Abstract
In the face of carbon, nitrogen, and phosphorus starvation, microorganisms have evolved adaptive mechanisms to maintain growth. In a previous study, we identified a protein predicted to contain acyl-CoA-binding domains in the plant pathogenic fungus Verticillium dahliae. The predicted protein, designated VdAcb1, possesses an atypical signal peptide. However, the functions of this acyl-CoA-binding protein in V. dahliae are not clear. In this research, in vivo or in vitro assays confirmed that VdAcb1 is secreted extracellularly from V. dahliae, although it does not have the typical signal peptide. Furthermore, the unconventional secretion of VdAcb1 was dependent on VdGRASP, a member of the compartment for unconventional protein secretion (CUPS). The deletion mutant strain of VdAcb1 (ΔVdAcb1) exhibited significant sensitivity to carbon starvation. RNA-seq revealed that the expression of genes related to filamentous growth (MSB2 pathway) and sugar transport were regulated by VdAcb1 under conditions of carbon starvation. Yeast one-hybrid experiments further showed that the expression of VdAcb1 was positively regulated by the transcription factor VdMsn4. The ΔVdAcb1 strain showed significantly reduced virulence on Gossypium hirsutum and Nicotiana benthamiana. We hypothesize that under conditions of carbon starvation, the expression of VdAcb1 is activated by VdMsn4 and VdAcb1 is secreted into the extracellular space. In turn, this activates the downstream MAPK pathway to enhance filamentous growth and virulence of V. dahliae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nutrient utilization is critically important for plant pathogens to infect and colonize host tissues. Successful pathogens have evolved mechanisms to manipulate the host to survive in a nutrient-poor environment. Pathogens infect plants in an environment lacking adequate nutrients, such as carbon, nitrogen, or phosphorus (Solomon et al. 2003; Thomas et al. 2001). Carbon is the most basic element to maintain biological life activities, and thus sufficient acquisition of sugars from plants is critical for successful invasion. In turn, the plant may modulate carbohydrate availability while activating defenses (Tauzin and Giardina 2014). The wheat stripe rust pathogen Puccinia striiformis f. sp. tritici (Pst) can uptake sucrose from the environment during infection by up-regulating the expression of hexose transporters and decomposing it by the unique invertase PsINV (Chang et al. 2017, 2020). Thus, nutrition is an important factor in regulating host–pathogen interactions.
Eukaryotes sense changes in the external environment and make required adjustments depending on the support by cell surface receptors and internal signal transduction networks (Carraway et al. 2003). Cell surface receptors usually couple downstream conserved signaling pathways, including the Ras/cAMP-PKA and the mitogen-activated protein kinase (MAPK) cascades, to regulate cell adaptability to stressful environments (Hamel et al. 2012; Lafon et al. 2006; Zhao et al. 2005). Signaling mucins are macromolecular transmembrane glycoproteins, which can sense external nutritional dynamics in the environment, and transmit signals to the interior of the cell (Carraway et al. 2003). In Aspergillus nidulans, starvation leads to the constitutive activation of the signaling mucin MsbA, which enhances cellulase activity by increasing the secretion of the cellobiohydrolase CbhA and improving substrate attachment, enhancing the starvation response (Brown et al. 2014). In Saccharomyces cerevisiae, nutrient deprivation activates the MAPK cascade modulating filamentous and invasive growth, and biofilm formation (Cullen et al. 2000; Karunanithi and Cullen 2012). And the starvation signal can be transmitted to transcription factors, through the cAMP-PKA signaling pathway, to regulate the expression of some genes related to nutrient utilization (Rolland et al. 2002). For example, when glucose is deficient, the transcription factor MSN2 is phosphorylated by PKA and then transferred from the cytoplasm to the nucleus, where it then regulates the expression of some metabolism-related genes in response to starvation (Mayordomo et al. 2002).
Verticillium dahliae is a notorious soil-borne phytopathogenic fungus that invades the roots and colonizes the xylem (Klosterman et al. 2009; Zhang et al. 2022). Verticillium wilt, caused by V. dahliae, is a major disease on cotton and other high value crops (Fradin and Thomma 2006). The mechanisms by which V. dahliae adapts to carbon starvation remain poorly characterized, although some starvation stress-responsive genes have been reported in V. dahliae. For example, VdNUC-2 is involved in phosphate-responsive signaling in V. dahliae and is required for the full virulence (Deng et al. 2015). The bZip transcription factor VdHapX is a conserved protein that mediates adaptation to iron starvation, affects microsclerotium formation, and is crucial for virulence of V. dahliae (Wang et al. 2018b). Detection of differentially expressed genes induced by carbon starvation, genes involved in utilization or production of acetyl-CoA, glycolysis, fatty acid biosynthesis or metabolism, and carbohydrate degradation can all be induced (Coradetti et al. 2013; Glass et al. 2013).
The acyl-CoA-binding protein (ACBP) contains a highly conserved ACBP domain, which plays an important role in acyl-CoA binding and transport (Fan et al. 2010; Xiao and Chye 2011). The basic function of the ACBP containing protein (ACB1) is to bind acyl-CoA and regulate other metabolic pathways (Schjerling et al. 1996). The acylation process is necessary for fatty acid production and oxidation. Long-chain acyl-CoA is a by-product of lipid metabolism and can also function as a signal molecule (Roduit et al. 2004). Whether in eukaryotes or prokaryotes, acetyl-CoA binding protein participation in lipid metabolism has been widely reported (Burton et al. 2005; Ferreira et al. 2017; Nie et al. 2018). Mutation of the Acb1 gene in yeast results in stearoyl-CoA accumulation in cells, and myristoyl-CoA, palmitoyl-CoA, and oleoyl-CoA were significantly reduced (Knudsen et al. 1994; Mandrup et al. 1993). MrACBP from the fungus Monascus ruber preferentially binds to myristoyl-CoA and can affect Monascus pigment biosynthesis (Long et al. 2018). In Magnaporthe oryzae, disruption of MoAcb1 causes delayed hyphal growth, significant reduction in conidial production and glycogen availability, delayed appressorium development, and reduced pathogenicity (Cao et al. 2023). Investigation of the roles of two ACBPs, PsACBP1 and PsACBP2, revealed that both are required for asexual development and virulence in Phytophthora sojae (Pei et al. 2022).
The acetyl-CoA binding protein is one of the most studied unconventionally secreted proteins. Acb1 lacks the conventional signal sequence targeting the ER. However, when yeast is cultured on media containing potassium acetate, Acb1 is secreted due to lack of nutrition (Charmpilas et al. 2020). There are two kinds of ACBP family proteins in A. oryzae, and AoAcb2 can also be secreted into the supernatant by an unconventional secretion mechanism under carbon starvation conditions (Kwon et al. 2017). Secretion of either Acb1 in S. cerevisiae or AcbA in D. discoideum are dependent on a Golgi membrane-associated protein (GRASP) (Manjithaya et al. 2010). And the secretion of Acb1 in S. cerevisiae also depends on autophagy genes and the plasma membrane t-SNARE, Sso1 (Duran et al. 2010).
In our previous research, a predicted V. dahliae acyl-CoA-binding protein (VdAcb1), lacking a signal peptide, was detected in the exoproteome of the fungus following its induction in cotton tissue culture medium (Chen et al. 2016). However, the function of VdAcb1 is not clear in V. dahliae. The objectives of this study were to: (1) confirm that VdAcb1 is secreted and to characterize the mechanism of its secretion; (2) determine whether VdAcb1 is involved in the response to starvation; and (3) ascertain the role of VdAcb1 in V. dahliae virulence.
Results
VdAcb1 encodes the acyl-CoA-binding protein
Exoproteome analysis of V. dahliae Vd991 induced by cotton medium identified a putative acyl-CoA-binding protein (VEDA_01681, Gene-ID in VdLs.17 genome: VDAG_00497). Based upon the conservation of amino acid residues in this protein and in the acyl-CoA-binding domain of this protein with other fungal ACBPs (Fig. S1A), some of which have been structurally and functionally characterized (Teilum et al. 2005), the protein was designated as VdAcb1. CD-Search of the NCBI revealed that VdAcb1 has an acyl-CoA-binding protein domain (ACBP, pfam00887, from S-6 to V-84) of 83 amino acids (Fig. 1A). The VdAcb1 CDS encodes a protein of 104 amino acids with a relative molecular weight of 11.29 kDa and an isoelectric point of 5.15 (http://www.expasy.org), consistent with the size of ACBPs of other species (Teilum et al. 2005). The 545 bp VdAcb1 gene contains three exons of 98, 71 and 143 bp and two introns of 156 and 81 bp (Fig. S1A). To further examine the evolutionary relationship of VdAcb1 in different fungi, BLASTp was performed at NCBI, along with sequence alignment, and phylogenetic tree construction with the VdAcb1 orthologs from Ascomycotina. The phylogeny revealed that VdAcb1 shared 98% identity to Verticillium spp and 70% to Colletotrichum spp, but was more distantly related to Pyricularia spp. and Fusarium spp, with 61% and 58% identities, respectively (Fig. 1B and S1B). VdAcb1 was previously detected in the exoproteome of V. dahliae (Chen et al. 2016), suggesting that VdAcb1 can be secreted. However, SignalP predictions resulted in no evidence of a signal peptide at the N-terminus of VdAcb1 (with scores of 0.9995, above the threshold of 0.5) (Fig. S1C). Furthermore, the SecretomeP 2.0 prediction suggested that VdAcb1 may be an unconventional secreted protein (with SecP scores of 0.937, above the threshold of 0.5) (Fig. S1D). These results suggested that VdAcb1 encodes a typical acyl-CoA-binding protein and the unconventional secreted protein was also conserved in the Ascomycotina.
Bioinformatic analysis of the Verticillium dahliae VdAcb1. A Structure of the VdAcb1 protein as determined in a conserved domain search of NCBI. ACBP, acyl-CoA-binding protein domain. Numbers indicate amino acid positions demarcating the signal peptide region and conserved acyl-CoA-binding protein (ACBP) domain. B Phylogenetic analysis of ACBPs from V. dahliae and other fungi in the Ascomycotina. Yellow box represents the genus Verticillium. Sequence IDs represent the GenBank (https://www.ncbi.nlm.nih.gov/genbank/) accession number of the different acyl-CoA-binding proteins in Ascomycotina
VdAcb1 is an unconventional secreted protein and its secretion is dependent on VdGRASP
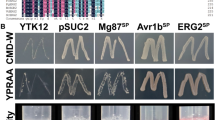
To verify the absence of signal peptide activity at the N-terminus of VdAcb1, a yeast signal sequence trap assay was employed. The region encoding the polypeptide sequence of 40 amino acids at the N-terminus of VdAcb1 was fused to the pSUC2 vector, which carries an invertase gene without the signal peptide, to generate pSUC2-VdAcb1N40. While strain YTK12::pSUC2-VdAcb1N40 containing recombinant plasmids grew normally on CMD-W medium containing sucrose as the sole carbon source, YTK12::pSUC2-VdAcb1N40 could not secrete invertase extracellularly, and therefore could not grow properly on YPRAA medium, which contains raffinose as the only carbon source (Fig. 2A). As predicted by SignalP, the result indicated that the N-terminus of VdAcb1 does not possess a signal peptide for typical secretion through Golgi–ER systems.
VdAcb1 is an unconventionally secreted protein and its secretion depends on VdGRASP in Verticillium dahliae. A Analysis of the function of N-terminal peptide of VdAcb1 by a yeast signal trap assay. The sequence of the 40 aa N-terminal of VdAcb1 was fused in frame to the invertase sequence in the vector pSUC2 and transformed into yeast strain YTK12. The YTK12 stain and YTK12 with empty pSUC2 vector were used as the negative controls. The YTK12 strain carrying the pSUC2 vector fused with the signal peptide of the oomycete effector Avr1b was used as the positive control. Four yeast strains were cultured on YPRAA and CMD-W media. B Detection of VdAcb1-HA in both mycelia and cultural filtrate of wild type (Vd991), ΔVdGRASP, and the ΔVdATG1 strains. The mycelia and culture filtrate proteins of the indicated strains were extracted and immunoblot analyses were carried out using anti-HA and anti-GADPH antibodies. C Observation of onion epidermal cells infected with V. dahliae strains WT::VdAcb1-GFP, ΔVdGRASP::VdAcb1-GFP, ΔVdATG1:: VdAcb1-GFP, WT::VdEG1-GFP (positive control), WT::GFP and (negative controls), respectively. Images were taken 5 days after infection at 25 °C using a confocal microscope with an excitation wavelength of 488 nm and emission of 510 nm for GFP. Scale bar = 50 μm
To examine whether VdAcb1 could be secreted from V. dahliae, an HA-tag was ligated to the C-terminus of VdAcb1 and expressed in the wild-type strain Vd991. Thus, the secretion of VdAcb1 was verified by western blotting of the culture filtrate of the WT::VdAcb1-HA overexpressing strain with an anti-HA antibody (Fig. 2B). VdAcb1-HA was detected in the mycelia and culture filtrate of WT::VdAcb1-HA strain. But the control GAPDH was only found in the mycelia, and not in the culture filtrate (Fig. 2B). To further confirm whether VdAcb1 is secreted in vivo, we examined the subcellular location of VdAcb1 using an onion epidermal cell system. To this end, VdAcb1 was GFP-tagged in the wild-type background to produce the WT::VdAcb1-GFP strain. As shown in Fig. 2C, the green fluorescence of the VdAcb1-GFP fusion was mainly distributed at the onion epidermal cell walls like the positive control VdEG1, which is a secreted protein in V. dahliae that possesses a signal peptide (Gui et al. 2017). However, the green fluorescence of the negative control WT::GFP was randomly distributed in the mycelia of V. dahliae. (Fig. 2C). Together, these results demonstrate that VdAcb1 is secreted extracellularly by V. dahliae as an unconventional secreted protein without a signal peptide.
In S. cerevisiae (Bruns et al. 2011), Pichia pastoris (Manjithaya et al. 2010) and Dictyostelium discoideum (Kinseth et al. 2007), Acb1 is secreted in an ER–Golgi independent manner. In S. cerevisiae, the secretion of Acb1 depends on the Golgi reassembly and stacking protein (GRASP), which can form a novel membrane compartment called CUPS (compartment for unconventional protein secretion) (Bruns et al. 2011). Thus, we constructed the VdGRASP deletion mutant (VdGRASP, VEDA_09322, Gene-ID in VdLs.17 genome: VDAG_03042) to determine whether the secretion of VdAcb1 requires the participation of VdGRASP in V. dahliae. Furthermore, the VdAcb1-HA and VdAcb1-GFP constructs were transformed into the ΔVdGRASP strain, respectively. The results showed that VdAcb1-HA was not detected in the culture filtrate of ΔVdGRASP as compared to the wild-type (Fig. 2B). Moreover, the green fluorescence was not detected on the onion inner epidermal cell walls infected by the ΔVdGRASP::VdAcb1-GFP strain (Fig. 2C). Taken together with the findings of VdAcb1-HA localization, these results indicate that the unconventional secretion of VdAcb1 relies on VdGRASP.
Autophagy proteins (ATGs) are also involved in the secretion of Acb1 in S. cerevisiae, which can fuse with recycling endosomes to release cargo (Manjithaya et al. 2010). Thus, we also overexpressed VdAcb1 in the ΔVdATG1 background (VdATG1, VEDA_06789, Gene-ID in VdLs.17 genome: VDAG_05745). VdAcb1 was detected in the culture filtrate of ΔVdATG1::VdAcb1-HA (Fig. 2B). And further GFP fluorescence was also observed clearly on the onion epidermal cell walls following incubation with ΔVdATG1::VdAcb1-GFP and WT::VdAcb1-GFP (Fig. 2C). Therefore, the secretion pathway of VdAcb1 in V. dahliae is independent of VdATG1, and differentiated from that of S. cerevisiae. In general, we conclude that the extracellular release of VdAcb1 depends on VdGRASP but not VdATG1.
VdAcb1 participates in the carbon starvation response of V. dahliae
To determine the function of VdAcb1 in V. dahliae, we constructed the VdAcb1 gene deletion mutants (ΔVdAcb1) and complemented strains (EC). The colony morphology and diameter of the ΔVdAcb1 did not differ from the wild-type (Vd991) on PDA and CM media (Fig. S2A and B). The number of conidia produced by different strains grown on PDA plates for 9 days revealed that the Vd991, ΔVdAcb1, and complemented strains (EC) produced similar amounts of conidia (Fig. S2C). Therefore, VdAcb1 did not affect the growth of V. dahliae under nutrient-rich culture conditions.
Starvation can induce the secretion of Acb1 in S. cerevisiae (Cruz-Garcia et al. 2018). To examine whether the effect of VdAcb1 on carbon source utilization is related to the starvation response of V. dahliae, sucrose at different concentration gradients was used to simulate the process of continuously amplifying the starvation signal of V. dahliae. The growth defects in the ΔVdAcb1 strain were gradually ameliorated with decreasing sucrose concentrations (from 1 × (30 g/L), 1/4 × (7.5 g/L) to 1/8 × (3.75 g/L) sucrose), and growth was comparable between the wild-type strain and ΔVdAcb1 strains in the zero sucrose media (Fig. 3A, B). Moreover, we analyzed the expression levels of VdAcb1 under varying concentrations of sucrose containing Czapeck-Dox medium, finding that starvation stress induces the expression of VdAcb1 and the transcriptional level of VdAcb1 was highest under the sucrose-free condition (Fig. 3C). These results indicated that starvation can complement the growth defects of ΔVdAcb1, and demonstrate the role of VdAcb1 in enhancing the adaptability of V. dahliae under nutritional stress. As V. dahliae colonizes plants within the nutrition-poor xylem tissue, where polysaccharides such as pectin and cellulose serve as a primary carbon source (Klosterman et al. 2011; Zhang et al. 2022), we examined the growth of wild-type (Vd991), ΔVdAcb1, and complemented strains (EC) on media supplemented with sucrose, pectin, cellulose, and lignin as carbon sources. The colony diameters of ΔVdAcb1 were reduced by 13%, 3%, 4% and 6% relative to wild-type, respectively (Fig. 3D, E). The growth defect of ΔVdAcb1 on the polysaccharide medium (pectin, cellulose and lignin) was significantly reduced compared to that of sucrose (Fig. 3D, E), a result similarly observed by reducing sucrose concentration (Fig. 3A, B). Therefore, we examined the expression of VdAcb1 under different carbon starvation conditions in V. dahliae. The expression of VdAcb1 induced by pectin and cellulose was significantly higher than that induced by sucrose (Fig. 3F). These results suggested that VdAcb1 may be involved in the response of V. dahliae to starvation during infection.
VdAcb1 positively regulates utilization of carbon sources of Verticillium dahliae. Radial growth (A) and diameter (B) of the wild type (Vd991), ΔVdAcb1 and complemented (EC) strains on Czapek salt medium containing different concentrations of sucrose for 12 days at 25 °C. C RT-qPCR analysis of VdAcb1 expression in Czapek salt medium containing different concentrations of sucrose. Error bars represent the standard deviation between triplicate experiments. Statistical significance was determined by one-way ANOVA with Tukey’s multiple comparisons test. Growth phenotype (D) and diameter (E) of Vd991, ΔVdAcb1, and complemented (EC) strains were analyzed on Czapek salt medium supplemented with sucrose (30 g/L), pectin (10 g/L), cellulose (10 g/L) and lignin (10 g/L) as carbon sources for 12 days at 25 °C. F RT-qPCR analysis of VdAcb1 expression in Czapek salt medium supplemented with sucrose, pectin, and cellulose as carbon sources. The statistical significance of B, E and F was calculated by an unpaired student t-test. *, ** and *** represent significance at P < 0.05, P < 0.01 and P < 0.001, respectively. The error bars of B and E represent the standard deviation of three biological replicates. The error bars of F represent the standard deviation between triplicate experiments. A and D scale bar = 20 mm
RNA-seq revealed that VdAcb1 could regulate the expression of starvation response-related genes
The defective growth of the ΔVdAcb1 strain under starvation conditions with polysaccharides as carbon sources was less than that under nutrient-rich media with sucrose as carbon source (Fig. 3D,E). Therefore, we compared differentially expression of genes (DEGs) in the Vd991 and ΔVdAcb1 strains treated with pectin and sucrose, respectively (Fig. 4A). MSB2, a signaling mucin, can induce the expression of cell-adhesion flocculin Flo11 through the MAPK pathway to regulate the filamentous growth (FG) in fungi (Cullen and Sprague 2012). In the wild-type strain Vd991, the FG pathway, including the MSB2-related genes and FLO11-related genes, were responsive to starvation (pectin) treatment (WT-P vs WT-S), but under the same conditions, these genes in ΔVdAcb1 showed the opposite trend in their expression (ΔVdAcb1-P vs WT-P) (Fig. 4B). This suggested that VdAcb1 can positively regulate the FG pathway in relation to the starvation response of V. dahliae.
VdAcb1 regulates the expression of starvation response genes in Verticillium dahliae. A Analysis of differential gene expression (DEGs) in VdAcb1 regulatory region by comparing expression of the wild-type grown on pectin medium (WT-P) versus wild type grown on sucrose medium (WT-S), the ΔVdAcb1 strain grown on pectin medium (ΔVdAcb1-P) versus the ΔVdAcb1 strain grown on sucrose medium (ΔVdAcb1-S), ΔVdAcb1-S vs WT-S and ΔVdAcb1-P vs WT-P. All samples were collected after 5 days of incubation at 25 °C. B Fold-change of differentially expressed MSB2-related genes and FLO11-related genes in the filamentous growth pathway. The gene IDs in Vd991 are shown on the right side of the colored blocks. The fold-change represents the value of the log2 ratio. C Expression pattern of sugar transport-related proteins in four comparison groups. The annotations corresponding to each group of color blocks are shown on the right. The gene ID corresponding to the sugar transport-related proteins are shown in Table S2. The fold change represents the value of the log2ratio
Pathogens can use sugar transporters to access nutrients from hosts (Chen et al. 2010; Sutton et al. 2007). Among the DEGs, we also found that some genes related to sugar transport (gene ID in Table S2) changed regularly under induction conditions (Fig. 4C). These genes were up-regulated in the wild-type strain Vd991 in response to pectin treatment as compared to the sucrose treatment (WT-P vs WT-S), but down-regulated in ΔVdAcb1 strain in response to pectin compared to the wild-type strain (ΔVdAcb1-P vs WT-P). This indicated that the starvation conditions examined could improve the sugar transport capacity of V. dahliae for energy metabolism; however, the sugar transport-related genes in the ΔVdAcb1 strain were down-regulated compared to the wild-type, even in response to the same carbon sources (ΔVdAcb1-P vs WT-P and ΔVdAcb1-S vs WT-S). Thus, VdAcb1 affects sugar transport processes in V. dahliae. In the summary, VdAcb1 can regulate the filamentous growth and sugar transport capacity during the starvation response by regulating the expression of starvation response-related genes.
VdAcb1 participates in the carbon starvation through the VdMsb2 pathway
Transcriptome analysis showed that VdAcb1 could regulate the expression of MSB2-pathway genes under carbon starvation (Fig. 4B). In order to further verify the results of the transcriptome, we cultured the wild type, ΔVdAcb1, and EC strains on the Czapek medium with sucrose or polysaccharides (pectin or cellulose) as carbon sources. In the ΔVdAcb1 strain, the fold change in the expression of VdMsb2 pathway genes in response to pectin and cellulose induction (ΔVdAcb1-pectin/ΔVdAcb1-sucrose, ΔVdAcb1-cellulose/ΔVdAcb1-sucrose) were significantly lower than that of the wild type (Vd991-pectin/Vd991-sucrose, Vd991-cellulose/Vd991-sucrose) (Fig. 5A, B). These results demonstrated that VdAcb1 positively regulates the VdMsb2 pathway in response to carbon starvation.
The Verticillium dahliae VdAcb1 participates in the carbon starvation responses through the VdMsb2 pathway. Expression analysis of VdMsb2-pathway genes in pectin (A) and cellulose medium (B). Wild-type (Vd991) and ΔVdAcb1 strains were cultured on Czapek salt medium supplemented with sucrose and polysaccharide (pectin or cellulose) for 4 days. RT-qPCR analysis of the relative expression of VdMsb2-pathway genes in different strains induced by polysaccharide (pectin or cellulose) compared to that induced by sucrose. VdEF-1α was used as an endogenous control for gene expression analysis. The statistical significance of A and B was calculated by an unpaired student t-test. *, ** and *** represent significance at P < 0.05, P < 0.01 and P < 0.001, respectively. C Yeast one-hybrid assay of VdMsn4 binding to the VdAcb1 promoter. The STRE elements of VdAcb1 promoter (Top). The yeast strains containing gene-pGADT7 (VdMsn2 and VdMsn4) vector and pAbAi vector containing VdAcb1 promoter were cultured on the MDO (SD–Leu) with 500 ng/mL AbA (Below). Growth can be observed in those strains with positive interactions. The pGADT7-empty vector was used as a negative control. D The expression of VdAcb1 in the wild type Vd991, ΔVdMsn2 and ΔVdMsn4 strains was detected under starvation induction conditions (Czapek salt medium). The expression level of VdAcb1 in the Vd991 was set to 1 and VdEF-1α was used as an endogenous control for gene expression analysis. Statistical significance was calculated by an unpaired student t-test with ** representing significance at P < 0.01. The error bars of A, B and D represent the standard deviation between triplicate experiments
We further evaluated deletion mutants of VdMsb2-regulated FG signaling pathway, including the membrane-localized sensors VdSho1 and VdMsb2, and the MAPK pathway adaptor Vst50. The growth diameter of each of these mutants on different carbon sources examined was significantly smaller than that of wild type (Fig. S3). These results suggested that the VdMsb2-involved FG signaling pathway can affect the growth of V. dahliae under starvation conditions. In S. cerevisiae, starvation can induce the expression and secretion of Acb1 (Cruz-Garcia et al. 2018). To explore whether VdAcb1 is regulated by the VdMsb2-involved FG signaling pathway under starvation conditions, the relative expression levels of VdAcb1 were examined in the above deletion mutant strains. The expression of VdAcb1 in the ΔVdSho1, ΔVdMsb2 and ΔVdVst50 were all significantly lower than in the wild-type under the induction conditions of either pectin or carbon source starvation (Fig. S3C). In S. cerevisiae, transcription factors MSN2 and MSN4 bind specifically to the stress response elements (STRE) 5′-AGGGG or 5′-GGGGA (Stewart-Ornstein et al. 2013) and thus participate in a variety of emergency responses including starvation stress (Gasch et al. 2000). We found three STRE binding sites also located at − 535, − 1085 and − 1742 bp upstream of VdAcb1 (Fig. 5C). Yeast one-hybrid (Y1H) assays showed that VdMsn2 and VdMsn4 can bind to the VdAcb1 promoter (Fig. 5C). However, the binding of VdMsn4 was stronger than with VdMsn2 (Fig. 5C). The expression of VdAcb1 in the different mutant strains was also examined under starvation induction conditions. The expression of VdAcb1 was positively regulated by the transcription factor VdMsn4. Compared to ΔVdMsn4, VdMsn2 did not significantly regulate the expression of VdAcb1, probably due to the functional redundancy between VdMsn2 and VdMsn4 (Fig. 5D). In summary, when V. dahliae is induced by starvation, VdAcb1 is induced in its expression by transcription factors VdMsn2 and VdMsn4 and secreted extracellularly to regulate the VdMsb2-pathway. In turn, this further activates the starvation stress response in V. dahliae.
VdAcb1 is required for virulence in V. dahliae
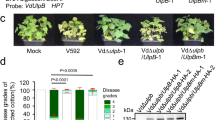
To investigate the role of VdAcb1 during the V. dahliae infection, we first examined the expression of VdAcb1 in cotton roots in a series of incubation times by RT-qPCR. This revealed that the expression of VdAcb1 was up-regulated during infection and reached the maximum at 3 days post-inoculation (Fig. S4A). To determine whether VdAcb1 plays a role in the initial penetration phase of V. dahliae, we analyzed the penetration ability on cellophane membranes. The penetration ability of the ΔVdAcb1 strain was not affected at 4 days, compared to the wild-type strain (Fig. S4B). Additionally, cotton (Gossypium hirsutum cv. Junmian 1) and tobacco (Nicotiana benthamiana) were inoculated with spore suspensions of wild type, ΔVdAcb1 and VdAcb1-complemented strains at the same concentration. The typical symptoms of Verticillium wilt caused by the wild-type strain Vd991, such as the yellowing of plant leaves, were reduced in plants inoculated with the ΔVdAcb1 strain (Fig. 6A, C), and correspondingly, the browning of cotton vascular tissue was also reduced (Fig. 6A). The qPCR assays of fungal biomass in cotton and tobacco revealed that the fungal biomass was significantly lower in plants inoculated with the ΔVdAcb1 strain than in the plants inoculated with the wild-type strain (Fig. 6B, C). In conclusion, VdAcb1 is an important virulence factor in V. dahliae.
Pathogenicity analysis of VdAcb1 deletion strains on cotton and Nicotiana benthamiana. A Disease symptoms (top) and discoloration in longitudinal sections (bottom) of cotton (Gossypium hirsutum cv. Junmian 1) inoculated with sterile water (CK), wild-type (Vd991), ΔVdAcb1, and the VdAcb1 complemented strains (EC#1 and #2). Plants were observed 21 days after inoculation. C Disease phenotypes of 5-weeks-old N. benthamiana plants inoculated with wild-type (Vd991), ΔVdAcb1, and the VdAcb1 complemented strains (EC#1 and #2). Plants were examined at 21 days after inoculation. Fungal biomass in cotton (B) and N. benthamiana (D) inoculated with different strains was anlayzed by qPCR. Statistical significance was calculated by an unpaired student t-test with * and *** representing significance at P < 0.05 and P < 0.001, respectively. Error bars represent the standard deviation between triplicate experiments
Discussion
Although acetyl-CoA binding protein is one of the most studied unconventional secreted proteins in eukaryotes (Charmpilas et al. 2020), its extracellular function in plant pathogenic fungi has not been elucidated. In this study, we found that VdAcb1 was induced by transcription factor VdMsn4 under starvation conditions and secreted into the extracellular space by an unconventional secretion. We also demonstrated that it can regulate the carbon starvation response during V. dahliae-plant interactions by VdMsb2-related MAPK pathway to enhance the downstream filamentous growth. VdAcb1 enhances the nutritional utilization ability of V. dahliae and is also a critical virulence factor as demonstrated on two host plants.
Secreted proteins are usually defined as a class of proteins with a signal peptide that can be secreted extracellularly through the ER–Golgi pathway. However, proteomics has revealed that there are a large number of proteins in fungal secretomes that lack signal peptides (González-Fernández et al. 2015; Rampitsch et al. 2013). Unconventionally-secreted proteins in plant pathogens are involved in pathogenic processes in a variety of ways. For example, Magnaporthe oryzae oxysterol-binding protein-related proteins (MoORPs) are detected in intercellular fluids of barley plants following M. oryzae infection (Chen et al. 2022). MoORPs act as PAMP molecules to regulate plant innate immunity and promote the virulence of M. oryzae (Chen et al. 2022). VdSOD1 and VdTrx1 lack signal peptides yet are important in scavenging intracellular and extracellular reactive oxygen species in V. dahliae (Tian et al. 2021, 2023). In this study, we determined that VdAcb1 lacks a typical signal peptide and the 40 amino acids at its N-terminus also do not have the secretory activity since this sequence failed to direct the secretion of sucrose invertase to extracellular space in the yeast signal sequence trap system (Fig. 2A). The secretion of VdAcb1 was detected in the culture supernatant of V. dahliae and green fluorescence was observed on the onion cell walls after co-incubation of onion cells and the V. dahliae strain overexpressing the VdAcb1-GFP fusion (Fig. 2B, C). Taken together, these results provide concrete evidence that VdAcb1 is an unconventionally secretion protein.
Studying the function of unconventionally secreted proteins in plant—microbe interactions is an emerging topic (Miura and Ueda 2018). The peroxisome protein, sterol carrier protein 2 (Scp2), was detected in the extracellular fluid of infected maize and may play a role in inhibiting the competitors in the extracellular fluid (Krombach et al. 2018). Our previous work showed that the exoproteome of V. dahliae strain Vd991 contains 99 proteins without signal peptides, suggesting that many more unconventionally secreted proteins probably play important roles in host–pathogen interactions (Chen et al. 2016; Wang et al. 2018a). The research on the pathogenic function of unconventionally secreted proteins in V. dahliae is still in the initial stages. For example, VdIsc1 can suppress salicylate-mediated innate immunity in planta by hydrolyzing isochorismate, a precursor of salicylate (Liu et al. 2014). VdSOD1 and VdTrx1, without signal peptides, can promote V. dahliae infection by scavenging reactive oxygen species (Tian et al. 2021, 2023). In this study, we further demonstrated the importance of the unconventionally secreted protein VdAcb1 during V. dahliae infection, and its role in responding to host carbon starvation (Fig. 3D, E) is likely critically important for its contribution to virulence (Fig. 6A, C). This work further sheds light on unconventionally secreted proteins in plant-pathogen interactions and additional focus on these proteins in plant-pathogen interactions may be fruitful.
Research on molecular mechanisms of the unconventional secretory pathway is still in its infancy. The unconventional secretory pathway is commonly categorized into four types (Rabouille et al. 2012). Among these, type III secretion is more studied in fungi. In type III unconventional protein secretion, the cargo can be integrated into the lumen of the cell inner membrane, and when fused with the plasma membrane, it mediates the transport of the cargo to the extracellular space. The membrane compartment called CUPS (compartment for unconventional protein secretion) is marked by Grh1 (homologous proteins of GRASP) in S. cerevesiae (Bruns et al. 2011). Acb1 and SOD1 of S. cerevisiae lacking signal peptides can be captured into CUPS under starvation and released into the extracellular space (Cruz-Garcia et al. 2017). However, some of the mechanisms of unconventional secretion of Acb1 can be differentiated among fungi. For example, in S. cerevisiae, P. pastoris and Cryptococcus neoformans, the secretion of Acb1 is not only affected by GRASP, but also regulated by autophagy-related genes (Duran et al. 2010; Manjithaya et al. 2010; Xu et al. 2016). In Aspergillus oryzae, the secretion of AoAcb1 is dependent on t-SNARE AoSso1, but independent of the autophagy-related protein AoAtg1 (Kwon et al. 2017). In this research, we observed the secretion efficiency of VdAcb1 in VdGRASP and VdATG1 deletion strains by combining in vivo live-cell imaging and in vitro western blots. The secretion of VdAcb1 in V. dahliae was regulated by VdGRASP but not VdATG1 (Fig. 2B, C). In addition, our previous research had shown that the unconventional secretion of VdSOD1 requires the participation of VdGRASP and the secretion of VdTrx1 is affected by VdVps36 (Tian et al. 2021, 2023). Therefore, V. dahliae also secretes some proteins important for pathogenicity by unconventional secretion, and the types of unconventional secretion may also be differentiated based on the protein types.
Acyl-CoA-binding proteins represent an evolutionarily conserved protein family with an acyl-CoA binding domain, which has high specificity and affinity for long-chain fatty acyl-CoA esters (LCACoAs) (Neess et al. 2015). The reports on the function of Acb1 in fungi mainly focus on growth and development. In Schizosaccharomyces pombe, Acb1 is essential for maintaining mitochondrial tubular morphology, mitochondrial respiration, and remodeling lipid droplets to promote cell survival under nutrient-rich conditions (He et al. 2022). MoAcb1 is involved in conidial germination and appressorium formation in M. oryzae (Cao et al. 2023). However, deletion of VdAcb1 did not affect growth of V. dahliae under optimal nutritional conditions (Fig. S2A, B), nor did it affect spore yield (Fig. S2C) or penetration ability (Fig. S4B). These results indicated that unlike reported functions for Acb1 in different fungi, VdAcb1 is not involved in the metabolic processes that influence a variety of functions in V. dahliae.
In S. cerevisiae and A. oryzae, Acb1 can be secreted into the extracellular space under carbon starvation (Cruz-Garcia et al. 2018; Kwon et al. 2017). In this study, we confirmed that VdAcb1 positively regulated the response of V. dahliae to carbon starvation stress when sucrose concentrations decreased or when pectin or cellulose was supplied as the carbon source (Fig. 3A–F). It is well established that V. dahliae colonizes the plant xylem, a nutrition-poor niche (Klosterman et al. 2011), and thus VdAcb1 may play a critical role during the colonization of this rather unique niche. Furthermore, RNA-seq analysis indicate that VdAcb1 could affect the expression of genes in the FG pathway, such as the MSB2 and FLO11 related genes, and also regulate the expression of sugar transport related genes (Fig. 4B, C). In fungal pathogens, filamentous growth and biofilm formation are required for virulence (Lo et al. 1997; Nobile and Mitchell 2006). Under nutrient-limited conditions, the adhesion molecule flocculin Flo11p can enhance cell–cell adhesion and cell surface properties to promote the filamentous growth of S. cerevisiae (Lo and Dranginis 1996). MSB2, as a cell membrane-localized signal mucin, senses a variety of external environmental changes and regulates different activities through the downstream coupling of MAPK signaling pathways (Carraway et al. 2003) including starvation signaling (Abdullah and Cullen 2009). We examined the expression levels of VdMsb2-related MAPK pathway genes under pectin and cellulose simulated starvation conditions and found that the expression levels of these genes were significantly reduced under starvation induction in the ΔVdAcb1 strain (Fig. 5A, B). Thus, VdAcb1 can positively regulate the growth of V. dahliae in a carbon-starved environment through the VdMsb2-related MAPK pathway. But how does VdAcb1 regulate this pathway? Since VdAcb1 is a secreted protein, we speculate whether there will be an interaction between VdAcb1 and cell membrane-localized signal mucin to regulate the starvation response, which needs to be further verified.
The activated/repressed genes specific to certain stress conditions in organisms are known collectively as “the environmental stress response genes” (ESR) (Garcia-Gimeno and Struhl 2000). The coordinated activation of hundreds of induced ESR (iESR) genes is regulated by two functionally redundant transcription factors Msn2 and Msn4 (Berry and Gasch 2008). We identified three STRE binding sites in the promoter of VdAcb1 that were bound by VdMsn2 and VdMsn4 (Fig. 5C). The Y1H analysis in this study further confirmed that VdMsn4 could directly bind to the promoter of VdAcb1 (Fig. 5C). Previous research also determined that the subcellular localization of Msn2 and Msn4 is regulated by the availability of carbon sources: if cells are grown in glucose, Msn2/4 is mainly located in the cytoplasm, but if grown in carbon-deficient medium, Msn2/4 is mainly located in the nucleus (Mayordomo et al. 2002). Once entering the nucleus, Msn2/4 regulates the expression of genes related to carbon source utilization such as a UDP-glucose pyrophosphorylase (Ugp1) (Yi and Huh 2015). Moreover, Msn2/4 were found to be regulated by the major signaling pathways including the cAMP-PKA to respond to starvation (Garmendia-Torres et al. 2007; Lee et al. 2008). In this study, we also observed that VdMsn4 can promote the expression of VdAcb1 (Fig. 5D). This further supports a role of VdAcb1 in carbon source utilization. Thereafter, VdAcb1 is secreted extracellularly via an unconventional secretion mechanism. Then it can promote theVdMsb2-related MAPK pathway to enhance the starvation response during infection (Fig. 7). Therefore, we speculate that VdAcb1 acts to link the starvation signaling functions of the cAMP-PKA and VdMsb2-related MAPK pathways.
Proposed model of VdAcb1 function during Verticillium dahliae-host interactions. Under the carbon starvation induction, the expression of VdAcb1 is induced by VdMsn4. VdAcb1 is then secreted extracellularly, under the guidance of VdGRASP, and it can then regulate the filamentous growth by the VdMsb2 pathway to enhance the virulence of V. dahliae
In summary, V. dahliae faces carbon starvation stress and carbon resources dominated by complex polysaccharides such as cellulose and pectin during infection. Starvation signals can be transmitted to transcription factor VdMsn4. And the expression of VdAcb1 can be activated by VdMsn4 and secreted extracellularly by an unconventional secretion pathway dependent on the Golgi membrane-associated protein VdGRASP. VdAcb1 is able to regulate the VdMsb2-related MAPK signaling pathway, thereby enhancing nutrient utilization and filamentous growth. Therefore, VdAcb1 is important for V. dahliae in adapting to a carbon starved environment during infection and thus contributes to virulence.
Materials and methods
Bioinformatics analysis
The DNA and cDNA sequences of VdAcb1 were identified in the genome database of Vd991 (Chen et al. 2018). Proteins homologous to VdAcb1 were searched by BLASTp on the NCBI database. Bioedit v7.2.0 (http://www.mbio.ncsu.edu/bioedit/) was used for the sequence alignment of homologous proteins. The phylogenetic tree of VdAcb1 was prepared using neighbor-joining in MEGA 6.0 (Tamura et al. 2013). The conserved domain of VdAcb1 was predicted using SMART (Letunic et al. 2021). SignalP v. 5.0 (Almagro Armenteros et al. 2019) was used in signal peptide prediction (Almagro Armenteros et al. 2019) and SecretomeP v.2.0 (Bendtsen et al. 2005) was used to predict unconventional secretion of VdAcb1.
Fungal strains and vector construction
All transgenic strains of V. dahliae in this study were constructed using an ATMT method. For the preparation of single gene knockout strains, the upstream and downstream (1.1–1.5 kb) sequences of the target genes were amplified from the wild-type Vd991 genomic DNA and integrated into EcoRI and XbaI restriction endonuclease sites of pGKO2-Hyg (Zhou et al. 2013), respectively. The plasmid was transferred into Vd991, and transformants were screened with 200 μg/mL cefotaxime, 50 μg/mL hygromycin, and 200 μg/mL 5-fluoro-2′-deoxyuridine. To construct the complementation vector for VdAcb1, the native promoter (1 kb) and terminator (0.5 kb) of the target gene was ligated to a XbaI-linearized pCOM vector (Zhou et al. 2013). The recombinant plasmid was transformed into the ΔVdAcb1 strain. To obtain the overexpression transformants of VdAcb1, the cDNA of VdAcb1 was inserted into the KpnI site of the pCOM-GFP vector (Tian et al. 2021) and SacI/XbaI sites of the pCOM-TrpC vector with an HA tag (Zhou et al. 2013). The recombinant plasmids were transferred into Vd991, ΔVdGRASP and ΔVdATG1. Positive overexpression strains were selected on PDA medium containing 200 μg/mL cefotaxime and 50 μg/ mL geneticin (G418). DNA of different strains was extracted and verified to contain the correct insertions by PCR. The primers used are shown in Table S1.
Colony growth phenotype and conidiation assays
The spore concentration of the different V. dahliae strains was adjusted to 5 × 106 conidia/mL using a hemocytometer. Two-microliters of the conidial suspension were placed on PDA and CM media and incubated at 25 °C for 9 days. Different carbon sources (sucrose 30 g/L, pectin 10 g/L, cellulose 10 g/L, lignin 10 g/L, glucose 10 g/L and fructose 10 g/L) and sucrose of different concentrations as 1 × (30 g/L), 1/4 × (7.5 g/L), 1/8 × (3.75 g/L) and zero in Czapek salts (NaNO3 3 g/L, MgSO4⋅7H2O 0.5 g/L, KCl 0.5 g/L, FeSO4⋅7H2O 0.01 g/L, K2HPO4 1 g/L, Agar 18 g/L) were used for carbon source utilization analysis. The colony diameters were measured at 9 days.
To analyse the conidiation, the wild type, ΔVdAcb1, and complementary strains were cultured on PDA at 25 °C. Three blocks of mycelia were cut out from the edge of a 9 day-old colony by using the 5 mm-diameter puncher. After shaking in sterile water for 1 min, the number of conidia was counted using a hemocytometer.
Yeast signal sequence trap system and infection of onion epidermal cells
Functional validation of the predicted signal peptide was performed as described previously (Jacobs et al. 1997). The N-terminal sequence of VdAcb1 was fused into the pSUC2 vector at the EcoRI and XhoI sites. The recombinant plasmid (VdAcb1N40) was transformed into yeast competent YTK12 and cultured on CMD-W (lacking tryptophan) medium. The yeast strain YTK12 with functional signal peptide of Avr1b was the positive control. The YTK12 strain and transformants with pSUC2 alone were negative controls. The growth of these strains on YPRAA (2% raffinose) medium was used to assay activity of the signal peptide.
The spore suspensions of the GFP-tagged VdAcb1 overexpressing strains (WT::VdAcb1-GFP, ΔVdGRASP::VdAcb1-GFP, ΔVdATG1::VdAcb1-GFP) were adjusted to 1 × 107 conidia/mL. The strains with GFP and fused proteins (VdEG1-GFP) were the negative and positive controls, respectively. The onion inner epidermis was immersed in the respective spore suspensions for 30 min. The onion inner epidermis was overlayed on 1% water agar for 5 days at 25 °C. GFP fluorescence in the epidermal cells was observed by laser confocal microscopy (ZEISS, LSM 880) at emission and excitation wavelengths of 488 and 510 nm, respectively.
Protein extraction and western blot
The HA-tagged VdAcb1 overexpressing strains of V. dahliae (WT::VdAcb1-HA, ΔVdGRASP::VdAcb1-HA, ΔVdATG1::VdAcb1-HA) were grown in CM liquid medium for 4 days at 25 °C. Total proteins were extracted from the collected mycelia by using RIPA lysate (Beyotime, P0013K) with 1 mM phenylmethylsulfonyl fluoride (PMSF) (Solarbio, P0100). To extract the secreted proteins, the supernatant of each of the respective strains was mixed with phenol, and the secreted proteins were precipitated with methanol containing 100 mM ammonium acetate. The secreted proteins were collected following centrifugation at 4000 rpm for 10 min.
To detect protein secretion, SDS–PAGE (10% separation gel and 5% concentrated gel) was prepared for western blotting. Proteins were transferred to Immobilon-P transfer membranes (Merck Millipore) at 110 V for 1 h. The membrane was blocked in 5% (w/v) nonfat dry milk for 1 h. The membrane was incubated with anti-HA (Abmart, M20003, 1:5000) and anti-GAPDH (Abclonal, AC035, 1:5000) primary antibody for 1 h. The secondary goat anti-mouse IgG-HRP (TransGen Biotech, HS201-01, 1:5000) was used for detection with an Immobilon Western Chemiluminescent HRP Substrate (Merck Millipore).
RNA extraction and RT-qPCR
To detect expression of VdAcb1 during the cotton root infection process, total RNA was extracted from cotton roots collected at 0 day, 1 day, 3 days, 5 days, 7 days, and 9 days after inoculations with Vd991 using a total RNA Miniprep kit (Aidlab). To detect the function of VdAcb1 in the starvation pathway, different strains were cultured in Czapek liquid medium (NaNO3, 3 g/L; K2HPO4, 1 g/L; MgSO4⋅7H2O, 0.5 g/L; KCl, 0.5 g/L; FeSO4, 0.01 g/L) with sucrose (10 g/L), pectin (10 g/L) and cellulose (10 g/L) as carbon sources and Czapek salt (no carbon sources) for 4 days at 25 °C. RNA was extracted from the collected mycelia.
The cDNA was reverse transcribed using TransScript II One-Step gDNA Removal and cDNA Synthesis SuperMix (TransGen Biotech). The qPCR was carried out using 2 × Taq Pro Universal SYBR qPCR Master Mix (Vazyme). The amplification reaction process included pre-denaturation at 95 °C for 10 min, followed by 40 cycles of 95 °C denaturation for 15 s, 60 °C annealing for 30 s, and 72 °C extension for 30 s. The expression of VdAcb1 at different infection times was detected by using the cotton 18S rRNA (Gh18S) as the internal reference gene. The V. dahliae VdEF-1α gene was used as endogenous reference gene to examine the expression of genes related to starvation pathways. The relative transcript levels of the above genes were analyzed by the 2−∆∆Ct method (Livak and Schmittgen 2001). Primers for the RT-qPCR expression analyses are listed in Table S1.
RNA-Seq
The wild-type and ΔVdAcb1 strains of V. dahliae were cultured in Czapek medium containing sucrose (10 g/L) and pectin (10 g/L) respectively for 4 days at 25 °C. Total RNA was extracted for RNA-seq using total RNA Miniprep kit (Aidlab, RN38). Transcriptome data analyses were conducted as previously described (Zhang et al. 2018).
Penetration and pathogenicity analysis
The conidial densities of the wild-type, ΔVdAcb1, and VdAcb1-complemented (EC) strains were adjusted to 1 × 107 conidia/mL using a hemocytometer. The spore suspensions were incubated on MM with a sterilized cellophane membrane. The respective strains were cultured for 4 days on MM at 25 °C, the membrane was removed, and the MM plates were incubated for four additional days at 25 °C.
Evaluation of strain virulence was made according to the method described by (Sun et al. 2021). Three-week-old cotton plants (Gossypium hirsutum cv. Junmian 1) were immersed in the spore suspensions (1 × 107 conidia/mL) of the different strains for 30 min. The inoculated cotton was replanted in the soil. Four-week-old tobacco (N. benthamiana) plants were inoculated by root-irrigation (Zhang et al. 2019). For each strain within each experiment, 20 cotton plants or five tobacco plants were inoculated. The disease symptoms on cotton and tobacco were observed 21 days after inoculation and the vascular discoloration in cotton root and stem was scored. The inoculation experiments were repeated once.
For analyses of fungal biomass, DNA was extracted from the root–stem junction of cotton and tobacco plants 21 days after inoculation. The V. dahliae EF-1α (VdEF-1α) was quantified by qPCR using the cotton 18S rRNA (Gh18S), and N. benthamiana EF-1α (NbEF-1α) as internal reference genes. The amplification reaction process included pre-denaturation at 95 °C for 10 min, followed by 40 cycles of 95 °C denaturation for 15 s, 60 °C annealing for 30 s, and 72 °C extension for 30 s. The primers used are listed in Table S1.
Yeast one-hybrid
Yeast one-hybrid assays the replicated STRE element (AGGGG × 3) was ligated into the pAbAi vector and the respective CDS of each gene (VdMsn2 and VdMsn4) was cloned into the pGADT7 vector. Recombinant plasmids were co-transformed into Y1HGold. Detection of gene activation by transcription factors was performed using the MDO (SD–Leu) medium with 500 ng/mL AbA.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Abdullah U, Cullen PJ (2009) The tRNA modification complex elongator regulates the Cdc42-dependent mitogen-activated protein kinase pathway that controls filamentous growth in yeast. Eukaryot Cell 8(9):1362–1372. https://doi.org/10.1128/ec.00015-09
Almagro Armenteros JJ, Tsirigo KD, Sønderby, et al (2019) SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat Biotechnol 37(4):420–423. https://doi.org/10.1038/s41587-019-0036-z
Bendtsen JD, Kiemer L, Fausbøll A, Brunak S (2005) Non-classical protein secretion in bacteria. BMC Microbiol 5:58. https://doi.org/10.1186/1471-2180-5-58
Berry DB, Gasch AP (2008) Stress-activated genomic expression changes serve a preparative role for impending stress in yeast. Mol Biol Cell 19(11):4580–4587. https://doi.org/10.1091/mbc.e07-07-0680
Brown NA, Dos Reis TF, Goinski AB et al (2014) The Aspergillus nidulans signalling mucin MsbA regulates starvation responses, adhesion and affects cellulase secretion in response to environmental cues. Mol Microbiol. https://doi.org/10.1111/mmi.12820
Bruns C, McCaffery JM, Curwin AJ, Duran JM, Malhotra V (2011) Biogenesis of a novel compartment for autophagosome-mediated unconventional protein secretion. J Cell Biol 195(6):979–992. https://doi.org/10.1083/jcb.201106098
Burton M, Rose TM, Faergeman NJ, Knudsen J (2005) Evolution of the acyl-CoA binding protein (ACBP). Biochem J 392(Pt 2):299–307. https://doi.org/10.1042/bj20050664
Cao N, Zhu XM, Bao JD et al (2023) Acyl-coenzyme a binding protein MoAcb1 regulates conidiation and pathogenicity in Magnaporthe oryzae. Front Microbiol 14:1179536. https://doi.org/10.3389/fmicb.2023.1179536
Carraway KL, Ramsauer VP, Haq B, Carothers Carraway CA (2003) Cell signaling through membrane mucins. BioEssays 25(1):66–71. https://doi.org/10.1002/bies.10201
Chang Q, Liu J, Lin X et al (2017) A unique invertase is important for sugar absorption of an obligate biotrophic pathogen during infection. New Phytol 215(4):1548–1561. https://doi.org/10.1111/nph.14666
Chang Q, Lin X, Yao M et al (2020) Hexose transporter PsHXT1-mediated sugar uptake is required for pathogenicity of wheat stripe rust. Plant Biotechnol J 18(12):2367–2369. https://doi.org/10.1111/pbi.13398
Charmpilas N, Ruckenstuhl C, Sica V et al (2020) Acyl-CoA-binding protein (ACBP): a phylogenetically conserved appetite stimulator. Cell Death Dis 11(1):7. https://doi.org/10.1038/s41419-019-2205-x
Chen LQ, Hou BH, Lalonde S et al (2010) Sugar transporters for intercellular exchange and nutrition of pathogens. Nature 468(7323):527–532. https://doi.org/10.1038/nature09606
Chen JY, Xiao HL, Gui YJ et al (2016) Characterization of the Verticillium dahliae exoproteome involves in pathogenicity from cotton-containing medium. Front Microbiol 7:1709. https://doi.org/10.3389/fmicb.2016.01709
Chen JY, Liu C, Gui YJ et al (2018) Comparative genomics reveals cotton-specific virulence factors in flexible genomic regions in Verticillium dahliae and evidence of horizontal gene transfer from Fusarium. New Phytol 217(2):756–770. https://doi.org/10.1111/nph.14861
Chen MM, Yang SR, Wang J, Fang YL, Peng YL, Fan J (2022) Fungal oxysterol-binding protein-related proteins promote pathogen virulence and activate plant immunity. J Exp Bot 73(7):2125–2141. https://doi.org/10.1093/jxb/erab530
Cruz-Garcia D, Brouwers N, Duran JM, Mora G, Curwin AJ, Malhotra V (2017) A diacidic motif determines unconventional secretion of wild-type and ALS-linked mutant SOD1. J Cell Biol 216(9):2691–2700. https://doi.org/10.1083/jcb.201704056
Cruz-Garcia D, Malhotra V, Curwin AJ (2018) Unconventional protein secretion triggered by nutrient starvation. Semin Cell Dev Biol 83:22–28. https://doi.org/10.1016/j.semcdb.2018.02.021
Cullen PJ, Sprague GF Jr (2012) The regulation of filamentous growth in yeast. Genetics 190(1):23–49. https://doi.org/10.1534/genetics.111.127456
Cullen PJ, Schultz J, Horecka J, Stevenson BJ, Jigami Y, Sprague GF Jr (2000) Defects in protein glycosylation cause SHO1-dependent activation of a STE12 signaling pathway in yeast. Genetics 155(3):1005–1018. https://doi.org/10.1093/genetics/155.3.1005
Deng S, Wang CY, Zhang X, Wang Q, Lin L (2015) VdNUC-2, the key regulator of phosphate responsive signaling pathway, is required for Verticillium dahliae infection. PLoS One 10(12):e0145190. https://doi.org/10.1371/journal.pone.0145190
Duran JM, Anjard C, Stefan C, Loomis WF, Malhotra V (2010) Unconventional secretion of Acb1 is mediated by autophagosomes. J Cell Biol 188(4):527–536. https://doi.org/10.1083/jcb.200911154
Fan J, Liu J, Culty M, Papadopoulos V (2010) Acyl-coenzyme A binding domain containing 3 (ACBD3; PAP7; GCP60): an emerging signaling molecule. Prog Lipid Res 49(3):218–234. https://doi.org/10.1016/j.plipres.2009.12.003
Ferreira NS, Engelsby H, Neess D et al (2017) Regulation of very-long acyl chain ceramide synthesis by acyl-CoA-binding protein. J Biol Chem 292(18):7588–7597. https://doi.org/10.1074/jbc.M117.785345
Fradin EF, Thomma BP (2006) Physiology and molecular aspects of Verticillium wilt diseases caused by V. dahliae and V. albo-atrum. Mol Plant Pathol 7(2):71–86. https://doi.org/10.1111/j.1364-3703.2006.00323.x
Garcia-Gimeno MA, Struhl K (2000) Aca1 and Aca2, ATF/CREB activators in Saccharomyces cerevisiae, are important for carbon source utilization but not the response to stress. Mol Cell Biol 20(12):4340–4349. https://doi.org/10.1128/mcb.20.12.4340-4349.2000
Garmendia-Torres C, Goldbeter A, Jacquet M (2007) Nucleocytoplasmic oscillations of the yeast transcription factor Msn2: evidence for periodic PKA activation. Curr Biol 17(12):1044–1049. https://doi.org/10.1016/j.cub.2007.05.032
Gasch AP, Spellman PT, Kao CM et al (2000) Genomic expression programs in the response of yeast cells to environmental changes. Mol Biol Cell 11(12):4241–4257. https://doi.org/10.1091/mbc.11.12.4241
Glass NL, Schmoll M, Cate JH, Coradetti S (2013) Plant cell wall deconstruction by ascomycete fungi. Annu Rev Microbiol 67:477–498. https://doi.org/10.1146/annurev-micro-092611-150044
González-Fernández R, Valero-Galván J, Gómez-Gálvez FJ, Jorrín-Novo JV (2015) Unraveling the in vitro secretome of the phytopathogen Botrytis cinerea to understand the interaction with its hosts. Front Plant Sci 6:839. https://doi.org/10.3389/fpls.2015.00839
Gui YJ, Chen JY, Zhang DD et al (2017) Verticillium dahliae manipulates plant immunity by glycoside hydrolase 12 proteins in conjunction with carbohydrate-binding module 1. Environ Microbiol 19(5):1914–1932. https://doi.org/10.1111/1462-2920.13695
Hamel LP, Nicole MC, Duplessis S, Ellis BE (2012) Mitogen-activated protein kinase signaling in plant-interacting fungi: distinct messages from conserved messengers. Plant Cell 24(4):1327–1351. https://doi.org/10.1105/tpc.112.096156
He J, Liu K, Zheng S et al (2022) The acyl-CoA-binding protein Acb1 regulates mitochondria, lipid droplets, and cell proliferation. FEBS Lett 596(14):1795–1808. https://doi.org/10.1002/1873-3468.14415
Jacobs KA, Collins-Racie LA, Colbert M et al (1997) A genetic selection for isolating cDNAs encoding secreted proteins. Gene 198(1–2):289–296. https://doi.org/10.1016/s0378-1119(97)00330-2
Karunanithi S, Cullen PJ (2012) The filamentous growth MAPK pathway responds to glucose starvation through the Mig1/2 transcriptional repressors in Saccharomyces cerevisiae. Genetics 192(3):869–887. https://doi.org/10.1534/genetics.112.142661
Kinseth MA, Anjard C, Fuller D, Guizzunti G, Loomis WF, Malhotra V (2007) The Golgi-associated protein GRASP is required for unconventional protein secretion during development. Cell 130(3):524–534. https://doi.org/10.1016/j.cell.2007.06.029
Klosterman SJ, Atallah ZK, Vallad GE, Subbarao KV (2009) Diversity, pathogenicity, and management of Verticillium species. Annu Rev Phytopathol 47:39–62. https://doi.org/10.1146/annurev-phyto-080508-081748
Klosterman SJ, Subbarao KV, Kang S et al (2011) Comparative genomics yields insights into niche adaptation of plant vascular wilt pathogens. PLoS Pathog 7(7):e1002137. https://doi.org/10.1371/journal.ppat.1002137
Knudsen J, Faergeman NJ, Skøtt H et al (1994) Yeast acyl-CoA-binding protein: acyl-CoA-binding affinity and effect on intracellular acyl-CoA pool size. Biochem J 302(Pt 2):479–485. https://doi.org/10.1042/bj3020479
Krombach S, Reissmann S, Kreibich S, Bochen F, Kahmann R (2018) Virulence function of the Ustilago maydis sterol carrier protein 2. New Phytol 220(2):553–566. https://doi.org/10.1111/nph.15268
Kwon HS, Kawaguchi K, Kikuma T, Takegawa K, Kitamoto K, Higuchi Y (2017) Analysis of an acyl-CoA binding protein in Aspergillus oryzae that undergoes unconventional secretion. Biochem Biophys Res Commun 493(1):481–486. https://doi.org/10.1016/j.bbrc.2017.08.166
Lafon A, Han KH, Seo JA, Yu JH, d’Enfert C (2006) G-protein and cAMP-mediated signaling in aspergilli: a genomic perspective. Fungal Genet Biol 43(7):490–502. https://doi.org/10.1016/j.fgb.2006.02.001
Lee P, Cho BR, Joo HS, Hahn JS (2008) Yeast Yak1 kinase, a bridge between PKA and stress-responsive transcription factors, Hsf1 and Msn2/Msn4. Mol Microbiol 70(4):882–895. https://doi.org/10.1111/j.1365-2958.2008.06450.x
Letunic I, Khedkar S, Bork P (2021) SMART: recent updates, new developments and status in 2020. Nucleic Acids Res 49(D1):D458-d460. https://doi.org/10.1093/nar/gkaa937
Liu T, Song T, Zhang X et al (2014) Unconventionally secreted effectors of two filamentous pathogens target plant salicylate biosynthesis. Nat Commun 5:4686. https://doi.org/10.1038/ncomms5686
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔC(T) method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Lo WS, Dranginis AM (1996) FLO11, a yeast gene related to the STA genes, encodes a novel cell surface flocculin. J Bacteriol 178(24):7144–7151. https://doi.org/10.1128/jb.178.24.7144-7151.1996
Lo HJ, Köhler JR, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink GR (1997) Nonfilamentous C albicans mutants are avirulent. Cell 90(5):939–949. https://doi.org/10.1016/s0092-8674(00)80358-x
Long C, Liu M, Chen X et al (2018) The acyl-CoA binding protein affects Monascus pigment production in Monascus ruber CICC41233. 3 Biotech 8(2):121. https://doi.org/10.1007/s13205-018-1147-9
Mandrup S, Jepsen R, Skøtt H et al (1993) Effect of heterologous expression of acyl-CoA-binding protein on acyl-CoA level and composition in yeast. Biochem J 290(Pt 2):369–374. https://doi.org/10.1042/bj2900369
Manjithaya R, Anjard C, Loomis WF, Subramani S (2010) Unconventional secretion of Pichia pastoris Acb1 is dependent on GRASP protein, peroxisomal functions, and autophagosome formation. J Cell Biol 188(4):537–546. https://doi.org/10.1083/jcb.200911149
Mayordomo I, Estruch F, Sanz P (2002) Convergence of the target of rapamycin and the Snf1 protein kinase pathways in the regulation of the subcellular localization of Msn2, a transcriptional activator of STRE (stress response element)-regulated genes. J Biol Chem 277(38):35650–35656. https://doi.org/10.1074/jbc.M204198200
Miura N, Ueda M (2018) Evaluation of unconventional protein secretion by Saccharomyces cerevisiae and other fungi. Cells 7(9):128. https://doi.org/10.3390/cells7090128
Neess D, Bek S, Engelsby H, Gallego SF, Færgeman NJ (2015) Long-chain acyl-CoA esters in metabolism and signaling: role of acyl-CoA binding proteins. Prog Lipid Res 59:1–25. https://doi.org/10.1016/j.plipres.2015.04.001
Nie Z, Wang Y, Wu C et al (2018) Acyl-CoA-binding protein family members in laticifers are possibly involved in lipid and latex metabolism of Hevea brasiliensis (the para rubber tree). BMC Genom 19(1):5. https://doi.org/10.1186/s12864-017-4419-6
Nobile CJ, Mitchell AP (2006) Genetics and genomics of Candida albicans biofilm formation. Cell Microbiol 8(9):1382–1391. https://doi.org/10.1111/j.1462-5822.2006.00761.x
Pei Y, Si J, Navet N et al (2022) Two typical acyl-CoA-binding proteins (ACBPs) are required for the asexual development and virulence of Phytophthora sojae. Fungal Genet Biol 161:103695. https://doi.org/10.1016/j.fgb.2022.103695
Rabouille C, Malhotra V, Nickel W (2012) Diversity in unconventional protein secretion. J Cell Sci 125(Pt 22):5251–5255. https://doi.org/10.1242/jcs.103630
Rampitsch C, Day J, Subramaniam R, Walkowiak S (2013) Comparative secretome analysis of Fusarium graminearum and two of its non-pathogenic mutants upon deoxynivalenol induction in vitro. Proteomics 13:1913–1921. https://doi.org/10.1002/pmic.201200446
Roduit R, Nolan C, Alarcon C et al (2004) A role for the malonyl-CoA/long-chain acyl-CoA pathway of lipid signaling in the regulation of insulin secretion in response to both fuel and nonfuel stimuli. Diabetes 53(4):1007–1019. https://doi.org/10.2337/diabetes.53.4.1007
Rolland F, Winderickx J, Thevelein JM (2002) Glucose-sensing and -signalling mechanisms in yeast. FEMS Yeast Res 2(2):183–201. https://doi.org/10.1111/j.1567-1364.2002.tb00084.x
Schjerling CK, Hummel R, Hansen JK et al (1996) Disruption of the gene encoding the acyl-CoA-binding protein (ACB1) perturbs acyl-CoA metabolism in Saccharomyces cerevisiae. J Biol Chem 271(37):22514–22521. https://doi.org/10.1074/jbc.271.37.22514
Solomon PS, Thomas SW, Spanu P, Oliver RP (2003) The utilisation of di/tripeptides by Stagonospora nodorum is dispensable for wheat infection. Physiol Mol Plant Pathol 63:191–199. https://doi.org/10.1016/j.pmpp.2003.12.003
Stewart-Ornstein J, Nelson C, DeRisi J, Weissman JS, El-Samad H (2013) Msn2 coordinates a stoichiometric gene expression program. Curr Biol 23(23):2336–2345. https://doi.org/10.1016/j.cub.2013.09.043
Sun M, Zhang Z, Ren Z et al (2021) The GhSWEET42 glucose transporter participates in Verticillium dahliae infection in cotton. Front Plant Sci 12:690754. https://doi.org/10.3389/fpls.2021.690754
Sutton PN, Gilbert MJ, Williams LE, Hall JL (2007) Powdery mildew infection of wheat leaves changes host solute transport and invertase activity. Physiol Plant 129(4):787–795. https://doi.org/10.1111/j.1399-3054.2007.00863.x
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30(12):2725–2729. https://doi.org/10.1093/molbev/mst197
Tauzin AS, Giardina T (2014) Sucrose and invertases, a part of the plant defense response to the biotic stresses. Front Plant Sci 5:293. https://doi.org/10.3389/fpls.2014.00293
Thomas SW, Rasmussen SW, Glaring MA, Rouster JA, Christiansen SK, Oliver RP (2001) Gene identification in the obligate fungal pathogen Blumeria graminis by expressed sequence tag analysis. Fungal Genet Biol 33(3):195–211. https://doi.org/10.1006/fgbi.2001.1281
Teilum K, Thormann T, Caterer NR et al (2005) Different secondary structure elements as scaffolds for protein folding transition states of two homologous four-helix bundles. Proteins 59(1):80–90. https://doi.org/10.1002/prot.20340
Tian L, Li J, Huang C et al (2021) Cu/Zn superoxide dismutase (VdSOD1) mediates reactive oxygen species detoxification and modulates virulence in Verticillium dahliae. Mol Plant Pathol 22(9):1092–1108. https://doi.org/10.1111/mpp.13099
Tian L, Zhuang J, Li JJ et al (2023) Thioredoxin VdTrx1, an unconventional secreted protein, is a virulence factor in Verticillium dahliae. Front Microbiol 14:1130468. https://doi.org/10.3389/fmicb.2023.1130468
Wang J, Tian L, Zhang DD et al (2018a) SNARE-encoding genes VdSec22 and VdSso1 mediate protein secretion required for full virulence in Verticillium dahliae. Mol Plant Microbe Interact 31(6):651–664. https://doi.org/10.1094/mpmi-12-17-0289-r
Wang Y, Deng C, Tian L, Xiong D, Tian C, Klosterman SJ (2018b) The transcription factor VdHapX controls iron homeostasis and is crucial for virulence in the vascular pathogen Verticillium dahliae. Msphere 3(5):e00400-e418. https://doi.org/10.1128/mSphere.00400-18
Xiao S, Chye ML (2011) New roles for acyl-CoA-binding proteins (ACBPs) in plant development, stress responses and lipid metabolism. Prog Lipid Res 50(2):141–151. https://doi.org/10.1016/j.plipres.2010.11.002
Xu X, Zhao Y, Kirkman E, Lin X (2016) Secreted Acb1 contributes to the yeast-to-hypha transition in Cryptococcus neoformans. Appl Environ Microbiol 82(4):1069–1079. https://doi.org/10.1128/aem.03691-15
Yi DG, Huh WK (2015) PKA, PHO and stress response pathways regulate the expression of UDP-glucose pyrophosphorylase through Msn2/4 in budding yeast. FEBS Lett 589(18):2409–2416. https://doi.org/10.1016/j.febslet.2015.07.015
Zhang WQ, Gui YJ, Short DPG et al (2018) Verticillium dahliae transcription factor VdFTF1 regulates the expression of multiple secreted virulence factors and is required for full virulence in cotton. Mol Plant Pathol 19(4):841–857. https://doi.org/10.1111/mpp.12569
Zhang DD, Wang J, Wang D et al (2019) Population genomics demystifies the defoliation phenotype in the plant pathogen Verticillium dahliae. New Phytol 222(2):1012–1029. https://doi.org/10.1111/nph.15672
Zhang DD, Dai XF, Klosterman SJ, Subbarao KV, Chen JY (2022) The secretome of Verticillium dahliae in collusion with plant defence responses modulates Verticillium wilt symptoms. Biol Rev Camb Philos Soc 97(5):1810–1822. https://doi.org/10.1111/brv.12863
Zhao X, Kim Y, Park G, Xu JR (2005) A mitogen-activated protein kinase cascade regulating infection-related morphogenesis in Magnaporthe grisea. Plant Cell 17(4):1317–1329. https://doi.org/10.1105/tpc.104.029116
Zhou L, Zhao J, Guo W, Zhang T (2013) Functional analysis of autophagy genes via Agrobacterium-mediated transformation in the vascular wilt fungus Verticillium dahliae. J Genet Genom 40(8):421–431. https://doi.org/10.1016/j.jgg.2013.04.006
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2022YFE0130800, 2022YFD1400300), the National Natural Science Foundation of China (32370213, 32302327), Shandong Provincial Natural Science Foundation (ZR2020MC115), and the Agricultural Sciences Talent Program CAAS to JYC, the Agricultural Science and Technology Innovation Program grant to J-YC.
Author information
Authors and Affiliations
Contributions
JYC and LT conceived and designed the experiments. JZ, YDZ, JZ, YDZ, JJL, and WXS performed the experiments. DDZ analyzed the data. DDZ, YDZ, JZ and JJL wrote the initial draft. JYC, KVS, SJK and XFD wrote, reviewed, and edited the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All authors declare that they have no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhuang, J., Zhang, YD., Sun, WX. et al. The acyl-CoA-binding protein VdAcb1 is essential for carbon starvation response and contributes to virulence in Verticillium dahliae. aBIOTECH (2024). https://doi.org/10.1007/s42994-024-00175-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42994-024-00175-3