Abstract
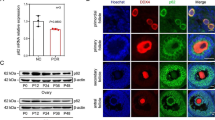
Numerous evidences suggested that microRNAs (miRs) could play an active and significant role during spermatogenesis. Cysteine-rich secretory protein (CRISP3) has a role in inflammatory response and is extremely over-expressed in adolescents with varicocele seminal plasma and modified semen analysis. Nowadays, the miRs expression’s association with their target genes is well recognized. The aim of this study was evaluating the association of CRISP3 and four candidate miRs among teratozoospermia (TZ) infertile men. First, we have selected four miRs, miR-182-5p, miR-192-5p, miR-204-5p, and miR-493-5p bioinformatically. After that, RNA was extracted from semen samples of 21 TZ patients and 20 normozoospermia (Norm). Then, their expression levels were assessed using real-time polymerase chain reaction method. In the next step, we quantified the expression of two CRISP3 protein isoforms, targeted by these miRs, using western blotting. According to our results, up-regulation of miR-182-5p, miR-192-5p, and miR-493-5p was observed. MiR-182-5p, miR-192-5p, and miR-493-5p showed good AUC values which can be introduced as possible biomarkers of TZ. In addition, the expression level of the CRISP3 glycosylated (31 kDa) isoform was significantly lower in TZ patients than Norm ones. Notably, in TZ patients, there was a possibly positive correlation of glycosylated CRISP3 expression with normal sperm morphology. According to our results, CRISP3 protein can play a significant role in male infertility especially in maturation formation of spermatozoa. Also, deregulation of the studied miRs, miR-182-5p, miR-92-5p, and miR-493-5p, can suggest a regulatory network between these miRs and CRISP3 isoforms and suggest their regulatory roles in male infertility.






Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Coutton C, Fissore RA, Palermo GD, Stouffs K, Touré A. Male infertility: genetics, mechanism, and therapies. Biomed Res Int. 2016;2016:1.
Coutton C, Escoffier J, Martinez G, Arnoult C, Ray PF. Teratozoospermia: spotlight on the main genetic actors in the human. Hum Reprod Update. 2015;21:455–85.
Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–5.
Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97.
Papaioannou MD, Nef S. microRNAs in the testis: building up male fertility. J Androl. 2010;31:26–33.
Abu-Halima M, Hammadeh M, Schmitt J, Leidinger P, Keller A, Meese E, et al. Altered microRNA expression profiles of human spermatozoa in patients with different spermatogenic impairments. Fertil Steril. 2013;99:1249–1255.e1216.
Lian J, Zhang X, Tian H, Liang N, Wang Y, Liang C, et al. Altered microRNA expression in patients with non-obstructive azoospermia. Reprod Biol Endocrinol. 2009;7:13.
Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer. 2007;7:192–201.
Zhou J-H, Zhou Q-Z, Yang J-K, Lyu X-M, Bian J, Guo W-B, et al. MicroRNA-27a-mediated repression of cysteine-rich secretory protein 2 translation in asthenoteratozoospermic patients. Asian J Androl. 2017;19:591.
Gholami D, Yazdi RS, Jami M-S, Ghasemi S, Gilani M-AS, Sadeghinia S, et al. The expression of cysteine-rich secretory protein 2 (CRISP2) and miR-582-5p in seminal plasma fluid and spermatozoa of infertile men. Gene. 2020;730:144261.
Udby L, Bjartell A, Malm J, Egesten A, Lundwall Å, Cowland JB, et al. Characterization and localization of cysteine-rich secretory protein 3 (CRISP-3) in the human male reproductive tract. J Androl. 2005;26:333–42.
Belardin L, Camargo M, Intasqui P, Antoniassi M, Fraietta R, Bertolla R. Cysteine-rich secretory protein 3: inflammation role in adult varicocoele. Andrology. 2019;7:53–61.
Zhou J, Xue K, Chen M, Zhou Q, Yang J, Bian J, et al. Expression of cysteine-rich secretory protein 2 in patients with asthenozoospermia and its clinical significance. Nan Fang Yi Ke Da Xue Xue Bao J South Med Univ. 2014;34:1528–33.
Menkveld R. Clinical significance of the low normal sperm morphology value as proposed in the fifth edition of the WHO Laboratory Manual for the Examination and Processing of Human Semen. Asian J Androl. 2010;12:47–58.
Mohammadi M, Amirmahani F, Goharrizi KJ, Pakzad R, Dolat H. Evaluating the expression level of Survivin gene in different groups of B-cell acute lymphoblastic leukemia patients of Iran. Mol Biol Rep. 2019;46:2679–84.
Bastian BC, LeBoit PE, Hamm H, Bröcker E-B, Pinkel D. Chromosomal gains and losses in primary cutaneous melanomas detected by comparative genomic hybridization. Cancer Res. 1998;58:2170–5.
Kurul NO, Ates F, Yilmaz I, Narli G, Yesildal C, Senkul T. The association of let-7c, miR-21, miR-145, miR-182, and miR-221 with clinicopathologic parameters of prostate cancer in patients diagnosed with low-risk disease. Prostate. 2019;79:1125–32.
Fang Y, Huang Z, Li H, Tan W, Zhang Q, Wang L, et al. Highly expressed miR-182-5p can promote preeclampsia progression by degrading RND3 and inhibiting HTR-8/SVneo cell invasion. Eur Rev Med Pharmacol Sci. 2018;22:6583–90.
Zhi E-L, Liang G-Q, Li P, Chen H-X, Tian R-H, Xu P, et al. Seminal plasma miR-192a: a biomarker predicting successful resolution of nonobstructive azoospermia following varicocele repair. Asian J Androl. 2018;20:396.
Liu T, Cheng W, Gao Y, Wang H, Liu Z. Microarray analysis of microRNA expression patterns in the semen of infertile men with semen abnormalities. Mol Med Rep. 2012;6:535–42.
Chen X, Li X, Guo J, Zhang P, Zeng W. The roles of microRNAs in regulation of mammalian spermatogenesis. J Anim Sci Biotechnol. 2017;8:35.
Niu B, Wu J, Mu H, Li B, Wu C, He X, et al. miR-204 regulates the proliferation of dairy goat spermatogonial stem cells via targeting to Sirt1. Rejuvenation Res. 2016;19:120–30.
Acknowledgements
We would like to acknowledge Royan Institute Infertility Clinic (Tehran, Iran), Hazrat Zahra Infertility Clinic (Shahrekord, Iran), and Vice Chancellor for Research and Technology Affairs of Shahrekord University of Medical Sciences for supporting this study.
Code Availability
Not applicable
Funding
This study has been supported financially by Royan Institute Infertility Clinic (Tehran, Iran) and Vice Chancellor for Research and Technology Affairs of Shahrekord University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data collection and experimental works were performed by Delnya Gholami. The first draft of the manuscript was written by Farzane Amirmahani. Reza Salman Yazdi and Tahereh Hasheminia analyzed data and edited the drafted manuscript. Hossein Teimori contributed to study design and supervised the project. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
All patients filled the informed consent form for participation. Ethical considerations were confirmed by the Shahrekord University of Medical Sciences ethical committee (ethics code: IR.SKUMS.REC.1395.214). The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Consent to Participate
A written informed consent was obtained from all individuals participated in this study.
Consent for Publication
Not applicable
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gholami, D., Amirmahani, F., Yazdi, R.S. et al. MiR-182-5p, MiR-192-5p, and MiR-493-5p Constitute a Regulatory Network with CRISP3 in Seminal Plasma Fluid of Teratozoospermia Patients. Reprod. Sci. 28, 2060–2069 (2021). https://doi.org/10.1007/s43032-021-00485-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-021-00485-7