Abstract
Information on the presence and abundance of a species is crucial for understanding key ecological processes but also for effective protection and population management. Collecting data on cryptic species, like small mustelids, is particularly challenging and often requires the use of non-invasive methods. Despite recent progress in the development of camera trap-based devices and statistical models to estimate the abundance of unmarked individuals, their application for studying this group of mammals is still very limited. We compared direct (live-trapping) and indirect (an enclosed camera-trapping approach—the Mostela system) survey methods to estimate the population size of weasels (Mustela nivalis) inhabiting open grasslands in Northeast Poland over a period of four years. We also live-trapped voles to determine prey availability. We used a Royle–Nichols model to estimate yearly (relative) abundance from the camera-trapping data in a Bayesian framework. The total number of live-captured weasels showed a similar change over time as the relative abundance of weasels estimated using camera-trap data. Moreover, estimates of weasel abundance increased with the availability of their main prey. Our study is part of a growing body of work showing that camera traps can provide a useful non-invasive method to estimate the relative abundance of small mustelids. Moreover, a combination of data from camera traps with statistical models allowed us to track the changes in weasel number over time. This information could be very useful for the conservation of small mustelids as well as their management in regions where they are invasive.
Similar content being viewed by others
Introduction
Understanding the determinants of the presence and abundance of a species remains one of the key questions in ecological studies (Hopkins and Kennedy 2004; MacKenzie et al. 2006). Small mustelids, such as weasel (Mustela nivalis) and stoat (Mustela erminea), play an important role in ecosystems as specialist predators of rodents (Jędrzejewski and Jędrzejewska 1993; Norrdahl and Korpimäki 1995; Korpela et al. 2014). There are increasing concerns about the conservation status of small mustelids globally, raising the need for accurate methods to estimate population densities of these species (Marneweck et al. 2021; Jachowski et al. 2021). The density of weasels is affected by many factors, including the availability of prey (Jędrzejewski et al. 1995; Zub et al. 2008), the structure of habitats (Zub et al. 2008) and individual variation in dispersal associated with phenotype (McDevitt et al. 2013). Currently, capture–mark–recapture (CMR) methods based on live trapping of weasel is the golden standard when it comes to density estimation for weasel (King 1975). However, due to the different responses of individual weasels to live traps, it is hard to define which individuals are residents or transients, but with a reasonable trapping effort, all the animals present in the area could be known (King 1975; Zub et al. 2008). Furthermore, live trapping of weasels is invasive, labour-intensive, and requires previous experience, making this method unsuitable for long-term and large-scale monitoring. There is thus a need for another, preferentially non-invasive, method that allows for the estimation of weasel abundance over space and time.
Weasels can be monitored with a variety of non-invasive methods including hair tubes (Bu et al. 2016), tracking tunnels (Graham 2002), and snow tracking (Jędrzejewski et al. 1995; Hellstedt et al. 2006). All of these methods have pros and cons. For example, snow tracking is considered a reliable method to estimate abundance, but it is limited to the winter season and is strongly affected by the local weather conditions (Zub et al. 2008). Furthermore, snow tracking is not feasible in countries that have little or no snowfall. Similarly, hair tubes in combination with genetic methods can be used to identify hairs to species and individuals, but application success varies among studies as it is hard to get high-quality hair samples of single individuals (García and Mateos 2009; Gleeson et al. 2010). Recent progress in the development of camera trap-based devices resulted in their application for studying small mustelids (Croose and Carter 2019; Mos and Hofmeester 2020; Croose et al. 2022). Among different systems, the ‘Mostela’ (a camera trap and a tracking tunnel inside a wooden box) seems to be most effective in detecting weasels (Mos and Hofmeester 2020) although the detection rate for stoats could be much lower (Croose and Carter 2019; Croose et al. 2022). The Mostela system thus seems a promising method to estimate the relative abundance of weasels.
Estimating densities with camera traps is relatively straightforward with CMR models when the identification of individuals is possible but becomes more challenging when individuals cannot be identified (Gilbert et al. 2021). Over the past decades, many methods have been developed to estimate the abundance of unmarked individuals from point samples such as camera-trapping data (Royle and Nichols 2003; Royle 2004; Rowcliffe et al. 2008; Chandler and Royle 2013; Howe et al. 2017; Nakashima et al. 2018). Some of these models cannot incorporate covariates (Nakashima et al. 2018), making them less useful when the aim is to study determinants of abundance in space and time. Another big distinction is that some models assume independence among detectors (Royle and Nichols 2003; Royle 2004) while others use the dependence among detectors to estimate densities (Chandler and Royle 2013). Due to the difficulty of obtaining data for abundance estimation, there generally is a lack of studies comparing these newly developed models to more traditional methods such as CMR (but see Martin-Garcia et al. (2022) for a recent comparison of methods for red fox Vulpes vulpes).
In this study, we aimed to test the application of the Mostela system to estimate (relative) abundance of weasels using the Royle–Nichols (RN) model (Royle and Nichols 2003). We used the RN model as is deals relatively well with false-positive and false-negative errors (Nakashima 2020). At the same time, we live-trapped weasels to estimate the minimum number of animals known to occur in the area as a measure of weasel abundance that has been successfully used in the past (Jędrzejewski et al. 1995; Zub et al. 2008). We compared these two estimates of weasel abundance to test how well the Mostela-derived estimates compared to the previously used standard. Furthermore, we live-trapped voles, the main prey of weasels in the study area (Jędrzejewski et al. 1995; Zub et al. 2008), and tested if the RN model estimates of weasel abundance correlated with the availability of prey.
Methods
Study area
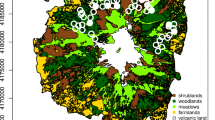
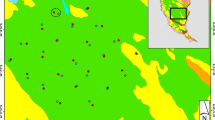
The study was carried out in the central part of the Białowieża Forest (23.86°E, 52.70°N), Northeast Poland (Fig. 1). The study area consists of meadows and unmanaged grasslands surrounded by forest, arable land and settlements (ca. 1.5 km2) and a mosaic of reeds, wet and moist meadows, mires, willow and willow-alder brushwood along the Narewka river valley (ca. 0.5 km2; for details see Zub et al. 2008). The landscape between the village and our study area consisted mostly of intensively used meadows and arable fields (grey in Fig. 1), where weasels were previously only found occasionally (Zub et al. 2008). We thus did not sample in this area during this study but focussed on the meadows and unmanaged grasslands (light grey in Fig. 1). Stoat (Mustela erminea) was not present in the study area during the study.
Study area on the border of Białowieża village in northeast Poland, with the locations surveyed with the Mostela system—an enclosed camera-trapping approach—and live traps depicted with their respective names. The fence (dashed line) running through the natural grasslands (light grey) formed a linear landscape feature that was targeted for our sampling to increase the chance of capturing weasels
Sampling set-up
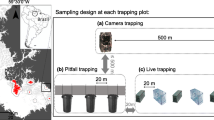
To monitor weasels in the whole study area, between 2015 and 2018, we deployed a Mostela at nine point locations, with the effort varying between locations and years (Fig. 1, Table 1). The Mostela system is a camera-trapping device specifically developed to monitor small mustelids (Mos and Hofmeester 2020). It consists of a camera trap (Bushnell NatureView HD, with 250 mm lens for close focus) placed inside a wooden box (610 × 300 × 150 mm) with an open tracking tunnel (ø 10 cm, PVC drainpipe) going through it. Mostela systems were spaced minimally 200 m apart to reduce the chance of double-counting individuals at more than one device. Due to logistics, Mostela systems were active during different parts of the study period (Table 1). We placed all Mostela systems close to linear landscape features, like fences or ditches, as these are frequently used by weasels and changed the memory cards and batteries in the camera trap monthly. We used these monthly deployments as the basis of our analysis (see below).
Live trapping of weasels was performed using the methodology described in Jędrzejewski et al. (1995), using modified live traps with a metal flap-door instead of the wooden ramp. In short, life traps contained two life laboratory mice with food and bedding as bait. Caught weasels were anaesthetised using a 2% mixture of isoflurane (Iso–Vet), and individually marked by punching ear marks. Live trapping was done using a clustered design using four to six traps at each of six locations (BNP_gate, BNP_gateW, Cegielnia, Dyrekcyjny, Kamienne Bagno and Browska, Fig. 1, Table 1). Live traps were also located next to linear features, such as the fence running across our study area in the east–west direction along the buffer zone of the Białowieża National Park (Fig. 1). Traps were deployed for 2 weeks (except for September 2015 when they were deployed for four weeks) and checked once daily (late afternoon/ early evening). In this way, we reduced the time animals spent in the traps as weasels in the study area are mostly active during the day (Jędrzejewski et al. 2000). Weasels were trapped between the second half of July and the first half of October, the post-breeding period for weasels, and occasionally with additional trapping sessions in spring and early winter. This pattern of trapping allowed us to reduce field-work effort and provide the most reliable estimate of the minimum number of weasels present in the study area, as weasel densities are highest during this time of year (Zub et al. 2008). In September 2018, three weasels were captured in the rodent traps (see below), located at two locations (BNP_gate and BNP_gateW), where we monitored small mammals and thus decided not to trap weasels at the same time.
Our assumption regarding the estimate of the minimum number of weasels present were based on the long-term monitoring (since 1997) of the weasel population in the same study area, and radio-tracking data providing reliable information about the home-range sizes and movement patterns (Zub et al. 2008). Weasels in this area are almost exclusively using natural grasslands as habitat and are avoiding any open areas (cultivated fields and mowed grasslands; Zub et al. 2008). Moreover, there is a very limited exchange of individuals between forests and grasslands (McDevitt et al. 2013). The intensive use of natural grasslands within the study area caused high fragmentation of habitats suitable for weasels and the area covered by natural grasslands decreased from 92% in 2005 to 36% in 2018. As a result, the six areas where we trapped weasels were the only suitable habitats left. Furthermore, most weasels were only caught once (see results) precluding us from using a capture–mark–recapture (CMR) approach to estimate the population size of weasels in the area. Thus, we used the number of weasels caught as an estimate of the minimum number of weasels known to occur in the area as our best available population estimate. Due to the optimal sampling of available habitat, we are, however, quite confident that this number is a good estimate of the weasel population in the area.
The number and the winter survival of weasels in our study area depend on the availability of voles (Microtus oeconomus, M. agrestis and M. arvalis; Zub et al. 2008, 2011). We thus also monitored these vole species by trapping rodents using wooden live traps in a clustered design at the same six locations where weasels were live-trapped. In September, at each location, 10 traps were deployed for 5 days and checked twice daily. Traps were baited with oats and carrots. Captured animals were marked by clipping some fur on the back (to avoid double-counting) and released in the place of capture. We present the results of rodent trapping as a standardized index (number of individuals captured per 100 trap nights).
Analytical framework
We used a Royle–Nichols (RN) model (Royle and Nichols 2003) to compare (relative) abundance estimates from Mostela systems to abundance estimates from live traps. In short, the RN model can be compared to an occupancy model (MacKenzie et al. 2002) or N-mixture model (Royle 2004) where the sampling process is split into a state process model and a detection model. The RN model uses the repeated detection–non-detection data similar to an occupancy model, while it uses a Poisson distribution for the state process model similar to an N-mixture model (Kéry and Royle 2016). The state process model estimating the abundance at site i (λi) is described as:
where Ni is the number of individuals at site i. To derive the number of individuals from the detection-non-detection data, the observation model is described as:
where yij are the binary observations at site i during survey j, and the probability to detect the species (P*ij) is expressed as a function of the number of individuals (Ni) and the per-individual detection probability (pij). It is thus assumed that all individuals at a site have the same probability of being detected in a survey period, which allows for the number of individuals to be estimated from the combined detection probabilities. The RN model thus allows the estimation of (relative) abundance from detection–non-detection data. Based on a previous simulation (Nakashima 2020), we interpret this (relative) abundance as the number of individuals whose home range included the Mostela location. As we cannot rule out that single weasels had home ranges overlapping multiple Mostela systems, we interpreted the estimate of λ as an average relative abundance for the whole study area.
We transformed the detection–non-detection data from each Mostela system to a vector with daily detection (1) or non-detection (0) for the duration of each monthly deployment. These vectors were combined into a detection history matrix with one row per deployment. Our detection–non-detection matrix thus consisted of 130 rows (one for each deployment) and 123 columns (one for each day/date during which at least one of the Mostela systems was active). All days (columns) outside of the active deployment of each row were specified as missing data (NA). We did this to ensure that Mostela systems that were active during the same time also ended up having detection–non-detection data during the same days.
Due to a limited amount of data, we were not able to use dynamic models that explicitly model changes in abundance within and among years. Instead, to utilize as much information as possible for the estimation of individual detection probability and the number of individuals from the data, we ran a single model using the data from all years. In this way, we assumed individual detection probability to be constant over the years and that differences in observed detection probability among years were a consequence of fluctuating abundance. To accommodate differences in detection probability within a year, we included month as a covariate in the detection model. We modelled the differences among years without explicitly modelling changes over time using a ‘single-season’ model with year as a covariate in the state process part of the model. Our estimates of λ are thus an average per location within a given year and are assumed independent of the estimates in other years. To account for repeated measurements at the same locations, we included a random intercept on the state process model per location.
To test if the (relative) abundance of weasels could (partially) be explained by the density of voles as their main prey, we ran a second RN model where we substituted the year covariate for the year-specific estimate of vole density in the study area. We standardized this parameter by subtracting the mean and dividing by one standard deviation. We used leaving-one-out cross-validation (Vehtari et al. 2017) to test the support of this model compared to the model with the year covariate.
We fitted the RN model using MCMC with Stan (Carpenter et al. 2017) called from R (version 4.0.2; R Core Team 2020) using the ubms package (Kellner et al. 2021). The ubms package makes the running of models in Stan accessible using the same syntax and functions as the unmarked package (Fiske and Chandler 2011) but with the added advantage of being able to add random intercepts and slopes. We ran models on three chains for 4000 iterations (including 2000 iterations burn-in) per chain and used the default weakly informative priors as provided in the ubms package. We checked model convergence using the \(\widehat{R}\) statistic as well as trace plots (Brooks and Gelman 1998). We used the MacKenzie–Bailey goodness-of-fit test (MacKenzie and Bailey 2004) as implemented in the ubms package to test model fit. We used the loo package (version 2.6.0) to perform the leaving-one-out cross-validation (Vehtari et al. 2023).
Results
The Mostela systems were deployed for a total of 5201 days, ranging from 448 days in 2018 to 2057 days in 2016 (Table 2), divided over 130 monthly deployments (27 in 2015, 52 in 2016, 32 in 2017 and 19 in 2018). Raw capture rates of weasels (observations/100 camera-days) ranged widely from 2.9 in 2018 to 12.4 in 2017 (Table 2). Most weasels were trapped in August or September, and only single individuals in other months (February, April, July, October, and December). The number of live-trapped weasels ranged from three individuals in 2015 and 2016 to seven individuals in 2017 (Table 3). None of the live-trapped weasels were captured in more than one location. Only one individual was recaptured, in the same location (Dyrekcyjny) after more than one month (first capture end of August and recapture beginning of October). The vole trapping index also ranged widely, from 15.3 individuals per 100 trap nights in 2015 to 30 individuals per 100 trap nights in 2017 (Table 3).
Individual daily detection probabilities in the Mostela system differed among months from 2.6 e−3 (95% credible interval: 3.5 e−4 to 0.012) in March to 0.090 (95% CI 0.058 to 0.13) in August (Fig. 2). The (relative) abundance estimates (\(\lambda\)) similarly differed among years as the other indices of weasel abundance (Table 2, Fig. 3). The Royle–Nichols model including year as covariate had a good fit to the data (MacKenzie–Bailey goodness-of-fit test: p = 0.48). In the model substituting the year covariate for vole abundance, weasel abundance increased with vole abundance (slope estimate: 0.63, 95% CI 0.39 to 0.88). This model had a good fit to the data (MacKenzie–Bailey goodness-of-fit test: p = 0.45), and its predictive power was similar to the model with year as covariate, with a difference in expected predictive accuracy based on leaving-one-out cross-validation of − 2.34 (standard error of the difference: 3.0).
Discussion
This is the first study quantitatively comparing live-trapping and camera-trapping methods to estimate the population size of weasels. The (relative) abundance of weasels estimated with data obtained using Mostela systems showed a similar trend as the total number of weasels life-trapped in a given year, especially when correcting for potential differences in detection probabilities in Mostela systems among different months of the year. The raw capture rates of weasels in the Mostela systems correlated less well with the number of live-trapped individuals, mainly due to a low capture rate in 2018, underestimating weasel abundance. This underestimation might be caused by the fact that Mostela systems were only deployed at the start of that year when detection probabilities were very low (Fig. 2). A similar result was found in a study comparing capture rates with other abundance indices for red foxes, where failing to account for differences in detection resulted in a mismatch between estimates (Martin-Garcia et al. 2022). This suggests that raw capture rates from Mostela systems could potentially be used as a measure of (relative) abundance when differences in detection probability can be minimized, for example by comparing similar months, preferentially at times of the year when detection probabilities are high.
We were not able to use capture–mark–recapture (CMR) models to estimate population size of weasels from the live-trapping data due to only one recapture. Weasels are notoriously difficult to catch (King 1975), which might be one of the reasons why we had so few recaptures. However, the presence of the fence crossing our study area, which was the main linear feature in the otherwise open habitat, as well as limited availability of suitable habitat, likely optimized our life-trapping effort (Zub et al. 2008). Thus, we are confident that our estimate of the minimum number of weasels in the study area was a representative estimate of the weasel population. However, we do acknowledge that we might have missed weasels, and that the proportion of individuals we caught might have fluctuated among years. Furthermore, we found that weasel abundance, both those estimated using RN models from the Mostela data, and the minimum number known alive from the life-trapping data, closely followed changes in their main prey. This was similar to results from previous studies in Poland (Jędrzejewski et al. 1995; Zub et al. 2008), and northern England (Graham 2002). This close correlation between weasel abundance and availability of main prey provides additional support for the reliability of our weasel-abundance estimates.
We found that the daily detection probability of individual weasels differed among months, with the highest detection probabilities in July to September. This pattern is very similar to what was previously reported from a study in the Netherlands (Mos and Hofmeester 2020), as well as a study using the Mostela system to monitor Irish stoats (Mustela erminea hibernica; Croose et al. 2022). Due to our limited amount of data, we were unable to disentangle true differences in detection probability over the year from changes in weasel abundance over the year that could lead to a similar pattern. Therefore, part of the change in detection probability might be caused by varying weasel abundance during the year. A previous study in northeast Poland showed that weasel abundance was highest at the end of summer (Jędrzejewski et al. 1995). Our estimates of (relative) abundance are thus an average over the year, but these numbers should be comparable to our live-trapping data as we compare them to the total number of weasels caught in the study area in a year. Based on these findings, we suggest that monitoring efforts to estimate the presence or (relative) abundance of weasels using the Mostela system focus their efforts on the period from July to September to ensure high detection probabilities.
The Royle–Nichols model provided an estimate of the average number of individuals whose home range overlapped a Mostela system. To estimate density based on this population size estimate, the size of the home range of the focal species is needed (Nakashima 2020). However, home range sizes of weasels might fluctuate over the year and are dependent on e.g. prey density (Jędrzejewski et al. 1995; Zub et al. 2008). Furthermore, we expect that the number of live-trapped weasels gave us an estimate of the total number of weasels in our study population (Zub et al. 2008). As the total abundance estimate from the RN model (summing the estimates for all locations) was higher than the number of caught weasels for each of the four years, this suggests that we either captured the same individuals at multiple locations or that we missed part of the weasel population with our life-trapping effort. Previous estimates of home range size from the study area suggest that weasels can have home-range sizes that with our set-up could overlap multiple locations (Jędrzejewski et al. 1995; Zub et al. 2008). We thus want to stress that our abundance estimates from the RN models should not be interpreted as absolute. More importantly, the similar trends of the RN model estimates and the number of live-trapped individuals gives a first indication that the RN model estimate of (relative) abundance can potentially be used to monitor changes in weasel abundance in space and time.
An alternative approach to RN models, would be to try and identify individual weasels based on the spot-pattern on the lateral line between brown and white (Mos and Hofmeester 2020). This would allow the use of CMR or spatial–capture–recapture (SCR) models to estimate density. The combination of camera traps and SCR models has successfully been implemented to estimate density of another mustelid species, the stone marten (Martes foina; Burgos et al. 2023). An added advantage of using SCR models is that the density estimate is spatially explicit and could therefore be used to answer questions about the spatial distribution of individuals over the study area. However, one big disadvantage is that weasel records in the Mostela system need to be of very high quality to be able to see the spot patterns, and individuals might not show both sides during every visit, increasing the chance of misidentifications and consequent biases in density estimates (Johansson et al. 2020).
Our study is part of a growing body of work showing that camera traps can provide a useful non-invasive method to provide abundance estimates of elusive species (Wearn and Glover-Kapfer 2019). We were able to track changes in weasel abundance over time using the Mostela method and RN models, suggesting that this combination of methods might be a promising way to survey changes in weasel abundance over space and time. However, further studies in different locations, with a variety of weasel densities would be needed to further test the ability of Mostela systems with RN models to detect changes in weasel abundance of space and time. The densities of weasels we found in this study are much lower than those recorded in the study area in the past (Zub et al. 2008). This might be an effect of the reduction in snow cover, negatively affecting the survival of weasels in the study area (Atmeh et al. 2018) resulting in lower population sizes. Thus, it is possible that at higher densities of weasels the relationship between the RN model estimate and true abundance could be different, e.g. due to a saturation effect. Similarly, it would be good to study this relationship in different landscapes and habitat types. We would thus encourage further studies into the usefulness of the Mostela system as a tool to monitor weasels in other areas with different abundances and habitats. Considering these limitations, our approach could be a useful tool to monitor spatial and temporal changes in weasel abundances both for conservation purposes, as well as management in areas where the species is invasive.
Data availability
All data and code used in the analyses presented in this article are available on GitHub: https://github.com/Tim-Hofmeester/mostela-poland. An archived version of this repository can be found on Zenodo: https://doi.org/10.5281/zenodo.10512568.
References
Atmeh K, Andruszkiewicz A, Zub K (2018) Climate change is affecting mortality of weasels due to camouflage mismatch. Sci Rep 8:1–7. https://doi.org/10.1038/s41598-018-26057-5
Brooks SP, Gelman A (1998) General methods for monitoring convergence of iterative simulations. J Comput Graph Stat 7:434–455. https://doi.org/10.1080/10618600.1998.10474787
Bu H, Hopkins JB III, Zhang D, Li S, Wang R, Yao M, Wang D (2016) An evaluation of hair-snaring devices for small-bodied carnivores in southwest China. J Mammal 97:589–598. https://doi.org/10.1093/jmammal/gyv205
Burgos T, Salesa J, Fedriani JM, Escribano-Ávila G, Jiménez J, Krofel M, Cancio I, Hernández-Hernández J, Rodríguez-Siles J, Virgós E (2023) Top-down and bottom-up effects modulate species co-existence in a context of top predator restoration. Sci Rep 13:4170. https://doi.org/10.1038/s41598-023-31105-w
Carpenter B, Gelman A, Hoffman MD, Lee D, Goodrich B, Betancourt M, Brubaker M, Guo J, Li P, Riddell A (2017) Stan: a probabilistic programming language. J Stat Softw 76:1–32. https://doi.org/10.18637/jss.v076.i01
Chandler RB, Royle JA (2013) Spatially explicit models for inference about density in unmarked or partially marked populations. Ann Appl Stat 7:936–954. https://doi.org/10.1214/12-AOAS610
Croose E, Carter SP (2019) A pilot study of a novel method to monitor weasels (Mustela nivalis) and stoats (M. erminea) in Britain. Mamm Comm 5:6–12. https://doi.org/10.59922/YIUK4739
Croose E, Hanniffy R, Hughes B, McAney K, MacPherson J, Carter SP (2022) Assessing the detectability of the Irish stoat Mustela erminea hibernica using two camera trap-based survey methods. Mammal Res 67:1–8. https://doi.org/10.1007/s13364-021-00598-z
Fiske I, Chandler R (2011) Unmarked: an R package for fitting hierarchical models of wildlife occurrence and abundance. J Stat Softw 43:1–23. https://doi.org/10.18637/jss.v043.i10
García P, Mateos I (2009) Evaluation of three indirect methods for surveying the distribution of the Least Weasel Mustela nivalis in a Mediterranean area. Small Carniv Conserv 40:22–26
Gilbert NA, Clare JD, Stenglein JL, Zuckerberg B (2021) Abundance estimation methods for unmarked animals with camera traps. Conserv Biol 35:88–100. https://doi.org/10.1111/cobi.13517
Gleeson DM, Byrom AE, Howitt RLJ (2010) Non-invasive methods for genotyping of stoats (Mustela erminea) in New Zealand: potential for field applications. New Zeal J Ecol 34:356–359
Graham I (2002) Estimating weasel Mustela nivalis abundance from tunnel tracking indices at fluctuating field vole Microtus agrestis density. Wildl Biol 8:279–287. https://doi.org/10.2981/wlb.2002.025
Hellstedt P, Sundell J, Helle P, Henttonen H (2006) Large-scale spatial and temporal patterns in population dynamics of the stoat, Mustela erminea, and the least weasel, M. nivalis, in Finland. Oikos 115:286–298. https://doi.org/10.1111/j.2006.0030-1299.14330.x
Hopkins HL, Kennedy ML (2004) An assessment of indices of relative and absolute abundance for monitoring populations of small mammals. Wildl Soc Bull 32:1289–1296. https://doi.org/10.2193/0091-7648(2004)032[1289:AAOIOR]2.0.CO;2
Howe EJ, Buckland ST, Després-Einspenner M-L, Kühl HS (2017) Distance sampling with camera traps. Methods Ecol Evol 8:1558–1565. https://doi.org/10.1111/2041-210x.12790
Jachowski D, Kays R, Butler A et al (2021) Tracking the decline of weasels in North America. PLoS ONE 16:e0254387. https://doi.org/10.1371/journal.pone.0254387
Jędrzejewski W, Jędrzejewska B (1993) Predation on rodents in Białowieza primeval forest, Poland. Ecography 16:47–64. https://doi.org/10.1111/j.1600-0587.1993.tb00058.x
Jędrzejewski W, Jędrzejewska B, Szymura L (1995) Weasel population response, home range, and predation on rodents in a deciduous forest in Poland. Ecology 76:179–195. https://doi.org/10.2307/1940640
Jędrzejewski W, Jędrzejewska B, Zub K, Nowakowski W (2000) Activity patterns of radio-tracked weasels Mustela nivalis in Bialowieza National Park (E Poland). Ann Zool Fenn 37:161–168
Johansson Ö, Samelius G, Wikberg E, Chapron G, Mishra C, Low M (2020) Identification errors in camera-trap studies result in systematic population overestimation. Sci Rep 10:1–10. https://doi.org/10.1038/s41598-020-63367-z
Kellner K, Fowler N, Petroelje T, Kautz T, Beyer D, Belang J (2021) ubms: an R package for fitting hierarchical occupancy and N-mixture abundance models in a Bayesian framework. Methods Ecol Evol 13:577–584. https://doi.org/10.1111/2041-210X.13777
Kéry M, Royle JA (2016) Applied hierarchical modeling in ecology: analysis of distribution, abundance and species richness in R and BUGS, 1st edn. Academic Press, London
King C (1975) Sex ratio of trapped weasels (Mustela nivalis). Mammal Rev 5:1–8. https://doi.org/10.1111/j.1365-2907.1975.tb00180.x
Korpela K, Helle P, Henttonen H, Korpimäki E, Koskela E, Ovaskainen O, Pietiäinen H, Sundell J, Valkama J, Huitu O (2014) Predator–vole interactions in northern Europe: the role of small mustelids revised. Proc R Soc B 281:20142119. https://doi.org/10.1098/rspb.2014.2119
MacKenzie DI, Bailey LL (2004) Assessing the fit of site-occupancy models. J Agr Biol Envir Stat 9:300–318. https://doi.org/10.1198/108571104X3361
MacKenzie DI, Nichols JD, Lachman GB, Droege S, Royle JA, Langtimm CA (2002) Estimating site occupancy rates when detection probabilities are less than one. Ecology 83:2248–2255. https://doi.org/10.1890/0012-9658(2002)083[2248:ESORWD]2.0.CO;2
MacKenzie DI, Nichols JD, Royle JA, Pollock KH, Bailey LL, Hines JE (2006) Occupancy estimation and modeling - inferring patterns and dynamics of species occurrence. Springer, New York
Marneweck C, Butler AR, Gigliotti LC, Harris SN, Jensen AJ, Muthersbaugh M, Newman BA, Saldo EA, Shute K, Titus KL, Yu SW, Jachowski DS (2021) Shining the spotlight on small mammalian carnivores: Global status and threats. Biol Conserv 255:109005. https://doi.org/10.1016/j.biocon.2021.109005
Martin-Garcia S, Rodríguez-Recio M, Peragón I, Bueno I, Virgós E (2022) Comparing relative abundance models from different indices, a study case on the red fox. Ecol Indic 137:108778. https://doi.org/10.1016/j.ecolind.2022.108778
McDevitt AD, Oliver MK, Piertney SB, Szafrańska PA, Konarzewski M, Zub K (2013) Individual variation in dispersal associated with phenotype influences fine-scale genetic structure in weasels. Conserv Genet 14:499–509. https://doi.org/10.1007/s10592-012-0376-4
Mos J, Hofmeester TR (2020) The Mostela: an adjusted camera trapping device as a promising non-invasive tool to study and monitor small mustelids. Mammal Res 65:843–853. https://doi.org/10.1007/s13364-020-00513-y
Nakashima Y (2020) Potentiality and limitations of N-mixture and Royle-Nichols models to estimate animal abundance based on noninstantaneous point surveys. Popul Ecol 62:151–157. https://doi.org/10.1002/1438-390X.12028
Nakashima Y, Fukasawa K, Samejima H (2018) Estimating animal density without individual recognition using information derivable exclusively from camera traps. J Appl Ecol 55:735–744. https://doi.org/10.1111/1365-2664.13059
Norrdahl K, Korpimäki E (1995) Mortality factors in a cyclic vole population. Proc R Soc B 261:49–53. https://doi.org/10.1098/rspb.1995.0116
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed 8 Nov 2020
Rowcliffe JM, Field J, Turvey ST, Carbone C (2008) Estimating animal density using camera traps without the need for individual recognition. J Appl Ecol 45:1228–1236. https://doi.org/10.1111/j.1365-2664.2008.01473.x
Royle JA (2004) N-mixture models for estimating population size from spatially replicated counts. Biometrics 60:108–115. https://doi.org/10.1111/j.0006-341X.2004.00142.x
Royle JA, Nichols JD (2003) Estimating abundance from repeated presence–absence data or point counts. Ecology 84:777–790. https://doi.org/10.1890/0012-9658(2003)084[0777:EAFRPA]2.0.CO;2
Vehtari A, Gelman A, Gabry J (2017) Practical Bayesian model evaluation using leave-one-out cross-validation and WAIC. Stat Comput 27:1413–1432. https://doi.org/10.1007/s11222-016-9696-4
Vehtari A, Gabry J, Magnusson M, Yao Y, Bürkner P, Paananen T, Gelman A (2023) Loo: efficient leave-one-out cross-validation and WAIC for Bayesian models. R package version 2.6.0, https://mc-stan.org/loo/. Accessed 17 July 2023
Wearn OR, Glover-Kapfer P (2019) Snap happy: camera traps are an effective sampling tool when compared with alternative methods. R Soc Open Sci 6:181748. https://doi.org/10.1098/rsos.181748
Zub K, Sönnichsen L, Szafrańska PA (2008) Habitat requirements of weasels Mustela nivalis constrain their impact on prey populations in complex ecosystems of the temperate zone. Oecologia 157:571–582. https://doi.org/10.1007/s00442-008-1109-8
Zub K, Szafrańska PA, Konarzewski M, Speakman JR (2011) Effect of energetic constraints on distribution and winter survival of weasel males. J Anim Ecol 80:259–269. https://doi.org/10.1111/j.1365-2656.2010.01762.x
Acknowledgements
We are grateful to Pablo Garcia-Diaz as well as two anonymous reviewers for valuable comments on an earlier version of this manuscript.
Funding
Open access funding provided by Swedish University of Agricultural Sciences. This study was funded by the National Science Centre of Poland, grant no. 2013/11/B/NZ8/00887 and by the Bekker programme of the Polish National Agency for Academic Exchange (NAWA), grant PPN/BEK/2019/1/00036 to KZ.
Author information
Authors and Affiliations
Contributions
Conceptualization: KZ; methodology: TH, JM, KZ; formal analysis and investigation: TH; data curation: TH, KZ; writing—original draft preparation: TH, KZ; writing—review and editing: TH, JM, KZ; funding acquisition: KZ; visualization: TH, KZ.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical approval
Weasels were captured and handled in strict accordance with the guidelines set by the Polish Committee on the Ethics of Animal Experiments. The protocol was approved by the Local Committee on the Ethics of Animal Experiments at the Medical University in Białystok (permit no. LKE 49/2014). Weasels are protected in Poland and were trapped under the auspices of Polish nature conservancy authorities.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Handling editor: Heiko Georg Rödel.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hofmeester, T.R., Mos, J. & Zub, K. Comparing direct (live-trapping) and indirect (camera-trapping) approaches for estimating the abundance of weasels (Mustela nivalis). Mamm Biol 104, 141–149 (2024). https://doi.org/10.1007/s42991-023-00394-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42991-023-00394-z