Abstract
Mariculture has been one of the fastest-growing global food production sectors over the past three decades. With the congestion of space and deterioration of the environment in coastal regions, offshore aquaculture has gained increasing attention. Atlantic salmon (Salmo salar) and rainbow trout (Oncorhynchus mykiss) are two important aquaculture species and contribute to 6.1% of world aquaculture production of finfish. In the present study, we established species distribution models (SDMs) to identify the potential areas for offshore aquaculture of these two cold-water fish species considering the mesoscale spatio-temporal thermal heterogeneity of the Yellow Sea. The values of the area under the curve (AUC) and the true skill statistic (TSS) showed good model performance. The suitability index (SI), which was used in this study to quantitatively assess potential offshore aquaculture sites, was highly dynamic at the surface water layer. However, high SI values occurred throughout the year at deeper water layers. The potential aquaculture areas for S. salar and O. mykiss in the Yellow Sea were estimated as 52,270 ± 3275 (95% confidence interval, CI) and 146,831 ± 15,023 km2, respectively. Our results highlighted the use of SDMs in identifying potential aquaculture areas based on environmental variables. Considering the thermal heterogeneity of the environment, this study suggested that offshore aquaculture for Atlantic salmon and rainbow trout was feasible in the Yellow Sea by adopting new technologies (e.g., sinking cages into deep water) to avoid damage from high temperatures in summer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As of 2020, at least 155 million people across the globe are facing severe hunger, exacerbated by political conflicts, pandemics, and climate change (Food Security Information Network 2021; Laborde et al. 2020; Myers et al. 2017; Puma et al. 2018). To make matters worse, the world’s population is expected to reach 9.7 billion by 2050 (United Nations 2019) and will put enormous pressure on food security. Fisheries, as a key sector of food supplies (Waite et al. 2014), can provide high-quality proteins and nutrients. Since the 1990s, the production of wild capture fisheries has reached a plateau and the production of aquaculture has risen steadily (FAO 2020). Accompanying the rapid development, aquaculture is facing intensive constraints from global change, environmental pollution, and resource conflicts (for example space, fresh water and energy) (Naylor et al. 2021).
Mariculture will be one of the most promising industries to provide food in the future (Costello et al. 2020). However, most mariculture activities have mainly taken place in coastal areas where environmental impacts and resource conflicts with other uses have been accentuated in recent decades (Gentry et al. 2017b; Sarà et al. 2021). To cope with the increasing demand for food, exploring offshore aquaculture has gained increased attention in recent years (Barillé et al. 2020; Gentry et al. 2017b; Lester et al. 2018b; Thomas et al. 2019). Yet, when compared with the rapidly increasing demand for sustainable seafood, the offshore aquaculture industry is still in its infancy (Buck et al. 2017).
Initially, “offshore” aquaculture mainly referred to aquaculture activities located in open waters, some kilometers from the coast, the so-called open-ocean aquaculture (Morro et al. 2021). Recently, the definition of offshore aquaculture has been modified and re-classified according to a variety of criteria, including depth, distance from shore, and wave exposure (Holmer 2010; Lester et al. 2018a; Kapetsky 2013). There is thus inconsistency in definitions of “offshore” aquaculture, and many “offshore” aquaculture activities are relatively closer to shore and in shallower waters than the definition outlined by the Food and Agricultural Organization of the United Nations (FAO) (Froehlich et al. 2017; Lovatelli et al. 2013). Here, we use a broad definition of offshore aquaculture that includes all mariculture that is located in waters that are not directly adjacent to land (Gentry et al. 2017b).
Successfully mapping the potential of offshore aquaculture under changing climatic conditions is essential for the sustainable development of such a sector. The potential regions and yields of some mariculture species have been evaluated on a global scale (Froehlich et al. 2018; Gentry et al. 2017a). The GIS-based Multi-Criteria Evaluation (MCE) combines multiple physical, economic, and socio-ecological factors into structured models, and provides a holistic overview of the potential for a site to successfully support offshore aquaculture (FAO and World Bank 2015; Radiarta et al. 2008). Physical carrying capacity focuses on the effect of physical environmental factors and the farming systems on aquaculture, and is the primary criterion and first stage for aquaculture mapping (Brigolin et al. 2017; Sarà et al. 2018a). Species distribution models (SDMs), both correlative and mechanistic functionally based, use algorithms to evaluate and predict the distribution of species across geographic space and time, and have been widely applied in the fields of conservation planning, resource management, impact evaluation of climate change, and invasive species assessment (Austin et al. 1990; Bosch-Belmar et al. 2022; Elith and Leathwick 2009; Ferrier 2002; Guisan and Thuiller 2005; Peterson 2003; Thomas et al. 2004; Vetaas 2002). An ensemble provides a method for resolving differences among multiple SDMs for the same species (Grenouillet et al. 2011; Pikesley et al. 2013; Scales et al. 2016) and is a reliable tool for assessing the suitability of habitat for the selection of aquaculture sites (Dong et al. 2020). Recently, more studies have suggested that risk assessments at smaller spatial scales helped reveal spatio-temporal heterogeneity that can be ignored in coarse assessments (Payne et al. 2021; Pinsky 2021).
Atlantic salmon (Salmo salar Linnaeus, 1758) and rainbow trout (Oncorhynchus mykiss Walbaum, 1792) are important aquaculture species, and are widely traded all over the world. The global aquaculture production of S. salar and O. mykiss in 2018 was 2435.9 and 848.1 kilotonnes, respectively (FAO 2020). However, outdoor farming of salmon and trout in China is facing great challenges due to the lack of appropriate aquaculture areas. In 2018, an offshore platform-based fish farming facility for culturing salmon and trout, Deep Blue 1, was launched in the Yellow Sea Cold Water Mass (YSCWM), 140 nautical miles from the coast (Dong 2019; Huang et al. 2021). With the expansion of aquaculture areas and the deployment of offshore aquaculture facilities, mapping suitable offshore aquaculture areas have been one of the most important priorities for the sustainable development of the industry.
The YSCWM extending from the Liaodong Peninsula to the East China Sea covers one-third of the deep Yellow Sea (Fig. 1B). It forms in May and June, peaks in July and August, and dissipates completely in December, with extremely highly seasonal and vertical variations (Fig. 1C) (Li et al. 2016b; Oh et al. 2013; Park et al. 2011; Yu et al. 2006; Zhang et al. 2008). In summer, the sea surface temperature ranges between 18 and 24 °C, but the temperature of the three cold-water centers (at 50 m or deeper) in the YSCWM is less than 10 °C. With the strong vertical mixing of seawater in autumn, there are large areas of water temperature above 18 °C in the south YSCWM (Li et al. 2016a; Yu et al. 2006), which may affect the aquaculture suitability of the South Yellow Sea. The high dynamic thermal environment in the YSCWM creates an opportunity for farming cold-water fish by providing thermal shelter. In such a heterogeneous thermal environment, it is crucial to explore the fine-scale spatio-temporal patterns of potential aquaculture areas for salmon and trout. For achieving this goal, we developed ensemble SDMs for S. salar and O. mykiss, and calculated the suitability index (SI) for offshore aquaculture taking into account the spatio-temporal patterns in different water layers. Our study provides useful tools for mapping potential offshore aquaculture areas and emphasizes the importance of considering environmental heterogeneity during the process.
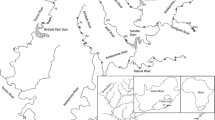
Study areas of the Salmo salar and Oncorhynchus mykiss. A The areas of data collection for developing species distribution models. The blue and orange circles represent the occurrence points for developing species distribution models (SDMs) for S. salar and O. mykiss, respectively. B Location of the Yellow Sea and the sea bottom temperature in September (mean from 1955 to 2017). C The vertical section of temperature (mean from 1955 to 2017) in the central Yellow Sea (Longitude: 123.375° E; Latitude: 33°N–38° N). The temperature data of the Yellow Sea were collected from the World Ocean Atlas 2018 dataset
Results
Model performances
The areas under the receiver-operating characteristic curve (AUC) and the true skill statistic (TSS) were used to assess the accuracy of SDMs. The four modeling algorithms, including random forest (RF), maximum entropy model (MaxEnt), support vector machine (SVM), and boosting regression tree (BRT), had high values of mean AUC (> 0.7) and mean TSS (> 0.4), indicating their good predictive capacities for S. salar and O. mykiss (Fig. 2A). Among the four modeling algorithms, the RF algorithm had the highest AUC (mean ± standard error, S. salar: 0.988 ± 0.001, O. mykiss: 0.986 ± 0.002) and TSS values (S. salar: 0.928 ± 0.002, O. mykiss: 0.923 ± 0.004). For S. salar, the BRT and MaxEnt algorithms had the lowest AUC (0.967 ± 0.002) and TSS values (0.852 ± 0.02), respectively. In addition, the MaxEnt algorithm had the lowest AUC (0.965 ± 0.002) and TSS values (0.845 ± 0.004) for O. mykiss. Overall, these four modeling algorithms are applicable in constructing the ensemble SDMs for S. salar and O. mykiss.
Model performance, variable characteristics, and distribution of areas with high suitability index (SI). A The values of the areas under the receiver-operating characteristic curve (AUC) and the true skill statistic (TSS) of the four modeling algorithms for Salmo salar (blue) and Oncorhynchus mykiss (orange). Results are expressed as mean ± 1 standard error (N = 10). B Importance of the four variables used to develop the ensemble species distribution models (SDMs) of S. salar (blue) and O. mykiss (orange). Results are expressed as mean ± 1 standard error (N = 10). C–D The mean response curves of predicted occurrence probability of S. salar (C) and O. mykiss (D) against the sea bottom temperature (SBT). Values are mean ± 1 standard error (N = 10). E–F The areas with SI ≥ 0.5 for culturing S. salar (E) and O. mykiss (F) in different water layers in the Yellow Sea every month. RF random forest, MaxEnt maximum entropy model, SVM support vector machine, BRT boosting regression tree. Four variables include sea surface temperature (SST), sea bottom temperature (SBT), sea surface salinity (SSS), and sea bottom salinity (SBS)
Variable importance and response curves
Among the four variables (sea surface temperature, SST; sea bottom temperature, SBT; sea surface salinity, SSS; and sea bottom salinity, SBS), SBT was the most important environmental variable regulating the potential distribution of S. salar (mean relative variable importance ± standard error: 0.736 ± 0.004) and O. mykiss (0.683 ± 0.009) (Fig. 2B). Response curves of S. salar and O. mykiss concerning SBT varied with algorithms (Fig. 2C, D). The mean response curves of S. salar and O. mykiss suggested that these species had higher probabilities of occurrence in areas with SBT ranging from 5 to 18 °C.
Suitability index (SI) for offshore aquaculture
SI was used to quantitatively assess the offshore aquaculture probability of each grid cell in different months and water layers. The SI values for culturing S. malar and O. mykiss in the Yellow Sea were highly dynamic in different months and different water layers (Figs. 3, 4).
The suitability index (SI) for Salmo salar at the layers of 0–15 m and 45–60 m every month in the Yellow Sea. The SI values at 0–15 m water layer and 45–60 m water layer are shown in blue and yellow, respectively. The deepening of green indicates that the values of SI in the two water layers increase isochronously
The suitability index (SI) for Oncorhynchus mykiss at the layers of 0–15 m and 30–45 m every month in the Yellow Sea. The SI values at 0–15 m water layer and 45–60 m water layer are shown in blue and yellow, respectively. The deepening of green indicates that the values of SI in the two water layers increase isochronously
For S. salar, the SI values over most areas of the Yellow Sea were less than 0.5 from June to November at the water layer of 0–15 m. At the water layer of 45–60 m, however, the SI values for culturing S. salar remained above 0.5 throughout the year (Fig. 3). The relatively high standard errors of SI values occurred at the 0–15 m layer of the northern Yellow Sea from January to April (Supplementary Figs. S1, S2). These results suggested that farming S. malar in the yellow Sea at a depth of 0–45 m was hard to succeed from June to November, but the relatively benign thermal environment at 45-60 m water layer provided a shelter during this period.
The SI values for culturing O. mykiss were relatively higher than those for S. salar. In most areas of the Yellow Sea, the SI values of O. mykiss also decreased in summer (from August to September) at the layer of 0–15 m. At the layer of 30–45 m, however, the SI values remained above 0.5 throughout the year (Fig. 4). The relatively high standard errors of SI values occurred at the 0–15 m layer in the northern Yellow Sea from January to April and at the layer of 30–45 m in March (Supplementary Figs. S3, S4).
Potential areas for offshore aquaculture
The areas with high SI values (threshold = 0.5) for culturing S. salar and O. mykiss expanded and contracted seasonally in the Yellow Sea, especially in the surface water layer of 0–15 m (Fig. 2E, F). The areas with high SI values for culturing S. salar remained stable at the layer of 45–60 m throughout the year but contracted at the other three shallower layers during the hot seasons. For culturing O. mykiss, the areas with high SI values contracted at the layer of 0–15 m from July to September, and the areas in other water layers remained relatively stable. It was worth noting that the areas with high SI values for culturing these two fish were small at the layer of 0-15 m in March (Fig. 2E, F). At this time, the regions with high SI values were mainly located in the southern Yellow Sea (Figs. 3, 4).
We counted the areas of the grid cells for estimating the offshore aquaculture potential based on the SI values (thresholds = 0.4, 0.5, and 0.6) (Table 1). For example, when the SI threshold was set as 0.5, the potential areas for offshore cultivation of S. salar and O. mykiss were 52,270 ± 3275 (95% confidence intervals, CI) and 146,831 ± 15,023 km2, respectively (Table 1).
Discussion
With extensive species occurrences data and environmental data, ensemble species distribution models (SDMs) were constructed for identifying the potential areas of offshore aquaculture for S. salar and O. mykiss. Our results show that the offshore aquaculture of the salmon and trout in the Yellow Sea is feasible taking into account the mesoscale spatio-temporal environmental heterogeneity. This study provides novel and important information for mapping offshore aquaculture areas in the aspect of physical environment, an essential element to address for aquaculture zoning.
Considering mesoscale environmental variations in mapping the potential of offshore aquaculture
SDMs can be reliable tools for selecting potential offshore aquaculture areas (Beard et al. 2020; Falconer et al. 2016). Previous studies have confirmed that SDMs were useful for identifying suitable aquaculture sites for farming Manila clam (Ruditapes philippinarum) (Dong et al. 2020) and for identifying the suitable locations for seaweeds aquaculture in the Spencer Gulf, South Australia (Wiltshire and Tanner 2020). In the present study, the high values of the areas under the receiver-operating characteristic curve (AUC) and the true skill statistic (TSS) suggest that the SDMs have a good predictive capacity for selecting suitable aquaculture areas for the cold-water fish S. salar and O. mykiss.
The following factors should consider during the selection of aquaculture areas using SDMs. First, the behavioral response of the aquaculture species is usually restricted to a limited area and then is more constricted than that of the wild species. Second, the offshore aquaculture system is generally a human-manipulated artificial system where the feed and dissolved oxygen usually are not limiting factors with artificial feed supply and aeration. On the other hand, some environmental factors which are difficult to regulate in the open ocean, such as temperature and salinity, are crucial variables for the success of aquaculture. Therefore, these uncontrollable factors should be considered. Third, SDMs for offshore aquaculture should be designed at a high resolution because most aquaculture activities were carried out in relatively small local areas, which can vary in their environmental conditions. The spatio-temporal environmental heterogeneity in the specific aquaculture areas is usually ignored in coarse assessments (Payne et al. 2021; Pinsky 2021; Sarà et al. 2018b). Finally, this study shows the potential value of farming operations that permit growing at variable depths, to ensure that fish remain within optimal temperatures.
Feasibility of offshore aquaculture of salmonids in the temperate region
Considering the spatio-temporal environmental heterogeneity, offshore aquaculture of salmon and trout is feasible in the Yellow Sea. In the present study, the suitability index (SI) was applied as an indicator to evaluate the suitability of offshore aquaculture for S. salar and O. mykiss, and quantitatively evaluate the potential farming areas. As indicated by the SI values, the deeper water layers in the Yellow Sea during the hot season provide ‘cool refugia’ for farming salmon and trout in the temperate regions. Sizes of the area with high SI values are highly variable in different water layers and months, and so closely tracking the dynamics of areas with high SI values can increase the feasibility of offshore aquaculture for salmon and trout in the Yellow Sea. In addition, the development of aquaculture facilities (e.g., Deep Blue 1) and the high demands for salmon and trout in the Chinese market are driving forces for the offshore aquaculture of salmonids in the Yellow Sea (Fishery Bureau of Ministry of Agriculture People’s Republic of China 2020; Shi et al. 2021).
Recommendations for offshore aquaculture of salmonids
The results of this study and other related work provide multiple recommendations for offshore aquaculture in the Yellow Sea.
-
1.
Sinking the net cages into the specific water layer during the hot seasons: restricted by policies, economic costs, and technologies, offshore aquaculture facilities are difficult to move around freely. Based on the results from the present study, vertical adjustments of the positions of the cages can avoid the thermal stress in summer in the Yellow Sea. It suggests that the cages can be sunk into the water layer of 45–60 m from June to November for culturing S. salar, and into the water layer of 30–45 m from August to September for culturing O. mykiss.
-
2.
Long-term environmental monitoring in the mariculture areas: environmental variations have huge impacts on mariculture, and so it is crucial to conduct in situ real-time monitoring of the aquaculture environmental factors, such as temperature (Elliott and Elliott 2010), dissolved oxygen (Oldham et al. 2019) or currents (Castro et al. 2011; Nilsen et al. 2019), for evaluating and predicting the effect of environmental factors on aquaculture and for making decisions to avoid or reduce losses in the face of environmental stresses.
-
3.
Assessing and controlling environmental impacts of offshore aquaculture: the impacts of mariculture on the surrounding environment are complex and diverse (Gentry et al. 2017b). Uneaten feed, feces, chemicals, antibiotics, and even dead fish can pollute the environment (Cao et al. 2007; Navedo and Vargas-Chacoff 2021; Price and Morris 2013; Reverter et al. 2020; Seymour and Bergheim 1991). For example, in March 2021, the mass death of farmed salmon threatened the coastal environment in Patagonia, South America (Navedo and Vargas-Chacoff 2021). Integrated multitrophic aquaculture (IMTA) may be an effective approach to mitigate pollution from aquaculture by imitating natural ecological nutrient cycling (Troell et al. 2009) overall increasing the resilience of the aquaculture systems as testified by farmers around the world under the COVID pandemic (Sarà et al. 2021). Studies such as these exploring the optimum growth conditions should be accompanied by studies to evaluate sustainability based on potential environmental damage (Sarà et al. 2018a).
-
4.
Preventing genetic risks from farmed fish escapes: large-scale escapes of farmed fish can cause serious genetic risks. From 2001 to 2009, 3.93 million S. salar, 0.98 million O. mykiss, and 1.05 million Gadus morhua had escaped in Norway (Jensen et al. 2010) and large-scale salmon escape also occurred in Chile in 2018 (Gomez-Uchida et al. 2018). Escapes of fish from aquaculture systems can affect the structure of the local food webs and gene pools (Weir and Grant 2005), and transfer aquaculture-associated diseases (Arechavala-Lopez et al. 2018; Bouwmeester et al. 2021; Jensen et al. 2010). Mandatory management, scientific assessment, high technical standards for mariculture facilities, and rigorous operation can effectively reduce the impacts of escape events (Jensen et al. 2010).
-
5.
Ensuring the health of farmed fishes: sea lice and amoebic gill disease (AGD) are common diseases in the aquaculture of salmon and trout. These diseases have serious effects on finfish health and cause economic losses (Carvalho et al. 2020; Shinn et al. 2015). Although the probability of sea lice infecting fish from their origin farm is low in offshore aquaculture sites (Kragesteen et al. 2018), the consequences of this disease should be serious due to the high density of farmed fish (Morro et al. 2021). Cutting the spread of diseases and conducting real-time health monitoring of farmed fish are essential to ensure the health and welfare of fish.
Limitations and perspectives
-
1.
Climate change: aquaculture has encountered dramatic changes in temperature, pH, dissolved oxygen, sea level, and extreme events in the face of climate change (Barange and Perry 2009; Handisyde et al. 2006; Ma et al. 2021). Climate change increases the complexity and uncertainty of aquaculture systems (Froehlich et al. 2018; Gentry et al. 2017a; Handisyde et al. 2017; Klinger et al. 2017). IPCC’s Sixth Assessment Report (AR6) has provided more reliable future environmental projections on the regional scale (Doblas-Reyes et al. 2021). Using these regional scenario projections of future climate, SDMs can be applied to assess the vulnerability of offshore aquaculture in the future for the sustainable development of offshore aquaculture.
-
2.
Mechanistic models: incorporating the mechanistic relationships between the functional traits of organisms and their environments into SDMs for aquaculture can provide more useful information (e.g., Bosch-Belmar et al. 2022; Liao et al. 2021). Thermal performance curves (TPCs) have been used to predict the potential productivity for three common aquaculture species (S. salar, Sparus aurata, and Rachycentron canadum), representing different thermal guilds across species and regions, in the context of global warming (Klinger et al. 2017). The species’ temperature tolerance range (maximum and minimum temperature) and von Bertalanffy growth function (VBGF) parameters (K and Linf) have been used to assess the relative potential productivity across countries (Gentry et al. 2017a). Based on the energy budget of an individual organism throughout its life cycle, the Dynamic Energy Budget (DEB) model can provide quantitative information for aquaculture, and has been used widely to estimate the potential for aquaculture species (Bertolini et al. 2021; Sarà et al. 2012, 2018a).
-
3.
Carrying capability: ecosystem Approach to Aquaculture (EAA) emphasizes the integration of aquaculture activities within the wider ecosystem (Corner and Aguilar-Manjarrez 2017). Our study focused on the effect of the physical environment on the mapping of aquaculture areas, while the Multi-Criteria Evaluation (MCE) also needs to fully consider the ecological carrying capability, production carrying capability, and social carrying capability in the process of actual aquaculture spatial planning (Filgueira et al. 2015). The production carrying capability can assess the maximum level of aquaculture production from a biomass or economics perspective. The assessment of ecological carrying capability considers the whole ecosystem involved in aquaculture (McKindsey et al. 2006), and the assessment of social carrying capacity limits the amount of aquaculture by mitigating potential conflicts across different uses of marine space or resources.
-
4.
Socio-economic factors: besides the physical environment, socio-economic factors also should be considered on this physical “base map”. For example, social opposition and complex and uncertain regulatory and permitting policies have hampered the development of aquaculture in the United States (Knapp and Rubino 2016; Lester et al. 2018a). Government regulations limited the commercial development of offshore aquaculture, particularly in the USA and European Union, due to disputes over its interactions with the environment, ecological damage, and conflicting uses of space resources (Gentry et al. 2017b; Ramos et al. 2017). Offshore aquaculture activities require large-scale farming systems (Naylor et al. 2021) and rely on high technology and costs for development, construction, and maintenance. Meanwhile, other marine management also need to be fully considered in the implementation of aquaculture projects, such as shipping activities, marine conservation areas, and fishing (Gentry et al. 2017b). For example, spatial conflicts between shipping and aquaculture as well as the potential for long-distance transport of pathogens are often considered in aquaculture spatial planning (Gimpel et al. 2018; Murray et al. 2002). Besides, co-location of aquaculture and other uses (e.g., offshore wind farms) could be an effective way to mitigate conflicts (Stelzenmüller et al. 2016). As the interactions between offshore aquaculture and other marine spatial management may be synergistic or conflicting (Lester et al. 2013), a holistic solution should be exploited for the sustainable development of offshore aquaculture in regions with suitable physical environments.
-
5.
Prerequisites for using aquaculture SDMs: moving to a new environment may lead to changes in a species’ niche from its original niche (Broennimann et al. 2007; Ørsted and Ørsted 2019; Torres et al. 2018), and cause transferability issue of SDMs. Previous studies have highlighted the usefulness of SDMs to predict species invasions (Peterson 2003; Peterson and Vieglais 2001; Ørsted and Ørsted 2019; Torres et al. 2018) based on “Niche conservatism” (Petitpierre et al. 2012). In the present study, we assumed that there were no niche shifts for S. salar and O. mykiss after being moved to the Yellow Sea. However, model transferability deserves more research attention.
Conclusion
To map the potential for offshore aquaculture of the salmonids in the Yellow Sea, we conducted ensemble species distribution models (SDMs) for Atlantic salmon (S. salar) and rainbow trout (O. mykiss) and assessed the suitability index (SI) for culturing these two cold-water fish in the Yellow Sea. Our results enabled us to estimate the potential areas for culturing S. salar and O. mykiss. Overall, the sizes of the area with high SI values for farming S. salar and O. mykiss are highly variable in different water layers and different months. The offshore aquaculture for salmonids should be feasible in the Yellow Sea by sinking cages into deep water to avoid damage from high temperatures. Therefore, SDMs are useful tools for estimating physical capability in aquaculture zoning. For the future expansion of offshore aquaculture, mesoscale spatio-temporal environmental heterogeneity needs to be fully considered. Notably, this study points to a potential optimum based on theoretical ecological suitability. The assumptions of niche conservation, local complex socio-economic factors and other marine spatial planning should be considered in offshore aquaculture zoning.
Materials and methods
Species occurrences data
The global distribution data of S. salar and O. mykiss was downloaded from the Global Biodiversity Information Facility (GBIF, https://www.gbif.org/) (GBIF 2021a, b), and 518,234 occurrences of S. salar and 202,792 occurrences of O. mykiss were acquired (Fig. 1A). The dataset provides a large dataset of occurrences data records around the world, covering the main distribution areas of S. salar and O. mykiss. To identify the potential areas for offshore aquaculture, only the marine occurrences data (terrestrial and freshwater occurrences data were eliminated) of target species with coordinates were retained, and the duplicate and wrong data were eliminated. To reduce the effects of sampling bias, and retain the greatest amount of useful information, the R package spThin was used to return a dataset with the maximum number of records for a thinning distance of 10 km (close to 5 arcmin resolution grids) with 100 iterations (Aiello-Lammens et al. 2015). Overall, 623 records and 181 records were used to develop species-specific species distribution models (SDMs) for S. salar and O. mykiss, respectively.
Environmental data
Dissolved oxygen, food, hydrodynamic characteristics, water temperature, and salinity are important variables for fish distribution patterns of natural populations (Pickens et al. 2021). It was evaluated whether these variables should be involved in the modeling. In the Yellow Sea Cold Water Mass (YSCWM), dissolved oxygen (DO) in most areas is above 6 mg/L throughout the year (Xin et al. 2013), which meets the physiological demands of dissolved oxygen for the cultured fish, and also aeration can be supplied in case of hypoxia in offshore aquaculture (Dong 2019). The high DO and artificial oxygen supply guarantee the requirements of farmed fish for oxygen. During the culturing period, fish usually were fed to excess with formulated diets, so food usually should not be a limiting factor. Farmed fish was limited in a net cage, and so hydrodynamic characteristics, e.g., wave and current velocity, were not involved in modeling although these factors can affect the distribution in the field. Seawater temperature is one of the biggest challenges for limiting the distribution of salmon and trout (Elliott and Elliott 2010; Mishra et al. 2021), and also for farming salmonids in the Yellow Sea. Salinity represents a critical environmental factor for the farmed fish and cannot be manipulated in the ocean, so it is potentially important for offshore aquaculture. Thus, in the present study, environmental factors including sea surface temperature (SST), sea bottom temperature (SBT), sea surface salinity (SSS), and sea bottom salinity (SBS) were selected as variables used to develop the ensemble species distribution models for mapping the potential of offshore aquaculture.
The R package sdmpredictors (Bosch and Fernandez 2021) were used to download the environmental data (from 2000 to 2014) represented on a latitude–longitude grid at 5 arcmin resolution from Bio-ORACLE v2.1 (https://www.bio-oracle.org/) for developing models (Assis et al. 2018). Dataset names included Sea water temperature (mean at mean depth), Sea surface temperature (mean), Seawater salinity (mean at mean depth), and Sea surface salinity (mean). To check the collinearity between environmental variables, the variance inflation factor (VIF) of the four environmental variables was calculated for S. salar and O. mykiss, respectively. All variables met the criteria (VIF ≤ 10) and were selected for modeling (Belsley et al. 1980).
For calculating the suitability index (SI) in different water layers in the Yellow Sea, we downloaded the monthly averaged global environmental data (from 2005 to 2017) in the NetCDF format from the World Ocean Atlas 2018 (https://www.ncei.noaa.gov/access/world-ocean-atlas-2018/), with a spatial resolution of 15 arcmins (Locarnini et al. 2018; Zweng et al. 2018), in different water depths at a 15 m interval, 0–15 m, 15–30 m, 15–45 m, and 45–60 m. Environmental data of the Yellow Sea were cropped from this global dataset to avoid the potential bias due to the scale effect between the global and regional climate models on SDMs.
All the environmental data were analyzed using R version 4.0.3 (R development Core Team 2020) with the following packages: ncdf4 (Pierce 2019), sp (Pebesma and Bivand 2022), and raster (Hijmans 2020).
Modeling and suitability index assessment
Previous studies have shown that the species’ niche may change due to the adaptation of species to a new environment, the behavior of aquaculture species, and the density-dependent effect when moving to new environments (Ørsted and Ørsted 2019; Torres et al. 2018), which may cause transferability issues of SDMs. In the present study, we aimed to identify suitable physical environments for S. salar and O. mykiss in the Yellow Sea using the correlative SDMs and developed SDMs under the assumption that the niches were conservative (Ackerly 2003).
We developed SDMs with four algorithms, including random forest (RF) (Breiman 2001), maximum entropy model (MaxEnt) (Phillips et al. 2006), support vector machine (SVM) (Cortes and Vapnik 1995), and boosting regression tree (BRT) (Elith et al. 2008) using R package sdm (Naimi and Araújo 2016). These algorithms have been widely used in SDM construction and application (Pendleton et al. 2020; Pouteau et al. 2011; Valavi et al. 2021). As the distribution information was presence-only data, using the argument “bg”, we randomly simulated pseudo-absence points for RF, SVM, and BRT in a 1:1 ratio, and also simulated 10,000 pseudo-absence points for MaxEnt (Phillips et al. 2009; Sillero and Barbosa 2021). In total, we generated 10 different pseudo-absence data sets and repeated the complete modeling and prediction process. We applied 75% of the dataset for training the models and the remaining 25% for testing using bootstrapping to generate 10 replicates for each algorithm. Meanwhile, the environmental variables were set to the “predictor” parameter.
The areas under the receiver-operating characteristic curve (AUC) and the true skill statistic (TSS) were used to assess the accuracy of SDMs (Allouche et al. 2006; Swets 1988). The AUC provides an indication of the usefulness of the models for prioritizing areas in terms of their relative importance as habitats for a particular species (Hanley and McNeil 1982). The TSS is presented as an improved measure of model accuracy, defining the average of the net prediction success rates for presence sites and absence sites (Allouche et al. 2006). The SDMs with TSS > 0.40 and AUC > 0.70 were selected for developing the weighted average ensemble models of S. salar and O. mykiss (Araújo et al. 2005; Engler et al. 2011). We used the function “getVarImp” to assess the contribution of each predictor variable to model fit, and the function “rcurve” to get the relationship between the probability of occurrence and each of the predictor variables.
The ensemble models of all four algorithms for each month and water layer were conducted by the function “ensemble”. The TSS values which have been shown to work better in previous studies were specified as a weighting factor for creating the ensemble SDMs (Alabia et al. 2016) and the environment variables were set to weight factor and predictors, respectively.
The suitability index (SI) values (between 0 and 1) were used to quantitatively assess the suitability of offshore aquaculture and estimated the potential areas for offshore aquaculture as the threshold. The SI value of each algorithm was calculated by the function “predict”, and the weighted average of SI was calculated based on the TSS values (Alabia et al. 2016, 2020; Mugo and Saitoh 2020). The higher value of SI represents a higher potential in the farming areas.
A grid was considered to have offshore aquaculture potential if a farmed species acquired habitats with SI values above the threshold only by adjusting the depth of the cage vertically throughout the year. Distinguished from previous SDMs studies, which often used a 10th percentile threshold to transform continuous habitat suitability into binary maps (Pearson et al. 2007), three different SI thresholds (0.4, 0.5, and 0.6) were used in the present study. The grid cells with SI values high the thresholds were regarded as suitable areas for offshore aquaculture. The area of each grid cell was ~ 623.75 km2 estimated by the actual area of the grid at 36°N.
All the statistical analyses on the results were performed in R version 4.0.3 (R development Core Team, 2020).
Data availability statements
The data underlying this article are available in the article and online supplementary materials.
References
Ackerly DD (2003) Community assembly, niche conservatism, and adaptive evolution in changing environments. Int J Plant Sci 164:165–184
Aiello-Lammens ME, Boria RA, Radosavljevic A, Vilela B, Anderson RP (2015) spThin: an R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 38:541–545
Alabia ID, Saitoh S-I, Igarashi H, Ishikawa Y, Usui N, Kamachi M, Awaji T, Seito M (2016) Ensemble squid habitat model using three-dimensional ocean data. ICES J Mar Sci 73:1863–1874
Alabia ID, Saitoh S-I, Igarashi H, Ishikawa Y, Usui N, Imamura Y (2020) Spatial habitat shifts of oceanic cephalopod (Ommastrephes bartramii) in oscillating climate. Remote Sens 12:521
Allouche O, Tsoar A, Kadmon R (2006) Assessing the accuracy of species distribution models: prevalence, kappa and the true skill statistic (TSS). J Appl Ecol 43:1223–1232
Araújo MB, Pearson RG, Thuiller W, Erhard M (2005) Validation of species-climate impact models under climate change. Global Change Biol 11:1504–1513
Arechavala-Lopez P, Toledo-Guedes K, Izquierdo-Gomez D, Šegvić-Bubić T, Sanchez-Jerez P (2018) Implications of sea bream and sea bass escapes for sustainable aquaculture management: a review of interactions, risks and consequences. Rev Fish Sci Aquac 26:214–234
Assis J, Tyberghein L, Bosch S, Verbruggen H, Serrão EA, De Clerck O (2018) Bio-ORACLE v2.0: extending marine data layers for bioclimatic modelling. Global Ecol Biogeogr 27:277–284
Austin MP, Nicholls AO, Margules CR (1990) Measurement of the realized qualitative niche: environmental niches of five Eucalyptus species. Ecol Monogr 60:161–177
Barange M, Perry RI (2009) Physical and ecological impacts of climate change relevant to marine and inland capture fisheries and aquaculture. In: Cochrane K, De Young C, Soto D, Bahri T (eds) Climate change implications for fisheries and aquaculture: Overview of current scientific knowledge. FAO Fisheries and Aquaculture Technical Paper. No. 530. FAO, Rome, pp 7–106
Barillé L, Le Bris A, Goulletquer P, Thomas Y, Glize P, Kane F, Falconer L, Guillotreau P, Trouillet B, Palmer S, Gernez P (2020) Biological, socio-economic, and administrative opportunities and challenges to moving aquaculture offshore for small French oyster-farming companies. Aquaculture 521:735045
Beard K, Kimble M, Yuan J, Evans KS, Liu W, Brady D, Moore S (2020) A method for heterogeneous spatio-temporal data integration in support of marine aquaculture site selection. J Mar Sci Eng 8:96
Belsley DA, Kuh E, Welsch RE (1980) Regression diagnostics: identifying influential data and sources of collinearity. Wiley, New York
Bertolini C, Brigolin D, Porporato EMD, Hattab J, Pastres R, Tiscar PG (2021) Testing a model of Pacific Oysters’ (Crassostrea gigas) growth in the Adriatic Sea: Implications for aquaculture spatial planning. Sustainability 13:3309
Bosch S, Fernandez S (2021) Package ‘sdmpredictors’. R package, pp 1–19
Bosch-Belmar M, Piraino S, Sarà G (2022) Predictive metabolic suitability maps for the thermophilic invasive hydroid Pennaria disticha under future warming Mediterranean Sea scenarios. Front Mar Sci 9:810555
Bouwmeester MM, Goedknegt MA, Pouli R, Thieltges DW (2021) Collateral diseases: aquaculture impacts on wildlife infections. J Appl Ecol 58:453–464
Breiman L (2001) Statistical modeling: the two cultures. Statist Sci 16:199–231
Brigolin D, Porporato EMD, Prioli G, Pastres R (2017) Making space for shellfish farming along the Adriatic coast. ICES J Mar Sci 74:1540–1551
Broennimann O, Treier UA, Müller-Schärer H, Thuiller W, Peterson AT, Guisan A (2007) Evidence of climatic niche shift during biological invasion. Ecol Lett 10:701–799
Buck BH, Nevejan N, Wille M, Chambers MD, Chopin T (2017) Offshore and multi-use aquaculture with extractive species: seaweeds and bivalves. In: Buck BH, Langan R (eds) Aquaculture perspective of multi-use sites in the open ocean. Springer, Cham, pp 23–69
Cao L, Wang WM, Yang Y, Yang CT, Yuan ZH, Xiong SB, Diana J (2007) Environmental impact of aquaculture and countermeasures to aquaculture pollution in China. Environ Sci Pollut Res Int 14:452–462
Carvalho LA, Whyte SK, Braden LM, Purcell SL, Manning AJ, Muckle A, Fast MD (2020) Impact of co-infection with Lepeophtheirus salmonis and Moritella viscosa on inflammatory and immune responses of Atlantic salmon (Salmo salar). J Fish Dis 43:459–473
Castro V, Grisdale-Helland B, Helland SJ, Kristensen T, Jørgensen SM, Helgerud J, Claireaux G, Farrell AP, Krasnov A, Takle H (2011) Aerobic training stimulates growth and promotes disease resistance in Atlantic salmon (Salmo salar). Comp Biochem Phys A 160:278–290
Corner RA, Aguilar-Manjarrez J (2017) Tools and models for aquaculture zoning, site selection and area management. In: Aguilar-Manjarrez J, Soto D, Brummett RD (eds) Aquaculture zoning, site selection and area management under the ecosystem approach to aquaculture. FAO, and World Bank Group, Washington, District of Columbia, USA, pp 95–145
Cortes C, Vapnik V (1995) Support-vector networks. Mach Learn 20:273–297
Costello C, Cao L, Gelcich S, Cisneros-Mata MÁ, Free CM, Froehlich HE, Golden CD, Ishimura G, Maier J, Macadam-Somer I, Mangin T, Melnychuk MC, Miyahara M, de Moor CL, Naylor R, Nøstbakken L, Ojea E, O’Reilly E, Parma AM, Plantinga AJ et al (2020) The future of food from the sea. Nature 588:95–100
Doblas-Reyes FJ, Sörensson AA, Almazroui M, Haarsma R, Hamdi R, Hewitson B, Kwon W-T, Lamptey BL, Maraun D, Stephenson TS, Takayabu I, Terray L, Turner Y, Zuo Z (2021) Linking global to regional climate change. In: Masson-Delmotte V, Zhai P, Pirani A, Connors SL, Péan C, Berger S, Caud Y, Goldfarb L, Gomis MI, Huang M, Leitzell K, Lonnoy E, Matthews JBR, Maycock TK, Waterfield T, Yelekçi O, Yu R, Zhou B (eds) Climate change 2021: The physical science basis. Contribution of working group I to the sixth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge pp 10.SM-1–10.SM-95.
Dong SL (2019) Researching progresses and prospects in large Salmonidae farming in cold water mass of Yellow Sea. Period Ocean Univ China 49:1–6 (in Chinese with English abstract)
Dong JY, Hu C, Zhang X, Sun X, Zhang P, Li WT (2020) Selection of aquaculture sites by using an ensemble model method: a case study of Ruditapes philippinarum in Moon Lake. Aquaculture 519:734897
Elith J, Leathwick JR (2009) Species distribution models: ecological explanation and prediction across space and time. Annu Rev Ecol Evol Syst 40:677–697
Elith J, Leathwick JR, Hastie T (2008) A working guide to boosted regression trees. J Anim Ecol 77:802–813
Elliott JM, Elliott JA (2010) Temperature requirements of Atlantic salmon Salmo salar, brown trout Salmo trutta and Arctic charr Salvelinus alpinus: predicting the effects of climate change. J Fish Biol 77:1793–1817
Engler R, Randin CF, Thuiller W, Dullinger S, Zimmermann NE, Araújo MB, Pearman PB, Lay GL, Piedallu C, Albert CH, Choler P, Clodea G, Lamo XD, Dirnböck T, Gégout JC, Gómez-García D, Grytnes JA, Heegaard E, Høistad F, Nogués-Bravo D et al (2011) 21st century climate change threatens mountain flora unequally across Europe. Global Change Biol 17:2330–2341
Falconer L, Telfer TC, Ross LG (2016) Investigation of a novel approach for aquaculture site selection. J Environ Manag 181:791–804
FAO (2020) The state of world fisheries and aquaculture (2020) Sustainability in action. Rome
FAO, World Bank (2015) Aquaculture zoning, site selection and area management under the ecosystem approach to aquaculture. Policy brief. FAO, Rome
Ferrier S (2002) Mapping spatial pattern in biodiversity for regional conservation planning: where to from here? Syst Biol 51:331–363
Filgueira R, Comeau LA, Guyondet T, McKindsey CW, Byron CJ (2015) Modelling carrying capacity of bivalve aquaculture: a review of definitions and methods. In: Meyers RA (ed) Encyclopedia of sustainability science and technology. Springer, New York, pp 1–33
Fishery Bureau of Ministry of Agriculture People’s Republic of China (2020) China fishery statistical yearbook. China Agriculture Press, Beijing
Food Security Information Network (2021) Global report on food crises 2021. Rome
Froehlich HE, Smith A, Gentry RR, Halpern BS (2017) Offshore aquaculture: I know it when I see it. Front Mar Sci 4:154
Froehlich HE, Gentry RR, Halpern BS (2018) Global change in marine aquaculture production potential under climate change. Nat Ecol Evol 2:1745–1750
GBIF (2021a) GBIF occurrence download. https://doi.org/10.15468/dl.bwhxu9
GBIF (2021b) GBIF occurrence download. https://doi.org/10.15468/dl.zt855u
Gentry RR, Froehlich HE, Grimm D, Kareiva P, Parke M, Rust M, Gaines SD, Halpern BS (2017a) Mapping the global potential for marine aquaculture. Nat Ecol Evol 1:1317–1324
Gentry RR, Lester SE, Kappel CV, White C, Bell TW, Stevens J, Gaines SD (2017b) Offshore aquaculture: spatial planning principles for sustainable development. Ecol Evol 7:733–743
Gimpel A, Stelzenmüllera V, Töpsch A, Galparsoro I, Gubbins M, Miller D, Murillas A, Murray AG, Pınarbaşı K, Roca G, Watret R (2018) A GIS-based tool for an integrated assessment of spatial planning trade-offs with aquaculture. Sci Total Environ 627:1644–1655
Gomez-Uchida D, Sepúlveda M, Ernst B, Contador TA, Neira S, Harrod C (2018) Chile’s salmon escape demands action. Science 361:857–858
Grenouillet G, Buisson L, Casajus N, Lek S (2011) Ensemble modelling of species distribution: the effects of geographical and environmental ranges. Ecography 34:9–17
Guisan A, Thuiller W (2005) Predicting species distribution: offering more than simple habitat models. Ecol Lett 8:993–1009
Handisyde N, Telfer TC, Ross LG (2017) Vulnerability of aquaculture-related livelihoods to changing climate at the global scale. Fish Fish 18:466–488
Handisyde N, Ross LG, Badjeck M-C, Allison EH (2006) The effects of climate change on world aquaculture: a global perspective. Aquaculture and Fish Genetics Research Programme, Stirling Institute of Aquaculture. Final Technical Report, DFID, Stirling
Hanley JA, McNeil BJ (1982) The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 143:29–36
Hijmans RJ (2020) Package ‘raster’. R package, pp 1–249
Holmer M (2010) Environmental issues of fish farming in offshore waters: perspectives, concerns and research needs. Aquacult Env Interac 1:57–70
Huang M, Yang XG, Zhou YG, Ge J, Davis A, Dong YW, Gao QF, Dong SL (2021) Growth, serum biochemical parameters, salinity tolerance and antioxidant enzyme activity of rainbow trout (Oncorhynchus mykiss) in response to dietary taurine levels. Mar Life Sci Technol 3:449–462
Jensen Ø, Dempster T, Thorstad EB, Uglem I, Fredheim A (2010) Escapes of fishes from Norwegian sea-cage aquaculture: causes, consequences and prevention. Aquacult Env Interac 1:71–83
Kapetsky JM (2013) From estimating global potential for aquaculture to selecting farm sites: perspectives on spatial approaches and trends. In: Site selection and carrying capacities for inland and coastal aquaculture. FAO/Institute of Aquaculture, University of Stirling, Expert Workshop 6–8 December 2010, Stirling, The United Kingdom of Great Britain and Northern Ireland, Rome, pp 129–146
Klinger DH, Levin SA, Watson JR (2017) The growth of finfish in global open-ocean aquaculture under climate change. Proc R Soc B 284:20170834
Knapp G, Rubino MC (2016) The political economics of marine aquaculture in the United States. Rev Fish Sci Aquac 24:213–229
Kragesteen TJ, Simonsen K, Visser AW, Andersen KH (2018) Identifying salmon lice transmission characteristics between Faroese salmon farms. Aquacult Env Interac 10:49–60
Laborde D, Martin W, Swinnen J, Vos R (2020) COVID-19 risks to global food security. Science 369:500–502
Lester SE, Costello C, Halpern BS, Gaines SD, White C, Barth JA (2013) Evaluating tradeoffs among ecosystem services to inform marine spatial planning. Mar Policy 38:80–89
Lester SE, Gentry RR, Kappel CV, White C, Gaines SD (2018a) Opinion: offshore aquaculture in the United States: untapped potential in need of smart policy. Proc Natl Acad Sci USA 115:7162–7165
Lester SE, Stevens JM, Gentry RR, Kappel CV, Bell TW, Costello CJ, Gaines SD, Kiefer DA, Maue CC, Rensel JE, Simons RD, Washburn L, White C (2018b) Marine spatial planning makes room for offshore aquaculture in crowded coastal waters. Nat Commun 9:945
Li GX, Qiao LL, Dong P, Ma YY, Xu JS, Liu SD, Liu Y, Li JC, Li P, Ding D, Wang N, Dada OA, Liu L (2016a) Hydrodynamic condition and suspended sediment diffusion in the Yellow Sea and East China Sea. J Geophys Res Oceans 121:6204–6222
Li JC, Li GX, Xu JS, Dong P, Qiao LL, Liu SD, Sun PK, Fan ZS (2016b) Seasonal evolution of the Yellow Sea cold water mass and its interactions with ambient hydrodynamic system. J Geophys Res Oceans 121:6779–6792
Liao ML, Li GY, Wang J, Marshall DJ, Hui TY, Ma SY, Zhang YM, Helmuth B, Dong YW (2021) Physiological determinants of biogeography: the importance of metabolic depression to heat tolerance. Global Change Biol 27:2561–2579
Locarnini RA, Mishonov AV, Baranova OK, Boyer TP, Zweng MM, Garcia HE, Reagan JR, Seidov D, Weathers K, Paver CR, Smolyar I (2018) World Ocean Atlas 2018, Volume 1: Temperature. NOAA Atlas NESDIS 81:1–43
Lovatelli A, Aguilar-Manjarrez J, Soto, D (2013) Expanding mariculture farther offshore: technical, environmental, spatial and governance challenges. FAO Technical Workshop, 22–25 March 2010, Orbetello, Italy. FAO Fisheries and Aquaculture Proceedings No. 24. FAO, Rome, pp 1–73
Ma CY, Zhu XL, Liao ML, Dong SL, Dong YW (2021) Heat sensitivity of mariculture species in China. ICES J Mar Sci 78:2922–2930
McKindsey CW, Thetmeyer H, Landry T, Silvert W (2006) Review of recent carrying capacity models for bivalve culture and recommendations for research and management. Aquaculture 261:451–462
Mishra BK, Khalid MA, Labh SN (2021) Assessment of water temperature on growth performance and protein profile of rainbow trout Oncorhynchus mykiss (Walbaum, 1792). J Aquacult Res Dev 12:585
Morro B, Davidson K, Adams TP, Falconer L, Holloway M, Dale A, Aleynik D, Thies PR, Khalid F, Hardwick J, Smith H, Gillibrand PA, Rey-Planellas S (2021) Offshore aquaculture of finfish: big expectations at sea. Rev Aquacult 14:791–815
Mugo R, Saitoh S-I (2020) Ensemble modelling of skipjack tuna (Katsuwonus pelamis) habitats in the western North Pacific using satellite remotely sensed data; a comparative analysis using machine-learning models. Remote Sens 12:2591
Murray A, Smith RJ, Stagg RM (2002) Shipping and the spread of infectious salmon anemia in Scottish aquaculture. Emerg Infect Dis 8:1–5
Myers SS, Smith MR, Guth S, Golden CD, Vaitla B, Mueller ND, Dangour AD, Huybers P (2017) Climate change and global food systems: potential impacts on food security and undernutrition. Annu Rev Publ Health 38:259–277
Naimi B, Araújo MB (2016) sdm: a reproducible and extensible R platform for species distribution modelling. Ecography 39:368–375
Navedo JG, Vargas-Chacoff L (2021) Salmon aquaculture threatens Patagonia. Science 372:695–696
Naylor RL, Hardy RW, Buschmann AH, Bush SR, Cao L, Klinger DH, Little DC, Lubchenco J, Shumway SE, Troell M (2021) A 20-year retrospective review of global aquaculture. Nature 591:551–563
Nilsen A, Hagen Ø, Johnsen CA, Prytz H, Zhou B, Nielsen KV, Bjørnevik M (2019) The importance of exercise: increased water velocity improves growth of Atlantic salmon in closed cages. Aquaculture 501:537–546
Oh K, Lee S, Song K, Lie H, Kim Y (2013) The temporal and spatial variability of the Yellow Sea cold water mass in the southeastern Yellow Sea, 2009–2011. Acta Oceanol Sin 32:1–10
Oldham T, Nowak B, Hvas M, Oppedal F (2019) Metabolic and functional impacts of hypoxia vary with size in Atlantic salmon. Comp Biochem Phys A 231:30–38
Ørsted IV, Ørsted M (2019) Species distribution models of the Spotted Wing Drosophila (Drosophila suzukii, Diptera: Drosophilidae) in its native and invasive range reveal an ecological niche shift. J Appl Ecol 56:423–435
Park S, Chu PC, Lee J (2011) Interannual-to-interdecadal variability of the Yellow Sea cold water mass in 1967–2008: characteristics and seasonal forcings. J Marine Syst 87:177–193
Payne MR, Kudahl M, Engelhard GH, Peck MA, Pinnegar JK (2021) Climate risk to European fisheries and coastal communities. Proc Natl Acad Sci USA 118:e2018086118
Pearson R, Raxworthy CJ, Nakamura M, Peterson AT (2007) Predicting species distributions from small numbers of occurrence records: a test case using cryptic geckos in Madagascar. J Biogeogr 34:102–117
Pebesma E, Bivand R (2022) Package ‘sp’. R package, pp 1–121
Pendleton DE, Holmes EE, Redfern J, Zhang JJ (2020) Using modelled prey to predict the distribution of a highly mobile marine mammal. Divers Distrib 26:1612–1626
Peterson AT (2003) Predicting the geography of species’ invasions via ecological niche modeling. Q Rev Biol 78:419–433
Peterson AT, Vieglais DA (2001) Predicting species invasions using ecological niche modeling: new approaches from bioinformatics attack a pressing problem. Bioscience 51:363–371
Petitpierre B, Kueffer C, Broennimann O, Randin C, Daehler C, Guisan A (2012) Climatic niche shifts are rare among terrestrial plant invaders. Science 335:1344–1348
Phillips SJ, Anderson RP, Schapire RE (2006) Maximum entropy modeling of species geographic distributions. Ecol Model 190:231–259
Phillips SJ, Dudík M, Elith J, Graham CH, Lehmann A, Leathwick J, Ferrier S (2009) Sample selection bias and presence-only distribution models: implications for background and pseudo-absence data. Ecol Appl 19:181–197
Pickens BA, Carroll R, Schirripa MJ, Forrestal F, Friedland KD, Taylor JC (2021) A systematic review of spatial habitat associations and modeling of marine fish distribution: a guide to predictors, methods, and knowledge gaps. PLoS ONE 16:e0251818
Pierce D (2019) Package ‘ncdf4’. R package, pp 1–37
Pikesley SK, Maxwell SM, Pendoley K, Costa DP, Coyne MS, Formia A, Godley BJ, Klein W, Makanga-Bahouna J, Maruca S, Ngouessono S, Parnell RJ, Pemo-Makaya E, Witt MJ (2013) On the front line: integrated habitat mapping for olive ridley sea turtles in the southeast Atlantic. Divers Distrib 19:1518–1530
Pinsky ML (2021) Mapping the climate risk for European fisheries. Proc Natl Acad Sci USA 118:e2115997118
Pouteau R, Meyer JY, Stoll B (2011) A svm-based model for predicting distribution of the invasive tree Miconia calvescens in tropical rainforests. Ecol Model 222:2631–2641
Price CS, Morris JA (2013) Marine cage culture & the environment: Twenty-first century science informing a sustainable industry. NOAA/National Centers for Coastal Ocean Science, pp 1–158
Puma MJ, Chon SY, Kakinuma K, Kummu M, Muttarak R, Seager R, Wada Y (2018) A developing food crisis and potential refugee movements. Nat Sustain 1:380–382
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org
Radiarta IN, Saitoh S-I, Miyazono A (2008) GIS-based multi-criteria evaluation models for identifying suitable sites for Japanese scallop (Mizuhopecten yessoensis) aquaculture in Funka Bay, southwestern Hokkaido, Japan. Aquaculture 284:127–135
Ramos J, Caetano M, Himes-Cornell A, dos Santos MN (2017) Stakeholders’ conceptualization of offshore aquaculture and small-scale fisheries interactions using a Bayesian approach. Ocean Coast Manage 138:70–82
Reverter M, Sarter S, Caruso D, Avarre J-C, Combe M, Pepey E, Pouyaud L, Vega-Heredía S, de Verdal H, Gozlan ER (2020) Aquaculture at the crossroads of global warming and antimicrobial resistance. Nat Commun 11:1870
Sarà G, Reid GK, Rinaldi A, Palmeri V, Troell M, Kooijman SALM (2012) Growth and reproductive simulation of candidate shellfish species at fish cages in the southern Mediterranean: dynamic energy budget (DEB) modelling for integrated multi-trophic aquaculture. Aquaculture 324–325:259–266
Sarà G, Gouhier TC, Brigolin D, Porporato EMD, Mangano MC, Mirto S, Mazzola A, Pastres R (2018a) Predicting shifting sustainability trade-offs in marine finfish aquaculture under climate change. Global Change Biol 24:3654–3665
Sarà G, Mangano MC, Johnson M, Mazzola A (2018b) Integrating multiple stressors in aquaculture to build the Blue Growth in a changing sea. Hydrobiologia 809:5–17
Sarà G, Mangano MC, Berlino M, Corbari L, Lucchese M, Milisenda G, Terzo S, Azaza MS, Babarro JMF, Bakiu R, Broitman BR, Buschmann AH, Christofoletti R, Deidun A, Dong Y, Galdies J, Glamuzina B, Luthman O, Makridis P, Nogueira AJA et al (2021) The synergistic impacts of anthropogenic stressors and COVID-19 on aquaculture: a current global perspective. Rev Fish Sci Aquac 30:1–13
Scales KL, Miller PI, Ingram SN, Hazen EL, Bograd SJ, Phillips RA (2016) Identifying predictable foraging habitats for a wide-ranging marine predator using ensemble ecological niche models. Divers Distrib 22:212–224
Seymour EA, Bergheim A (1991) Towards a reduction of pollution from intensive aquaculture with reference to the farming of salmonids in Norway. Aquacult Eng 10:73–88
Shi J, Yu W, Lu B, Cheng S (2021) Development status and prospect of Chinese deep-sea cage. J Fish China 45:992–1005 (in Chinese with English abstract)
Shinn AP, Pratoomyot J, Bron JE, Paladini G, Brooker EE, Brooker AJ (2015) Economic costs of protistan and metazoan parasites to global mariculture. Parasitology 142:196–270
Sillero N, Barbosa AM (2021) Common mistakes in ecological niche models. Int J Geogr Inf Sci 35:213–226
Stelzenmüller V, Diekmann R, Bastardie F, Schulze T, Berkenhagen J, Kloppmann M, Krause G, Pogoda B, Buck BH, Kraus G (2016) Co-location of passive gear fisheries in offshore wind farms in the German EEZ of the North Sea: a first socio-economic scoping. J Environ Manage 183:94–805
Swets JA (1988) Measuring the accuracy of diagnostic systems. Science 240:1285–1293
Thomas CD, Cameron A, Green RE, Bakkenes M, Beaumont LJ, Collingham YC, Erasmus BFN, Siqueira MFD, Grainger A, Hannah L, Hughes L, Huntley B, Jaarsveld ASV, Midgley GF, Miles L, Ortega-Huerta MA, Peterson AT, Phillips OL, Williams SE (2004) Extinction risk from climate change. Nature 427:145–148
Thomas LR, Clavelle T, Klinger DH, Lester SE (2019) The ecological and economic potential for offshore mariculture in the Caribbean. Nat Sustain 2:62–70
Torres U, Godsoe W, Buckley HL, Parry M, Lustig A, Worner SP (2018) Using niche conservatism information to prioritize hotspots of invasion by non-native freshwater invertebrates in New Zealand. Divers Distrib 24:1802–1815
Troell M, Joyce A, Chopin T, Neori A, Buschmann AH, Fang J-G (2009) Ecological engineering in aquaculture—potential for integrated multi-trophic aquaculture (IMTA) in marine offshore systems. Aquaculture 297:1–9
United Nations, Department of Economic and Social Affairs, Population Division (2019) World population prospects 2019: highlights. ST/ESA/SER.A/423
Valavi R, Elith J, Lahoz-Monfort JJ, Guillera-Arroita G (2021) Modelling species presence-only data with random forests. Ecography 44:1731–1742
Vetaas OR (2002) Realized and potential climate niches: a comparison of four Rhododendron tree species. J Biogeogr 29:545–554
Waite R, Beveridge M, Brummett R, Castine S, Chaiyawannakarn N, Kaushik S, Mungkung R, Nawapakpilai S, Phillips M (2014) Improving productivity and environmental performance of aquaculture. In Working Paper. Installment 5 of creating a sustainable food future. World Resources Institute, Washington, District of Columbia, USA
Weir LK, Grant JW (2005) Effects of aquaculture on wild fish populations: a synthesis of data. Environ Rev 13:145–168
Wiltshire KH, Tanner JE (2020) Comparing maximum entropy modelling methods to inform aquaculture site selection for novel seaweed species. Ecol Model 429:109071
Xin M, Wang BD, Ma DY (2013) Chemicohydrographic characteristics along the vertical section in the Yellow Sea. Adv Mar Sci 31:377–390 (in Chinese with English abstract)
Yu F, Zhang ZX, Diao XY, Gou JS, Tang YX (2006) Analysis of evolution of the Huanghai Sea Cold Water Mass and its relationship with adjacent water masses. Acta Oceanol Sin 28:26–34 (in Chinese with English abstract)
Zhang SW, Wang QY, Lü Y, Cui H, Yuan YL (2008) Observation of the seasonal evolution of the Yellow Sea Cold Water Mass in 1996–1998. Cont Shelf Res 28:442–457
Zweng MM, Reagan JR, Seidov D, Boyer TP, Locarnini RA, Garcia HE, Mishonov AV, Baranova OK, Weathers K, Paver CR, Smolyar I (2018) World Ocean Atlas 2018, Volume 2: salinity. NOAA Atlas NESDIS 82:1–41
Acknowledgements
We thank Ming-Ling Liao and Shu-Yang Ma of Ocean University of China, and Brian Helmuth of Northeastern University for the discussions. This study is supported by the National Natural Science Foundation of China (U1906206 and 42025604), the National Key Research and Development Program of China (Project 2019YFD0901002), and the Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Contributions
SY and YD conceived and designed the study; SY collected and analyzed the data; SY, SD, and YD wrote the manuscript; ZZ and GS provided professional advice in the modeling process; YZ, GS, and JW provided valuable insights for the study. All the authors approved the final version for submission.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Animal and human rights statement
No human or animal subjects were used during the course of this research.
Additional information
Edited by Xin Yu.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yu, SE., Dong, SL., Zhang, ZX. et al. Mapping the potential for offshore aquaculture of salmonids in the Yellow Sea. Mar Life Sci Technol 4, 329–342 (2022). https://doi.org/10.1007/s42995-022-00141-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42995-022-00141-2