, Sakurai M, Yu L, et al. Generation of blastocyst-like structures from mouse embryonic and adult cell cultures. Cell. 2019;179(3):687–702 e18. https://doi.org/10.1016/j.cell.2019.09.029.
Xiang L, Yin Y, Zheng Y, Ma Y, Li Y, Zhao Z, et al. A developmental landscape of 3D-cultured human pre-gastrulation embryos. Nature. 2019;577:537–42. https://doi.org/10.1038/s41586-019-1875-y.
Christodoulou N, Weberling A, Strathdee D, Anderson KI, Timpson P, Zernicka-Goetz M. Morphogenesis of extra-embryonic tissues directs the remodelling of the mouse embryo at implantation. Nat Commun. 2019;10(1):3557. https://doi.org/10.1038/s41467-019-11482-5.
Takata N, Sakakura E, Kasukawa T, Sakuma T, Yamamoto T, Sasai Y. Establishment of functional genomics pipeline in mouse epiblast-like tissue by combining transcriptomic analysis and gene knockdown/knockin/knockout, using RNA interference and CRISPR/Cas9. Hum Gene Ther. 2016;27(6):436–50. https://doi.org/10.1089/hum.2015.148.
Haider S, Meinhardt G, Saleh L, Fiala C, Pollheimer J, Knofler M. Notch1 controls development of the extravillous trophoblast lineage in the human placenta. Proc Natl Acad Sci U S A. 2016;113(48):E7710–E9. https://doi.org/10.1073/pnas.1612335113.
Okae H, Toh H, Sato T, Hiura H, Takahashi S, Shirane K, et al. Derivation of human trophoblast stem cells. Cell Stem Cell. 2018;22(1):50–63 e6. https://doi.org/10.1016/j.stem.2017.11.004.
McConkey CA, Delorme-Axford E, Nickerson CA, Kim KS, Sadovsky Y, Boyle JP, et al. A three-dimensional culture system recapitulates placental syncytiotrophoblast development and microbial resistance. Sci Adv. 2016;2(3):e1501462. https://doi.org/10.1126/sciadv.1501462.
Novakovic B, Saffery R. The ever growing complexity of placental epigenetics - role in adverse pregnancy outcomes and fetal programming. Placenta. 2012;33(12):959–70. https://doi.org/10.1016/j.placenta.2012.10.003.
Cotechini T, Komisarenko M, Sperou A, Macdonald-Goodfellow S, Adams MA, Graham CH. Inflammation in rat pregnancy inhibits spiral artery remodeling leading to fetal growth restriction and features of preeclampsia. J Exp Med. 2014;211(1):165–79. https://doi.org/10.1084/jem.20130295.
Renaud SJ, Cotechini T, Quirt JS, Macdonald-Goodfellow SK, Othman M, Graham CH. Spontaneous pregnancy loss mediated by abnormal maternal inflammation in rats is linked to deficient uteroplacental perfusion. J Immunol. 2011;186(3):1799–808. https://doi.org/10.4049/jimmunol.1002679.
Hibaoui Y, Feki A. Organoid models of human endometrial development and disease. Front Cell Dev Biol. 2020;8:84. https://doi.org/10.3389/fcell.2020.00084.
Boers SN, Bredenoord AL. Consent for governance in the ethical use of organoids. Nat Cell Biol. 2018;20(6):642–5. https://doi.org/10.1038/s41556-018-0112-5.
Hyun I, Munsie M, Pera MF, Rivron NC, Rossant J. Toward guidelines for research on human embryo models formed from stem cells. Stem Cell Reports. 2020;14(2):169–74. https://doi.org/10.1016/j.stemcr.2019.12.008.
Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8(6):e1000412. https://doi.org/10.1371/journal.pbio.1000412.
Moore HM, Kelly AB, Jewell SD, McShane LM, Clark DP, Greenspan R, et al. Biospecimen reporting for improved study quality (BRISQ). Cancer Cytopathol. 2011;119(2):92–101. https://doi.org/10.1002/cncy.20147.
El-Shamayleh Y, Horwitz GD. Primate optogenetics: progress and prognosis. Proc Natl Acad Sci U S A. 2019;116:26195–203. https://doi.org/10.1073/pnas.1902284116.
Schwank G, Koo BK, Sasselli V, Dekkers JF, Heo I, Demircan T, et al. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell. 2013;13(6):653–8. https://doi.org/10.1016/j.stem.2013.11.002.
Karzbrun E, Kshirsagar A, Cohen SR, Hanna JH, Reiner O. Human brain organoids on a chip reveal the physics of folding. Nat Phys. 2018;14(5):515–22. https://doi.org/10.1038/s41567-018-0046-7.
Fatehullah A, Tan SH, Barker N. Organoids as an in vitro model of human development and disease. Nat Cell Biol. 2016;18(3):246–54. https://doi.org/10.1038/ncb3312.
Takebe T, Enomura M, Yoshizawa E, Kimura M, Koike H, Ueno Y, et al. Vascularized and complex organ buds from diverse tissues via mesenchymal cell-driven condensation. Cell Stem Cell. 2015;16(5):556–65. https://doi.org/10.1016/j.stem.2015.03.004.
Worsdorfer P, Dalda N, Kern A, Kruger S, Wagner N, Kwok CK, et al. Generation of complex human organoid models including vascular networks by incorporation of mesodermal progenitor cells. Sci Rep. 2019;9(1):15663. https://doi.org/10.1038/s41598-019-52204-7.
Homan KA, Gupta N, Kroll KT, Kolesky DB, Skylar-Scott M, Miyoshi T, et al. Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat Methods. 2019;16(3):255–62. https://doi.org/10.1038/s41592-019-0325-y.
Warmflash A, Sorre B, Etoc F, Siggia ED, Brivanlou AH. A method to recapitulate early embryonic spatial patterning in human embryonic stem cells. Nat Methods. 2014;11(8):847–54. https://doi.org/10.1038/nmeth.3016.
Lancaster MA, Corsini NS, Wolfinger S, Gustafson EH, Phillips AW, Burkard TR, et al. Guided self-organization and cortical plate formation in human brain organoids. Nat Biotechnol. 2017;35(7):659–66. https://doi.org/10.1038/nbt.3906.
Knight GT, Lundin BF, Iyer N, Ashton LM, Sethares WA, Willett RM, et al. Engineering induction of singular neural rosette emergence within hPSC-derived tissues. Elife. 2018;7. https://doi.org/10.7554/eLife.37549.
Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, et al. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell. 2016;165(5):1238–54. https://doi.org/10.1016/j.cell.2016.04.032.
Li M, Izpisua Belmonte JC. Organoids - preclinical models of human disease. N Engl J Med. 2019;380(6):569–79. https://doi.org/10.1056/NEJMra1806175.
Niu Y, Sun N, Li C, Lei Y, Huang Z, Wu J, et al. Dissecting primate early post-implantation development using long-term in vitro embryo culture. Science. 2019;366(6467). https://doi.org/10.1126/science.aaw5754.
Acknowledgements
Many thanks to Alyssa Meng of UCSB and Ryan Lee of UCLA, for their proof reading of the draft.
Funding
This work was supported by “23456” Talent Project of Henan Provincial People’s Hospital.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wei, Y., Zhang, C., Fan, G. et al. Organoids as Novel Models for Embryo Implantation Study. Reprod. Sci. 28, 1637–1643 (2021). https://doi.org/10.1007/s43032-021-00501-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-021-00501-w