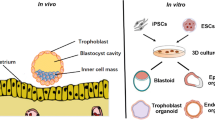
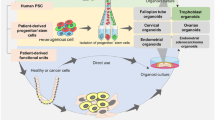
Abstract
Organoid technique has achieved significant progress in recent years, owing to the rapid development of the three-dimensional (3D) culture techniques in adult stem cells (ASCs) and pluripotent stem cells (PSCs) that are capable of self-renewal and induced differentiation. However, our understanding of human female reproductive system organoids is in its infancy. Recently, scientists have established self-organizing 3D organoids for human endometrium, fallopian tubes, oocyte, and trophoblasts by culturing stem cells with a cocktail of cytokines in a 3D scaffold. These organoids express multicellular biomarkers and show functional characteristics similar to those of their origin organs, which provide potential avenues to explore reproductive system development, disease modelling, and patient-specific therapy. Nevertheless, advanced culture methods, such as co-culture system, 3D bioprinting and organoid-on-a-chip technology, remain to be explored, and more efforts should be made for further elucidation of cell–cell crosstalk. This review describes the development and applications of human female reproductive system organoids.

Figure: Applications in developmental biology, disease modelling, and drug discovery of human female reproductive system organoids. ASCs: adult stem cells; PSCs: pluripotent stem cells.



Similar content being viewed by others
References
Giuliana, R., Andrea, M., & Lutolf, M. P. (2018). Progress and potential in organoid research. Nature Reviews Genetics, 19, 671–687.
Kai, K., & Hans, C. (2016). Organoids: Modeling development and the stem cell niche in a dish. Developmental Cell, 38, 590–600.
Turco, M. Y., Lucy, G., Jasmine, H., et al. (2017). Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nature Cell Biology, 19, 568–577.
Sandra, H., Gudrun, M., Leila, S., et al. (2018). Self-renewing trophoblast organoids recapitulate the developmental program of the early human placenta. Stem Cell Reports, 11, 537–551.
Lancaster, M. A., & Knoblich, J. A. (2014). Organogenesis in a dish: Modeling development and disease using organoid technologies. Science, 345, 1247125.
Kazutoshi, T., Koji, T., Mari, O., et al. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell, 131, 861–872.
In-Hyun, P., Natasha, A., Hongguang, H., et al. (2008). Disease-specific induced pluripotent stem cells. Cell, 134, 877–886.
Narasimman, G., Abdulrhman, A., Sheeja, R., et al. (2018). Adult stem cells for regenerative therapy. Progress in Molecular Biology and Translational Science, 160, 1–22.
Youssef, H., & Anis, F. (2020). Organoid models of human endometrial development and disease. Frontiers in Cell and Developmental Biology, 8, 84.
Thomson, J. A., Itskovitz-Eldor, J., Shapiro, S., et al. (1998). Embryonic stem cell lines derived from human blastocysts. Science, 282, 1145–1147.
Mototsugu, E., Nozomu, T., Hiroki, I., et al. (2011). Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature, 472, 51–56.
Toshiro, S., Vries, R. G., Snippert, H. J., et al. (2009). Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature, 459, 262–265.
Rinehart, C. A., Lyn-Cook, B. D., & Kaufman, D. G. (1988). Gland formation from human endometrial epithelial cells in vitro. In Vitro Cellular & Developmental Biology, 24, 1037–1041.
Bläuer, M., Heinonen, P. K., Martikainen, P. M., Tomás, E., & Ylikomi, T. (2005). A novel organotypic culture model for normal human endometrium: Regulation of epithelial cell proliferation by estradiol and medroxyprogesterone acetate. Human Reproduction, 20, 864–871.
Nguyen, H. P. T., Xiao, L., Deane, J. A., et al. (2017). N-cadherin identifies human endometrial epithelial progenitor cells by in vitro stem cell assays. Human Reproduction, 32, 2254–2268.
Fitzgerald, H. C., Dhakal, P., Behura, S. K., et al. (2019). Self-renewing endometrial epithelial organoids of the human uterus. Proceedings of the National Academy of Sciences of the United States of America, 116, 23132–23142.
Valentijn, A. J., Saretzki, G., Tempest, N., Critchley, H. O., & Hapangama, D. K. (2015). Human endometrial epithelial telomerase is important for epithelial proliferation and glandular formation with potential implications in endometriosis. Human Reproduction, 30, 2816–2828.
Syed, S. M., Kumar, M., Ghosh, A., et al. (2020). Endometrial axin2 cells drive epithelial homeostasis, regeneration, and cancer following oncogenic transformation. Cell Stem Cell, 26, 64–80.e13.
Sandra, H., Magdalena, G., Burkard, T. R., et al. (2019). Estrogen signaling drives ciliogenesis in human endometrial organoids. Endocrinology, 160, 2282–2297.
Murphy, A. R., Teerawat, W., Lu, Z., et al. (2019). Generation of multicellular human primary endometrial organoids. Journal of Visualized Experiments, 152. https://doi.org/10.3791/60384.
Bishop, E. A., Stan, L., Elangovan, T., et al. (2014). Insulin exerts direct effects on carcinogenic transformation of human endometrial organotypic cultures. Cancer Investigation, 32, 63–70.
Paweł, Ł., Gomez, A., Hire, G., et al. (2017). Human three-dimensional endometrial epithelial cell model to study host interactions with vaginal bacteria and Neisseria gonorrhoeae. Infection and Immunity, 85, e01049-16.
Benbrook, D. M., Stan, L., James, R.-M., et al. (2008). Gene expression analysis of biological systems driving an organotypic model of endometrial carcinogenesis and chemoprevention. Gene Regulation and Systems Biology, 2, 21–42.
Kamelle, S., Sienko, A., & Benbrook, D. M. (2002). Retinoids and steroids regulate menstrual phase histological features in human endometrial organotypic cultures. Fertility and Sterility, 78, 596–602.
Fayazi, M., Salehnia, M., & Ziaei, S. (2017). In-vitro construction of endometrial-like epithelium using CD146 mesenchymal cells derived from human endometrium. Reproductive Biomedicine Online, 35, 241–252.
Johnson, M. H. (2017). First evidence that endometrial-like organoids can develop from the endometrial mesenchymal stem/stromal cell population. Reproductive Biomedicine Online, 35, 239–240.
Wiwatpanit, T., Murphy, A. R., Lu, Z., et al. (2020). Scaffold-free endometrial organoids respond to excess androgens associated with polycystic ovarian syndrome. The Journal of Clinical Endocrinology and Metabolism, 105, 769–780.
Boretto, M., Maenhoudt, N., Luo, X., et al. (2019). Patient-derived organoids from endometrial disease capture clinical heterogeneity and are amenable to drug screening. Nature Cell Biology, 21, 1041–1051.
Classen-Linke, I., Kusche, M., Knauthe, R., & Beier, H. M. (1997). Establishment of a human endometrial cell culture system and characterization of its polarized hormone responsive epithelial cells. Cell and Tissue Research, 287, 171–185.
Yang, H., Sungwon, H., Haekwon, K., et al. (2002). Expression of integrins, cyclooxygenases and matrix metalloproteinases in three-dimensional human endometrial cell culture system. Experimental & Molecular Medicine, 34, 75–82.
Aurélie, H., Katharina, H., Matteo, B., et al. (2019). Functional expression of the mechanosensitive PIEZO1 channel in primary endometrial epithelial cells and endometrial organoids. Scientific Reports, 9, 1779.
Barros, F. S. V. (2017). Characterization of human endometrial glandular epithelium in vitro and in vivo (pp. 1–252). Warwick: Division of Biomedical Sciences Warwick Medical School University of Warwick.
Boretto, M., Cox, B., Noben, M., et al. (2017). Development of organoids from mouse and human endometrium showing endometrial epithelium physiology and long-term expandability. Development, 144, 1775–1786.
Hapangama, D. K., Drury, J., Da Silva, L., et al. (2019). Abnormally located SSEA1+/SOX9+ endometrial epithelial cells with a basalis-like phenotype in the eutopic functionalis layer may play a role in the pathogenesis of endometriosis. Human Reproduction, 34, 56–68.
Łaniewski, P., & Herbst-Kralovetz, M. M. (1997). Analysis of host responses to Neisseria gonorrhoeae using a human three-dimensional endometrial epithelial cell model. Methods in Molecular Biology, 2019, 347–361.
Zambuto, S. G., Clancy, K. B. H., & Harley, B. A. C. (2019). A gelatin hydrogel to study endometrial angiogenesis and trophoblast invasion. Interface Focus, 9, 20190016.
Miyazaki, K., Dyson, M. T., Coon, V., John, S., et al. (2018). Generation of progesterone-responsive endometrial stromal fibroblasts from human induced pluripotent stem cells: Role of the WNT/CTNNB1 pathway. Stem Cell Reports, 11, 1136–1155.
Kessler, M., Hoffmann, K., Brinkmann, V., et al. (2015). The Notch and Wnt pathways regulate stemness and differentiation in human fallopian tube organoids. Nature Communications, 6, 8989.
Yucer, N., Holzapfel, M., Vogel, T. J., et al. (2017). Directed differentiation of human induced pluripotent stem cells into fallopian tube epithelium. Scientific Reports, 7, 10741.
Lawrenson, K., Notaridou, M., Lee, N., et al. (2013). In vitro three-dimensional modeling of fallopian tube secretory epithelial cells. BMC Cell Biology, 14, 43.
Kessler, M., Hoffmann, K., Fritsche, K., et al. (2019). Chronic Chlamydia infection in human organoids increases stemness and promotes age-dependent CpG methylation. Nature Communications, 10, 1194.
Yu-Hsun, C., Tang-Yuan, C., & Dah-Ching, D. (2020). Human fallopian tube epithelial cells exhibit stemness features, self-renewal capacity, and Wnt-related organoid formation. Journal of Biomedical Science, 27, 32.
Turco, M. Y., Gardner, L., Kay, R. G., et al. (2018). Trophoblast organoids as a model for maternal-fetal interactions during human placentation. Nature, 564, 263–267.
Li, Z., Kurosawa, O., & Iwata, H. (2018). Development of trophoblast cystic structures from human induced pluripotent stem cells in limited-area cell culture. Biochemical and Biophysical Research Communications, 505, 671–676.
Dajung, J., Xiong, J., Min, Y., et al. (2017). In vitro differentiation of human embryonic stem cells into ovarian follicle-like cells. Nature Communications, 8, 15680.
Lawrenson, K., Benjamin, E., Turmaine, M., Jacobs, I., Gayther, S., & Dafou, D. (2009). In vitro three-dimensional modelling of human ovarian surface epithelial cells. Cell Proliferation, 42, 385–393.
Ohtake, H., Katabuchi, H., Matsuura, K., et al. (1999). A novel in vitro experimental model for ovarian endometriosis: The three-dimensional culture of human ovarian surface epithelial cells in collagen gels. Fertility and Sterility, 71, 50–55.
Girda, E., Huang, E. C., Leiserowitz, G. S., et al. (2017). The use of endometrial cancer patient-derived organoid culture for drug sensitivity testing is feasible. The International Journal of Gynecological Cancer, 27, 1701–1707.
Shuang, Z., Igor, D., Tao, Z., et al. (2019). Both fallopian tube and ovarian surface epithelium are cells-of-origin for high-grade serous ovarian carcinoma. Nature Communications, 10, 5367.
Paik, D. Y., Janzen, D. M., Schafenacker, A. M., et al. (2012). Stem-like epithelial cells are concentrated in the distal end of the fallopian tube: A site for injury and serous cancer initiation. Stem Cells, 30, 2487–2497.
Abbas, Y., Oefner, C. M., Polacheck, W. J., et al. (2017). A microfluidics assay to study invasion of human placental trophoblast cells. The Journal of the Royal Society Interface, 14, 20170131.
Ma, L., Li, G., Cao, G., et al. (2017). dNK cells facilitate the interaction between trophoblastic and endothelial cells via VEGF-C and HGF. Immunology and Cell Biology, 95, 695–704.
McConkey, C. A., Delorme-Axford, E., Nickerson, C. A., et al. (2016). A three-dimensional culture system recapitulates placental syncytiotrophoblast development and microbial resistance. Science Advances, 2, e1501462.
Kotomi, S., Yasuhisa, M., Michiya, S., et al. (2018). Aggregation of human trophoblast cells into three-dimensional culture system enhances anti-inflammatory characteristics through cytoskeleton regulation. The International Journal of Molecular Sciences, 19, 2322.
Wong, M. K., Shawky, S. A., Aryasomayajula, A., et al. (2018). Extracellular matrix surface regulates self-assembly of three-dimensional placental trophoblast spheroids. PLoS One, 13, e199632.
Kalkunte, S., Huang, Z., Lippe, E., et al. (2017). Polychlorinated biphenyls target Notch/Dll and VEGF R2 in the mouse placenta and human trophoblast cell lines for their anti-angiogenic effects. Scientific Reports, 7, 39885.
Nhan, P., Hong, J. J., Tofig, B., et al. (2019). A simple high-throughput approach identifies actionable drug sensitivities in patient-derived tumor organoids. Communications Biology, 2, 78.
Jabs, J., Zickgraf, F. M., Park, J., et al. (2017). Screening drug effects in patient-derived cancer cells links organoid responses to genome alterations. Molecular Systems Biology, 13, 955.
Moore, C. A., Shah, N. N., Smith, C. P., et al. (1842). 3D bioprinting and stem cells. Methods in Molecular Biology, 2018, 93–103.
Zhang, Y. S., Arneri, A., Bersini, S., et al. (2016). Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials, 110, 45–59.
Han, F., Utkan, D., & Pu, C. (2019). Emerging organoid models: leaping forward in cancer research. Journal of Hematology & Oncology, 12, 142.
Mittal, R., Woo, F. W., Castro, C. S., et al. (2019). Organ-on-chip models: Implications in drug discovery and clinical applications. Journal of Cellular Physiology, 234, 8352–8380.
Acknowledgements
No funding or financial support has been received.
No manuscript preparation assistance was provided or paid for.
All persons who contributed to the work reported in the manuscript are included as authors.
The contents of this manuscript have never been presented at a meeting.
No earlier version of this manuscript has been published on a preprint server.
All tables and figures are original, and no material has been adapted or modified from another source.
Disclaimer
The ideas and opinions expressed herein are those of the authors. The views expressed in the review are those of the authors and do not necessarily reflect the official policy or position of the Department of Maternal and Child Health of the National Health and Family Planning Commission of the People’s Republic of China.
Contributors
All authors contributed to the conception and design of this review. Cui drafted the first version of the article, and Zhao, Wu, and Li revised it critically for important intellectual content. Final approval of the version to be published was given by all authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest Disclosures
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cui, Y., Zhao, H., Wu, S. et al. Human Female Reproductive System Organoids: Applications in Developmental Biology, Disease Modelling, and Drug Discovery. Stem Cell Rev and Rep 16, 1173–1184 (2020). https://doi.org/10.1007/s12015-020-10039-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-020-10039-0