Abstract
Mammalian spermatogenesis contains three continuous and organized processes, by which spermatogonia undergo mitosis and differentiate to spermatocytes, follow on meiosis to form haploid spermatids and ultimately transform into spermatozoa. These processes require an accurately, spatially and temporally regulated gene expression patterns. The microRNAs are a novel class of post-transcriptional regulators. Cumulating evidences have demonstrated that microRNAs are expressed in a cell-specific or stage-specific manner during spermatogenesis. In this review, we focus on the roles of microRNAs in spermatogenesis. We highlight that N6-methyladenosine (m6A) is involved in the biogenesis of microRNAs and miRNA regulates the m6A modification on mRNA, and that specific miRNAs have been exploited as potential biomarkers for the male factor infertility, which will provide insightful understanding of microRNA roles in spermatogenesis.
Similar content being viewed by others
Background
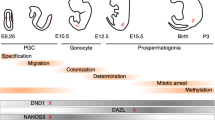
Male fertility is dependent upon the successful perpetuation of spermatogenesis that is a highly organized process of germ cell differentiation occurring within the seminiferous tubules in the testes. Spermatogonial stem cells (SSCs) are a subset of undifferentiated spermatogonia that are capable of self-renewal to maintain the pool of SSCs or differentiation to give rise to spermatogenic lineage, thus supporting the continuous production of spermatozoa. Spermatogenesis initiates once SSCs enter differentiation process [1]. The spermatogonia go into the meiotic phase and become spermatocytes. After a long-lasting meiosis I, preleptotene spermatocytes transform into second spermatocytes and enter meiosis II to produce haploid round spermatids [2], which undergo spermiogenesis including acrosomal biogenesis, flagellum development, chromatin condensation, cytoplasmic reorganization and exclusion [3]. Ultimately, the round spermatids transform into spermatozoa, which are released into the lumen of seminiferous tubules [4].
This highly organized spermatogenesis requires accurate, spatial and temporal regulation of gene expression governed by transcriptional, post-transcriptional and epigenetic processes [5, 6]. More than a thousand of protein coding genes that are involved in the spermatogenesis have been identified [7, 8]. However, the mechanisms that mediate the expression of these spermatogenesis-related genes have not been fully uncovered. The microRNAs (miRNAs, miR), small (~22 nucleotides) single-strand noncoding RNAs, are linked to cell proliferation, differentiation and apoptosis [9–11]. Transcriptome data indicate that miRNAs are extensively transcribed during spermatogenesis. The miRNAs are differentially expressed in a cell-specific and step-specific manner ([12, 13], Chen et al. unpublished data). Some miRNAs are specifically expressed in certain type of male germ cells, while the others are universally expressed among different types of cells in the testes. Growing evidences have showed that the miRNAs are essential for male germ cell development and differentiation [14–17]. A few recent reviews have reported the roles of miRNAs in spermatogenesis and fertility [5, 6, 10, 11]. In this article, we briefly summarize the most recent progress of miRNAs in the regulation of spermatogenesis.
miRNA biogenesis
At present, there are 1881 miRNA loci having been annotated in the human genome in the miRNA database (http://www.mirbase.org). Analysis has revealed that 1% of the human genome is miRNA genes [18–20], of which about half of miRNA genes located in the introns (intronic miRNAs) of host genes [21]. However, some intronic miRNAs exhibit low correlated expression level with their host genes. It is likely these miRNAs are transcribed from unique transcription units independent of host genes [22–24].
The biogenesis of miRNAs is modulated at a few levels, including miRNA transcription, processing by Drosha and Dicer, RNA methylation, uridylation and adenylation (Fig. 1) [25–27]. The initial transcripts are termed the primary miRNAs (pri-miRNAs) that are variable in length from several hundreds to thousands of nucleotides [25]. The pri-miRNAs are methylated by the methyltransferase like 3 (METTL3), marking them for recognition and processing by the DiGeorge syndrome critical region 8 (DGCR8) [28]. The pri-miRNAs are thus processed by drosha ribonuclease III (Drosha) and its cofactor DGCR8 into ~ 70 nucleotides (nt) long miRNA precursor (pre-miRNAs) [29, 30]. The pre-miRNAs are then transported into the cytoplasm by exportin 5 (EXP5) in accompanied with Ran-GTP [31, 32] and cleaved by Dicer into ~22 base pair (bp) double-strands RNAs (dsRNAs) [33–35]. These dsRNAs are loaded onto an Argonaute protein (AGO) so as to form miRNA-induced silencing complex (miRISC), in which one strand of the ~22-nt RNA duplex remains in AGO as a mature miRNA, whereas the other strand is degraded [36]. Interestingly, Alarcon et al. recently reported that RNA-binding protein heterogeneous nuclear ribonucleoprotein A2/B1 (HNRNPA2B1) binds m6A-bearing pri-miRNAs, interacts with DGCR8 and thus facilitates the processing of pri-miRNAs [37]. In consistent with this, loss of HNRNPA2B1 or depletion of METTL3 led to concomitant accumulation of unprocessed pri-miRNAs and decrease of the global mature miRNAs [28, 37]. Therefore, the methylation mark acts as a key post-transcriptional modification that enhances the initiation of miRNA biogenesis.
Mechanisms of miRNA action
Usually, a specific base-pairing between miRNAs and mRNAs induces mRNA degradation or translational repression [38]. In mammals, the overall complementarity between a miRNA and its target is usually imperfect, which allows each miRNA to potentially regulate multiple RNAs [39]. It is estimated that one miRNA may target as many as 400 genes on average [19]. Conversely, the expression of a single gene can also be modulated by multiple miRNAs [40].
Interestingly, it has been reported recently that miRNAs regulate the m6A modification in mRNAs via a sequence pairing mechanism. As a result, manipulation of miRNA expression leads to change of m6A modification through modulating the binding of METTL3 to mRNAs (Fig. 1) [41]. The m6A modification, in turn, modulates mRNA metabolism and thus is another key post-transcriptional control of gene expression [37, 42, 43]. Evidences have indicated that m6A methylation determines stem cell fate by regulating pluripotent transition toward differentiation [41, 44, 45]. Intriguingly, deficiency of ALKBH5, a m6A demethylase, leads to aberrant spermatogenesis and apoptosis in mouse testis through the demethylation of m6A on mRNAs [46].
Functions of miRNAs in spermatogenesis
Conditional Dicer knockout mouse models
The overall importance of miRNA signaling for regulation of spermatogenesis has been demonstrated using conditional knockout of Dicer in germ cells. Dicer1 ablation in prospermatogonia just before birth using Ddx4 promoter-driven Cre expression led to an alteration in meiotic progression, significant increase of apoptosis in pachytene spermatocytes, a reduced number of round spermatids and morphological defects in spermatozoa [47]. Moreover, Ngn3 is expressed endogenously in type A spermatogonia starting from postnatal d 5 [48, 49]. In the mouse model of selective deletion of Dicer1 in type A spermatogonia by Ngn3 promoter-driven Cre, the first clear defects were displayed in haploid round spermatids. The spermiogenesis was severely compromised [50]. Similarly, conditional depletion of Dicer1 using the Stra8Cre transgene in early spermatogonia resulted in the comparable phenotype to the Ngn3Cre-driven Dicer1 deletion [51, 52]. In addition, deletion of Dicer1 in haploid spermatids using the protamine 1 (Prm1)-Cre transgene led to abnormal morphology in the elongated spermatids and spermatozoa [53]. But, the Prm1Cre-Dicer1 knockout caused a less severe phenotype compared to those in which Dicer1 was deleted from prospermatogonia and spermatogonia [53].
Collectively, the earlier the ablation of Dicer occurs, the more severe side effects on spermatogenesis are found. Therefore, miRNA-mediated post-transcriptional control is an important regulator for spermatogenesis.
The roles of miRNAs in SSC self-renewal and differentiation
SSCs are the foundation of spermatogenesis that involves a delicate balance between self-renewal and differentiation of SSCs to ensure the lifelong production of spermatozoa. In the testes, the SSCs reside in a unique microenvironment or ‘niche’. The niche factor glial cell line-derived neurotropic factor (GDNF) is the first well-defined paracrine factor that promotes SSC self-renewal [54]. GDNF signaling acts via the RET tyrosine kinase [55] and requires a ligand-specific co-receptor GFRα1 [56] in mouse SSCs [57]. Evidences have shown that through the PI3K/AKT-dependent pathway [58] or the SRC family kinase (SFK) pathway [59], GDNF regulates the expression of the transcription factors B cell CLL/lymphoma 6 member B (BCL6B), ETS variant 5 (ETV5), DNA-binding protein 4 (ID4), LIM homeobox 1 (LHX1) and POU class 3 homeobox 1 (POU3F1) to drive SSC self-renewal [59].
miRNAs conduce maintenance of the pool of SSCs. It has been shown that miR-20 along with miR-21, −34c, −135a, −146a, −182, −183, −204, −465a-3p, −465b-3p, −465c-3p, −465c-5p and −544 were preferentially expressed in the SSC-enriched population (Fig. 2) [60, 61]. Importantly, miR-20, miR-21 and miR-106a contribute to maintenance of mouse SSC homeostasis [61]. miR-135a mediates the maintenance of rat SSCs by regulating FOXO1 that promotes high levels of Ret protein on the cell surface of SSCs [62]. Moreover, miR-544 regulates self-renewal of goat SSCs by targeting the promyelocytic leukemia zinc finger gene (PLZF), which is the first transcription factor to be identified as being involved in SSC self-renewal [63]. Similarly, miR-224 regulates mouse SSC self-renewal via modulating PLZF and GFRα1 [64]. Interestingly, miR-34c is expressed in goat SSCs and promotes SSC apoptosis in a p53-depemdent manner [65]. Recently, it was found that miR-204 was involved in the regulation of dairy goat SSC proliferation via targeting Sirt1 [66]. Collectively, miRNAs are involved in regulating SSCs fate.
On the other hand, some miRNAs have been identified to mediate spermatogonia differentiation. It is well-known that retinoic acid (RA) directs the sequential programs of spermatogonial differentiation and the entry into meiosis [67, 68]. miR-146 [69], miR-let7 family miRNAs [70], miR-17-92 and miR-106b-25 clusters [71] are downregulated during RA-induced spermatogonial differentiation. Importantly, male germ cell-specific knockout of miR-17-92 cluster resulted in the reduced number of SSCs and spermatogonia, and impaired spermatogenesis [71, 72]. Interestingly, exposure to RA downregulates miR-221/222 expression, while GDNF upregulates miR-221/222 abundance. Over-expression of miR-221/222 in undifferentiated spermatogonia made them resisting to RA-induced transition into c-kit-positive differentiated spermatogonia [73]. In addition, miR-34c promotes SSC differentiation and meiosis process by targeting NANOS2 and up-regulating meiosis regulated genes Stra8, Scp3 and Dazl [74]. Taken together, miRNAs are related to the post-transcriptional regulation of spermatogonia differentiation.
The roles of miRNAs in meiosis and spermiogenesis
Growing evidences have also demonstrated that specific miRNAs regulate meiosis (Fig. 2). The expression of miR-449 cluster is abundant and is upregulated upon meiotic initiation during testis development and in adult testes. The expression pattern of the miR-449 cluster is similar to that of miR-34b/c. Moreover, miR-34b/c and miR-449 cluster share the same seed region and thus target same sets of mRNAs [75–78]. Depletion of either miR-34 cluster or miR-449 cluster displays no apparent defect in male germ cell development. However, simultaneous knockout of these two clusters led to sexually dimorphic and infertility, suggesting that miR-34b/c and the miR-449 cluster function redundantly in the regulation of spermatogenesis [71]. Furthermore, miR-18, one of the miR-17-92 cluster, is abundantly expressed in spermatocytes. miR-18 targets heat shock factor2 (Hsf2), which is a critical transcription factor for spermatogenesis [79]. Finally, miR-34b-5p regulates meiotic progression by targeting Cdk6 [80].
A unique chromatin remodelling occurs during spermatogenesis when histones are replaced by DNA packing proteins, such as transition proteins (TPs) and protamines (PRMs), which are exclusive to male germ cells [81, 82]. In the post-mitotic germ cells, the timely expression of TPs and PRMs is prerequisite for compaction and condensation of chromatin during spermiogenesis [83]. To secure this timed expression pattern, Tp and Prm are subjected to an efficiently post-transcriptional control. It has been demonstrated that miR-469 suppresses the translation of TP2 and PRM2 by targeting mRNA of Tp2 and Prm2 in pachytene spermatocytes and round spermatids [84]. On the contrary, miR-122a that is abundantly expressed in late-stage male germ cells reduces the Tp2 mRNA expression by RNA cleavage [85].
Although the majority of miRNAs disappear during spermiogenesis, the sperm born miRNAs have also been demonstrated to play important roles. miR-34 is present in mouse spermatozoa and zygotes but not in the oocytes or in embryos beyond the one-cell stage [86]. Upon fertilization, miR-34c is transferred from spermatozoa to zygote where it reduces the expression of Bcl-2 and p27, leading to S-phase entry and the first cleavage. Moreover, injection of miR-34c inhibitor into the zygotes inhibits DNA synthesis and suppresses the first cleavage division, suggesting that the sperm-borne miR-34c is required for zygote cleavage [86]. In addition, dysregulation of miR-424/322 induces DNA double-strand breaks in spermatozoa [87]. Importantly, a set of sperm miRNAs are differentially expressed in asthenozoospermic and oligoastheno- zoospermic males compared with normozoospermic males [88, 89]. Furthermore, miR-151a-5p is abundant in severe asthenozoospermia cases compared with healthy controls and participates in mitochondrial biological functions [53, 90]. Therefore, specific miRNAs have been exploited as potential biomarkers for male factor infertility [91].
miRNAs in testicular somatic cells
Spermatogenesis is supported by the testicular Sertoli cells, peritubular myoid (PTM) cells and Leydig cells [92–94]. The extrinsic factors derived from these somatic cells trigger specific events in germ cells that dictate or influence spermatogenesis. It has been shown that miRNAs are highly abundant in Sertoli cells (Fig. 2) [12, 95, 96]. MiR-133b and miR-202 are involved in pathogenesis of azoospermia or Sertoli-cell-only syndrome [97, 98]. Importantly,conditional depletion of Dicer1 from Sertoli cells, using the Anti-Müllerian hormone (Amh) promoter-driven Cre in mice, results in disrupted spermatogenesis and progressive testis degeneration, indicating that miRNAs in Sertoli cells play critical roles in spermatogenesis [99, 100]. Specifically, miR-133b promotes the proliferation of human Sertoli cells by targeting GLI3 and mediating expression of Cyclin B1 and Cyclin D1 [97]. Moreover, miR-762 promotes porcine immature Sertoli cell growth via the ring finger protein 4 (RNF4) [101].
FSH and androgens are fundamentally important for spermatogenesis. To elucidate the molecular mechanisms by which FSH and androgen act in the Sertoli cells, Nicholls et al. [102] investigated the expression and regulation of micro-RNAs (miRNAs). The authors have found that a subset of miRNAs were up-regulated after hormone suppression in rat model and in vitro culture of primary rat Sertoli cells. Interestingly, Pten, an intracellular phosphatase, and Eps15, a mediator of endocytosis, were down-regulated by the withdrawal of hormones [102]. In consistent with it, overexpression of miR-23b in vitro resulted in decreased translation of PTEN and EPS15 protein. Similarly, by using androgen suppression and androgen replacement, Chang et al. [53] identified that androgen regulated the expression of several microRNAs in mouse Sertoli cells [103]. One of the miRNAs targets found in this study is desmocollin-1 (Dsc1), which plays an essential role in cell-cell adhesion in epithelial cells [104]. On the other hand, elevated estradiol level is associated with male infertility [105]. Evidences indicate that estradiol regulates proliferation of Sertoli cells in a dose-dependent manner, in which miR-17 family and miR-1285 are involved in the regulation [106, 107]. Collectively, miRNA transcription is a new paradigm in the hormone dependence of spermatogenesis.
Leydig cells are responsible for androgen production that is essential for sperm production [108]. Basic fibroblast growth factor (bFGF) promotes the development of stem Leydig cells and inhibits LH-stimulated androgen production by regulating miRNAs [109]. Interestingly, miR-140-5p/140-3p control mouse Leydig cell numbers in the developing testis. Deletion of miR-140-5p/miR-140-3p results in an increase of number of Leydig cells, indicating that the miRNAs are likely to regulate the expression of factors produced by Sertoli cells that regulate differentiation of Leydig cells [110].
Collectively, these findings indicate that miRNAs regulate the development and functions of Sertoli cells and Leydig cells, which create the niche for SSCs and thus provide structural and nutritional support for germ cells. Therefore, miRNAs in somatic cells play important roles in spermatogenesis.
Conclusion and perspectives
Extensive and accurate regulation of gene expression is prerequisite for spermatogenesis. miRNAs are expressed in a cell-specific or stage-specific manner during spermatogenesis. However, the roles and underlying mechanisms of many of those miRNAs in spermatogenesis remain largely unknown. Future studies should primarily focus on uncovering the roles of germ-cell specific miRNAs in spermatogenesis. The powerful single-cell small RNA sequencing would help to more accurately profile the miRNAs for certain type of germ cells. Meanwhile, the establishment of long-term culture of SSCs and in vitro induction of differentiation of male germ cells make it possible to elucidate the role of a certain miRNA or miRNA cluster in vitro. The application of CRISPR/Cas9 system and conditional knockout strategies would speed up the understanding of miRNA functions. Secondly, growing evidences have been demonstrated that some specific miRNAs are preferentially expressed in testicular somatic cells. But it is not clear whether these miRNAs act as secreted paracrine factors in the SSC niche, or whether they indirectly mediate the secretion of growth factors, GDNF for instance, which then affect germ cells. More somatic cell expressed miRNAs are needed to be functionally characterized. Thirdly, it has been demonstrated that some transcription factors promote SSC self-renewal (for example, BCL6B, BRACHYURY, ETV5, ID4, LHX1, and POU3F1), while several transcription factors stimulate spermatogonia differentiation (DMRT1, NGN3, SOHLH1, SOHLH2, SOX3, and STAT3) [111]. However, it is unclear which and how miRNA/miRNA cluster regulates the expression of these transcription factors. Fourthly, it has been discovered recently that RNA methylation is involved in pri-miRNA processing [28, 37], opening the door for exploring RNA methylation in the biogenesis and function of the miRNAs. Future research will pay increasing attention on the understanding of biological functions of epigenetic changes (or marks) during germ cell development. Finally, specific miRNAs in spermatozoa or seminal plasma will be exploited as potential biomarkers for male factor infertility. The annotation of the miRNAs and the elucidation of their regulating mechanisms in pathogenesis will provide insight into the etiology of male sterility and infertility. Together, uncovering these questions will shed new light on the pivotal roles of miRNA in spermatogenesis and fertility.
Abbreviations
- Aal:
-
Aalign
- Ad:
-
Dark type A
- AGO:
-
Argonaute protein
- ALKBH5:
-
alkB homolog5
- Apr:
-
Apair
- As:
-
A single
- ATF1:
-
Activating transcription factor 1
- bFGF:
-
Basic fibroblast growth factor
- DGCR8:
-
DiGeorge syndrome critical region 8
- DMRT1:
-
Doublesex and mab-3 related transcription factor 1
- Drosha:
-
Drosha ribonuclease III
- Dsc1:
-
Desmocollin-1
- E2F1:
-
E2F transcription factor 1
- ETV5:
-
ETS variant 5
- EXP5:
-
Exportin5
- FOXO1:
-
Forkhead box protein O1
- FSH:
-
Follicle-stimulating hormone
- FTO:
-
Fat mass and obesity-associated protein (FTO)
- GDNF:
-
Glial cell line-derived factor
- GFRα1:
-
GDNF family receptor alpha-1
- GLI3:
-
GLI family zinc finger3
- HNRNPA2B1:
-
Heterogeneous nuclear ribonucleoprotein A2/B1
- Hsf2:
-
Heat shock factor2
- LH:
-
Luteinizing hormone
- METTL3:
-
Methyltransferase like 3
- miRISC:
-
miRNA-induced silencing complex
- miRNA:
-
Micro RNA
- mTOR:
-
Mechanistic target of rapamycin
- PLZF:
-
Promyelocytic leukemia zinc finger gene
- Prm1:
-
Protamine 1
- RA:
-
Retinoic acid
- SR-BI:
-
Scavenger receptor class B type I
- SSCs:
-
Spermatogonial Stem Cells
- STAT3:
-
Signal transducer and activator of transcription 3
- Stra8:
-
Retinoic acid 8
- TPs:
-
Transition proteins
References
Kanatsu-Shinohara M, Shinohara T. Spermatogonial stem cell self-renewal and development. Annu Rev Cell Dev Biol. 2013;29:163–87.
Miller MP, Amon A, Unal E. Meiosis I: when chromosomes undergo extreme makeover. Curr Opin Cell Biol. 2013;25:687–96.
Rathke C, Baarends WM, Awe S, Renkawitz-Pohl R. Chromatin dynamics during spermiogenesis. Biochim Biophys Acta. 2014;1839:155–68.
Guyonnet B, Dacheux F, Dacheux JL, Gatti JL. The epididymal transcriptome and proteome provide some insights into new epididymal regulations. J Androl. 2011;32:651–64.
Kimmins S, Sassone-Corsi P. Chromatin remodelling and epigenetic features of germ cells. Nature. 2005;434:583–9.
Papaioannou MD, Nef S. microRNAs in the testis: building up male fertility. J Androl. 2010;31:26–33.
da Cruz I, Rodriguez-Casuriaga R, Santinaque FF, Farias J, Curti G, Capoano CA, et al. Transcriptome analysis of highly purified mouse spermatogenic cell populations: gene expression signatures switch from meiotic-to postmeiotic-related processes at pachytene stage. BMC Genomics. 2016;17:294.
Zhu Z, Li C, Yang S, Tian R, Wang J, Yuan Q, et al. Dynamics of the transcriptome during human spermatogenesis: predicting the potential key genes regulating male gametes generation. Sci Rep. 2016;6:19069.
Brennecke J, Hipfner DR, Stark A, Russell RB, Cohen SM. bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell. 2003;113:25–36.
Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303:83–6.
Ambros V. MicroRNA pathways in flies and worms: growth, death, fat, stress, and timing. Cell. 2003;113:673–6.
Tan T, Zhang Y, Ji W, Zheng P. miRNA signature in mouse spermatogonial stem cells revealed by high-throughput sequencing. Biomed Res Int. 2014;2014:154251.
Liu Y, Niu MH, Yao CC, Hai YN, Yuan QQ, Liu Y, et al. Fractionation of human spermatogenic cells using STA-PUT gravity sedimentation and their miRNA profiling. Scientific Reports. 2015;5:8084.
Yao CC, Liu Y, Sun M, Niu MH, Yuan QQ, Hai YA, et al. MicroRNAs and DNA methylation as epigenetic regulators of mitosis, meiosis and spermiogenesis. Reproduction. 2015;150:R25–34.
Khazaie Y, Nasr Esfahani MH. MicroRNA and male infertility: a potential for diagnosis. Int J Fertil Steril. 2014;8:113–8.
Wang L, Xu C. Role of microRNAs in mammalian spermatogenesis and testicular germ cell tumors. Reproduction. 2015;149:R127–137.
Kotaja N. MicroRNAs and spermatogenesis. Fertil Steril. 2014;101:1552–62.
Bentwich I, Avniel A, Karov Y, Aharonov R, Gilad S, Barad O, et al. Identification of hundreds of conserved and nonconserved human microRNAs. Nat Genet. 2005;37:766–70.
Friedman RC, Farh KKH, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105.
John B, Enright AJ, Aravin A, Tuschl T, Sander C, Marks DS. Human MicroRNA targets. PLoS Biol. 2004;2:e363.
Shomron N, Levy C. MicroRNA-biogenesis and Pre-mRNA splicing crosstalk. J Biomed Biotechnol. 2009;2009:594678.
Ramalingam P, Palanichamy JK, Singh A, Das P, Bhagat M, Kassab MA, et al. Biogenesis of intronic miRNAs located in clusters by independent transcription and alternative splicing. RNA. 2014;20:76–87.
Wang D, Lu M, Miao J, Li TT, Wang E, Cui QH. Cepred: Predicting the Co-Expression Patterns of the Human Intronic microRNAs with Their Host Genes. PLoS One. 2009;4(2):e4421.
Radfar MH, Wong W, Morris Q. Computational Prediction of Intronic microRNA Targets using Host Gene Expression Reveals Novel Regulatory Mechanisms. PLoS One. 2011;6(6):e19312.
Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15:509–24.
Castel SE, Martienssen RA. RNA interference in the nucleus: roles for small RNAs in transcription, epigenetics and beyond. Nat Rev Genet. 2013;14:100–12.
Ji L, Chen X. Regulation of small RNA stability: methylation and beyond. Cell Res. 2012;22:624–36.
Alarcon CR, Lee H, Goodarzi H, Halberg N, Tavazoie SF. N6-methyladenosine marks primary microRNAs for processing. Nature. 2015;519:482–5.
Cai XZ, Hagedorn CH, Cullen BR. Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. RNA. 2004;10:1957–66.
Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek SH, et al. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004;23:4051–60.
Yi R, Qin Y, Macara IG, Cullen BR. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003;17:3011–6.
Lund E, Guttinger S, Calado A, Dahlberg JE, Kutay U. Nuclear export of microRNA precursors. Science. 2004;303:95–8.
Shomron N, Levy C. MicroRNA-biogenesis and pre-mRNA splicing crosstalk. J Biomed Biotechnol. 2009.
Truscott M, Islam AB, Frolov MV. Novel regulation and functional interaction of polycistronic miRNAs. RNA. 2016;22:129–38.
Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol. 2009;10:126–39.
Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–33.
Alarcon CR, Goodarzi H, Lee H, Liu XH, Tavazoie S, Tavazoie SF. HNRNPA2B1 is a mediator of m(6)a-dependent nuclear RNA processing events. Cell. 2015;162:1299–308.
Thomas M, Lieberman J, Lal A. Desperately seeking microRNA targets. Nat Struct Mol Biol. 2010;17:1169–74.
Pasquinelli AE. MicroRNAs and their targets: recognition, regulation and an emerging reciprocal relationship. Nat Rev Genet. 2012;13:271–82.
Sood P, Krek A, Zavolan M, Macino G, Rajewsky N. Cell-type-specific signatures of microRNAs on target mRNA expression. Proc Natl Acad Sci U S A. 2006;103:2746–51.
Wang Y, Li Y, Toth JI, Petroski MD, Zhang ZL, Zhao JC. N-6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nat Cell Biol. 2014;16:191–8.
Berulava T, Rahmann S, Rademacher K, Klein-Hitpass L, Horsthemke B. N6-adenosine methylation in MiRNAs. PLoS One. 2015;10:e0118438.
Chen T, Hao YJ, Zhang Y, Li MM, Wang M, Han WF, et al. m(6)a RNA methylation is regulated by MicroRNAs and promotes reprogramming to pluripotency. Cell Stem Cell. 2015;16:289–301.
Batista PJ, Molinie B, Wang JK, Qu K, Zhang JJ, Li LJ, et al. m(6)a RNA modification controls cell fate transition in mammalian embryonic stem cells. Cell Stem Cell. 2014;15:707–19.
Geula S, Moshitch-Moshkovitz S, Dominissini D, Mansour AA, Kol N, Salmon-Divon M, et al. Stem cells. m6A mRNA methylation facilitates resolution of naive pluripotency toward differentiation. Science. 2015;347:1002–6.
Zheng GQ, Dahl JA, Niu YM, Fedorcsak P, Huang CM, Li CJ, et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell. 2013;49:18–29.
Romero Y, Meikar O, Papaioannou MD, Conne B, Grey C, Weier M, et al. Dicer1 Depletion in Male Germ Cells Leads to Infertility Due to Cumulative Meiotic and Spermiogenic Defects. PLoS One. 2011;6(10):e25241.
Yoshida S, Takakura A, Ohbo K, Abe K, Wakabayashi J, Yamamoto M, et al. Neurogenin3 delineates the earliest stages of spermatogenesis in the mouse testis. Dev Biol. 2004;269:447–58.
Suzuki H, Ahn HW, Chu T, Bowden W, Gassei K, Orwig K, et al. SOHLH1 and SOHLH2 coordinate spermatogonial differentiation. Dev Biol. 2012;361:301–12.
Korhonen HM, Meikar O, Yadav RP, Papaioannou MD, Romero Y, Da Ros M, et al. Dicer is required for haploid male germ cell differentiation in mice. PLoS One. 2011;6:e24821.
Wu QX, Song R, Ortogero N, Zheng HL, Evanoff R, Small CL, et al. The RNase III. Enzyme DROSHA is essential for MicroRNA production and spermatogenesis. J Biol Chem. 2012;287:25173–90.
Zimmermann C, Romero Y, Warnefors M, Bilican A, Borel C, Smith LB, et al. Germ Cell-Specific Targeting of DICER or DGCR8 Reveals a Novel Role for Endo-siRNAs in the Progression of Mammalian Spermatogenesis and Male Fertility. PLoS One. 2014; 9(9):e107023.
Chang YF, Lee-Chang JS, Imam JS, Buddavarapu KC, Subaran SS, Sinha-Hikim AP, et al. Interaction between microRNAs and actin-associated protein Arpc5 regulates translational suppression during male germ cell differentiation. Proc Natl Acad Sci U S A. 2012;109:5750–5.
Yang QE, Oatley JM. Spermatogonial stem cell functions in physiological and pathological conditions. Curr Top Dev Biol. 2014;107:235–67.
Naughton CK, Jain S, Strickland AM, Gupta A, Milbrandt J. Glial cell-line derived neurotrophic factor-mediated RET signaling regulates spermatogonial stem cell fate. Biol Reprod. 2006;74:314–21.
Jing SQ, Wen DZ, Yu YB, Holst PL, Luo Y, Fang M, et al. GDNF-induced activation of the Ret protein tyrosine kinase is mediated by GDNFR-alpha, a novel receptor for GDNF. Cell. 1996;85:1113–24.
Oatley JA, Brinster RL. Regulation of spermatogonial stem cell self-renewal in mammals. Annu Rev Cell Dev Biol. 2008;24:263–86.
Lee J, Kanatsu-Shinohara M, Inoue K, Ogonuki N, Miki H, Toyokuni S, et al. Akt mediates self-renewal division of mouse spermatogonial stem cells. Development. 2007;134:1853–9.
Oatley JM, Avarbock MR, Brinster RL. Glial cell line-derived neurotrophic factor regulation of genes essential for self-renewal of mouse spermatogonial stem cells is dependent on src family kinase signaling. J Biol Chem. 2007;282:25842–51.
Niu ZY, Goodyear SM, Rao S, Wu X, Tobias JW, Avarbock MR, et al. MicroRNA-21 regulates the self-renewal of mouse spermatogonial stem cells. Proc Natl Acad Sci U S A. 2011;108:12740–5.
He ZP, Jiang JJ, Kokkinaki M, Tang L, Zeng WX, Gallicano I, et al. MiRNA-20 and mirna-106a regulate spermatogonial stem cell renewal at the post-transcriptional level via targeting STAT3 and Ccnd1. Stem Cells. 2013;31:2205–17.
Moritoki Y, Hayashi Y, Mizuno K, Kamisawa H, Nishio H, Kurokawa S, et al. Expression profiling of microRNA in cryptorchid testes: miR-135a contributes to the maintenance of spermatogonial stem cells by regulating FoxO1. J Urol. 2014;191:1174–80.
Song W, Mu H, Wu J, Liao M, Zhu H, Zheng L, et al. miR-544 regulates dairy goat male germline stem cell self-renewal via targeting PLZF. J Cell Biochem. 2015;116:2155–65.
Cui N, Hao G, Zhao Z, Wang F, Cao J, Yang A. MicroRNA-224 regulates self-renewal of mouse spermatogonial stem cells via targeting DMRT1. J Cell Mol Med. 2016;20(8):1503–12.
Li M, Yu M, Liu C, Zhu H, He X, Peng S, et al. miR-34c works downstream of p53 leading to dairy goat male germline stem-cell (mGSCs) apoptosis. Cell Prolif. 2013;46:223–31.
Niu BW, Wu J, Mu HL, Li B, Wu CY, He X, et al. miR-204 regulates the proliferation of dairy goat spermatogonial stem cells via targeting to Sirt1. Rejuvenation Res. 2016;19:120–30.
Busada JT, Geyer CB. The role of retinoic acid (RA) in spermatogonial differentiation. Biol Reprod. 2016;94:10.
Zhou Q, Nie R, Li Y, Friel P, Mitchell D, Hess RA, et al. Expression of stimulated by retinoic acid gene 8 (Stra8) in spermatogenic cells induced by retinoic acid: an in vivo study in vitamin A-sufficient postnatal murine testes. Biol Reprod. 2008;79:35–42.
Huszar JM, Payne CJ. MicroRNA 146 (Mir146) modulates spermatogonial differentiation by retinoic acid in mice. Biol Reprod. 2013;88:15.
Tong MH, Mitchell D, Evanoff R, Griswold MD. Expression of Mirlet7 family microRNAs in response to retinoic acid-induced spermatogonial differentiation in mice. Biol Reprod. 2011;85:189–97.
Tong MH, Mitchell DA, McGowan SD, Evanoff R, Griswold MD. Two miRNA Clusters, Mir-17-92 (Mirc1) and Mir-106b-25 (Mirc3), Are Involved in the Regulation of Spermatogonial Differentiation in Mice. Biol Reprod. 2012;86(3):72.
Xie RY, Lin XL, Du T, Xu K, Shen HF, Wei F, et al. Targeted Disruption of miR-17-92 ImpairsMouse Spermatogenesis by Activating mTOR Signaling Pathway. Medicine. 2016;95(7):e2713.
Yang QE, Racicot KE, Kaucher AV, Oatley MJ, Oatley JM. MicroRNAs 221 and 222 regulate the undifferentiated state in mammalian male germ cells. Development. 2013;140:280–90.
Yu M, Mu H, Niu Z, Chu Z, Zhu H, Hua J. miR-34c enhances mouse spermatogonial stem cells differentiation by targeting Nanos2. J Cell Biochem. 2014;115:232–42.
Choi YJ, Lin CP, Ho JJ, He XY, Okada N, Bu PC, et al. miR-34 miRNAs provide a barrier for somatic cell reprogramming. Nat Cell Biol. 2011;13:1353–U1154.
Yuan S, Tang C, Zhang Y, Wu J, Bao J, Zheng H, et al. mir-34b/c and mir-449a/b/c are required for spermatogenesis, but not for the first cleavage division in mice. Biol Open. 2015;4:212–23.
Bao J, Li D, Wang L, Wu J, Hu Y, Wang Z, et al. MicroRNA-449 and microRNA-34b/c function redundantly in murine testes by targeting E2F transcription factor-retinoblastoma protein (E2F-pRb) pathway. J Biol Chem. 2012;287:21686–98.
Marcet B, Chevalier B, Luxardi G, Coraux C, Zaragosi LE, Cibois M, et al. Control of vertebrate multiciliogenesis by miR-449 through direct repression of the Delta/Notch pathway. Nat Cell Biol. 2011;13:693–U157.
Bjork JK, Sandqvist A, Elsing AN, Kotaja N, Sistonen L. miR-18, a member of Oncomir-1, targets heat shock transcription factor 2 in spermatogenesis. Development. 2010;137:3177–84.
Smorag L, Zheng Y, Nolte J, Zechner U, Engel W, Pantakani DVK. MicroRNA signature in various cell types of mouse spermatogenesis: Evidence for stage-specifically expressed miRNA-221, −203 and -34b-5p mediated spermatogenesis regulation. Biol Cell. 2012;104:677–92.
Kleene KC. Patterns, mechanisms, and functions of translation regulation in mammalian spermatogenic cells. Cytogenet Genome Res. 2003;103:217–24.
Pradeepa MM, Manjunatha S, Sathish V, Agrawal S, Rao MRS. Involvement of importin-4 in the transport of transition protein 2 into the spermatid nucleus. Mol Cell Biol. 2008;28:4331–41.
Meikar O, Da Ros M, Kotaja N. Epigenetic regulation of male germ cell differentiation. Subcell Biochem. 2013;61:119–38.
Dai LS, Tsai-Morris CH, Sato H, Villar J, Kang JH, Zhang JB, et al. Testis-specific miRNA-469 Up-regulated in gonadotropin-regulated testicular RNA helicase (GRTH/DDX25)-null mice silences transition protein 2 and protamine 2 messages at sites within coding region: implications of its role in germ cell development. J Biol Chem. 2011;286:44306–18.
Yu ZR, Raabe T, Hecht NB. MicroRNA Mirn122a reduces expression of the posttranscriptionally regulated germ cell transition protein 2 (Tnp2) messenger RNA (mRNA) by mRNA cleavage. Biol Reprod. 2005;73:427–33.
Liu WM, Pang RTK, Chiu PCN, Wong BPC, Lao KQ, Lee KF, et al. Sperm-borne microRNA-34c is required for the first cleavage division in mouse. Proc Natl Acad Sci U S A. 2012;109:490–4.
Zhao K, Chen YP, Yang RF, Bai Y, Li CL, Li HG, et al. miR-424/322 is downregulated in the semen of patients with severe DNA damage and may regulate sperm DNA damage. Reprod Fertil Dev. 2016;28:1598–607.
Liu T, Cheng WW, Gao YT, Wang H, Liu ZX. Microarray analysis of microRNA expression patterns in the semen of infertile men with semen abnormalities. Mol Med Rep. 2012;6:535–42.
Abu-Halima M, Hammadeh M, Schmitt J, Leidinger P, Keller A, Meese E, Backes C. Altered microRNA expression profiles of human spermatozoa in patients with different spermatogenic impairments. Fertil Steril. 2013;99:1249.
Zhou R, Wang R, Qin YF, Ji J, Xu MF, Wu W, Chen MJ, Wu D, Song L, Shen HB, Sha JH, Miao DS, et al. Mitochondria-related miR-151a-5p reduces cellular ATP production by targeting CYTB in asthenozoospermia. Sci Rep. 2015;5:17743.
Yadav RP, Kotaja N. Small RNAs in spermatogenesis. Mol Cell Endocrinol. 2014;382:498–508.
Yoshida S, Sukeno M, Nabeshima YI. A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science. 2007;317:1722–6.
Li LH, Xie T. Stem cell niche: structure and function. Annu Rev Cell Dev Biol. 2005;21:605–31.
Oatley JM, Brinster RL. The germline stem cell niche unit in mammalian testes. Physiol Rev. 2012;92:577–95.
Ortogero N, Hennig GW, Langille C, Ro S, McCarrey JR, Yan W. Computer-assisted annotation of murine Sertoli cell small RNA transcriptome. Biol Reprod. 2013;88:3.
Wainwright EN, Jorgensen JS, Kim Y, Truong V, Bagheri-Fam S, Davidson T, et al. SOX9 Regulates MicroRNA miR-202-5p/3p Expression During Mouse Testis Differentiation. Biol Reprod. 2013;89(2):34.
Yao C, Sun M, Yuan Q, Niu M, Chen Z, Hou J, et al. MiRNA-133b promotes the proliferation of human Sertoli cells through targeting GLI3. Oncotarget. 2016;7:2201–19.
Dabaja AA, Mielnik A, Robinson BD, Wosnitzer MS, Schlegel PN, Paduch DA. Possible germ cell-Sertoli cell interactions are critical for establishing appropriate expression levels for the Sertoli cell-specific MicroRNA, miR-202-5p, in human testis. Basic Clin Androl. 2015;25:2.
Papaioannou MD, Pitetti JL, Ro S, Park C, Aubry F, Schaad O, et al. Sertoli cell Dicer is essential for spermatogenesis in mice. Dev Biol. 2009;326:250–9.
Kim GJ, Georg I, Scherthan H, Merkenschlager M, Guillou F, Scherer G, et al. Dicer is required for Sertoli cell function and survival. Int J Dev Biol. 2010;54:867–75.
Ma CP, Song HB, Yu L, Guan KF, Hu PD, Li Y, et al. miR-762 promotes porcine immature Sertoli cell growth via the ring finger protein 4 (RNF4) gene. Sci Rep. 2016;6:32783.
Nicholls PK, Harrison CA, Walton KL, McLachlan RI, O’Donnell L, Stanton PG. Hormonal regulation of sertoli cell micro-RNAs at spermiation. Endocrinology. 2011;152:1670–83.
Panneerdoss S, Chang YF, Buddavarapu KC, Chen HIH, Shetty G, Wang HZ, et al. Androgen-Responsive MicroRNAs in Mouse Sertoli Cells. Plos One. 2012;7(7):e41146.
King IA, OBrien TJ, Buxton RS. Expression of the “skin-type” desmosomal cadherin DSC1 is closely linked to the keratinization of epithelial tissues during mouse development. J Investig Dermatol. 1996;107:531–8.
Leavy M, Trottmann M, Liedl B, Reese S, Stief C, Freitag B, et al. Effects of elevated beta-estradiol levels on the functional morphology of the testis - new insights. Sci Rep. 2017;7:39931.
Kumar N, Srivastava S, Burek M, Forster CY, Roy P. Assessment of estradiol-induced gene regulation and proliferation in an immortalized mouse immature Sertoli cell line. Life Sci. 2016;148:268–78.
Jiao ZJ, Yi W, Rong YW, Kee JD, Zhong WX. MicroRNA-1285 regulates 17beta-estradiol-inhibited immature boar sertoli cell proliferation via adenosine monophosphate-activated protein kinase activation. Endocrinology. 2015;156:4059–70.
Ge RS, Hardy MP. Variation in the end products of androgen biosynthesis and metabolism during postnatal differentiation of rat Leydig cells. Endocrinology. 1998;139:3787–95.
Liu H, Yang Y, Zhang L, Liang R, Ge RS, Zhang YF, et al. Basic fibroblast growth factor promotes stem Leydig cell development and inhibits LH-stimulated androgen production by regulating microRNA expression. J Steroid Biochem Mol Biol. 2014;144:483–91.
Rakoczy J, Fernandez-Valverde SL, Glazov EA, Wainwright EN, Sato T, Takada S, et al. MicroRNAs-140-5p/140-3p Modulate Leydig Cell Numbers in the Developing Mouse Testis. Biol Reprod. 2013;88(6):143.
Song HW, Wilkinson MF. Transcriptional control of spermatogonial maintenance and differentiation. Semin Cell Dev Biol. 2014;30:14–26.
Acknowledgements
We thank Dr. Bin Wu at Arizona Center for Reproductive Endocrinology and Infertility, USA for reviewing and editing the manuscript. This project is funded, in part, by the National Basic Research Program of China (973 program; No.2013CB943103), the National Natural Science Foundation of China (No. 31272439, No. 31230048 and No. 31572401), and Programs Foundation of Ministry of Education of China (No. 20130204110017).
Funding
Not applicable.
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Authors’ contribution
CXX have searched primary sources, written the manuscript. LXL, GJY, ZPF and ZWX revised and edited the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they no competing interests.
Consent for publication
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Chen, X., Li, X., Guo, J. et al. The roles of microRNAs in regulation of mammalian spermatogenesis. J Animal Sci Biotechnol 8, 35 (2017). https://doi.org/10.1186/s40104-017-0166-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40104-017-0166-4