Abstract
The European wildcat is a threatened carnivore, whose ecology is still scarcely studied, especially in Mediterranean areas. In this study, we estimated activity rhythm patterns of this felid, by means of camera-trapping at three spatial scales: (i) whole country (Italy); (ii) biogeographical areas; (iii) latitudinal zones. The activity rhythms patterns were also calculated according to temporal scales: (1) warm semester; (2) cold semester and (3) seasonal scales. Lastly, we also tested whether the effect of moon phases affected the wildcat activity. We conducted the analysis on a total of 975 independent events collected in 2009–2021, from 285 locations, in ~ 65,800 camera days. We showed that the wildcat in Italy exhibits a > 70% nocturnal behaviour, with 20% of diurnal activity, at all spatial scales, and throughout the whole year, with peaks at 10.00 p.m. and 04.00 a.m. We observed a high overlap of wildcat activity rhythms between different biogeographical and latitudinal zones. The wildcat was mainly active on the darkest nights, reducing its activity in bright moonlight nights. Diurnal activity was greater in the warm months and decreased with the distance from shrubs and woodlands, most likely according to activity rhythms of its main prey, water presence in summer, the care of offspring and the availability of shelter sites. Conversely, the distance to paved roads seems to have no significant effects on diurnal activity, suggesting that, in presence of natural shelters, the wildcat probably may tolerate these infrastructures. We suggested limited plasticity in activity rhythm patterns of the wildcat, emphasizing the importance of dark hours for this species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ecological studies on elusive carnivores are particularly challenging, as these mammals are often threatened species that generally live in low population densities, with a fragmented distribution (Gese 2001). The European wildcat Felis silvestris is widely reported as a strictly nocturnal carnivore, living at low population densities throughout Europe and with a discontinuous distribution range (Daniels et al. 2001; Germain et al. 2008; Soyumert 2020; Anile et al. 2021; Migli et al. 2021). During daylight hours, the wildcat usually exploits resting sites, i.e., shelter structures, mainly at the limits of the forests (Jerosch et al. 2009). Some aspects of the wildcat ecology including population dynamics, population density (Anile et al. 2014, 2020; Kilshaw et al. 2015; Fonda et al. 2022) and spatial behaviour (Monterroso et al. 2009; Anile et al. 2019) have already been studied in detail, although large scale evaluations are still sparse. Conversely, the temporal activity level has been partially overlooked, in particular on a large scale (Anile et al. 2021; Migli et al. 2021). Indeed, patterns of the temporal behaviour of species may change with habitat, latitude, presence of competitors or mating opportunities (Pearman et al. 2008; Pratas-Santiago et al. 2016; Karanth et al. 2017). Moreover, geographic differences in genetic structure and ethological features (i.e.: valerian lures response) have been reported (Mattucci et al 2013; Velli et al. 2015). Therefore, activity patterns need to be considered in different environmental contexts (e.g. Ashby 1972; Kerr 1997; Jordan et al. 2007; Brivio et al. 2017; Mori et al. 2020a). The European wildcat is fully protected over most of its range, under national and international legislations. This felid is included in CITES (Convention on International Trade of Endangered Species) Appendix II, listed in the European Union Habitats and Species Directive Annex IV and the Bern Convention Appendix II. Currently, some European wildcat populations are locally expanding, partially recovering the species’ historical distribution range (Ragni and Mandrici 2003; Steyer et al. 2016; Tormen et al. 2020; Gavagnin 2021). The process of colonization or recolonization exposes wildcats to even greater consequences of direct and indirect anthropogenic threats, such as habitat loss and fragmentation, hybridization with domestic cats and direct persecution (Yamaguchi et al. 2015; Mattucci et al. 2013). Anthropogenic structures and disturbances might also influence the activity patterns of the wildcat (see Anile et al. 2021). For instance, roads have negative effects on wildlife, acting as barriers to movement and source of disturbance, injuries and mortality (Spellerberg 2002; Roedenbeck et al. 2007; Bastianelli et al. 2021).
Camera trapping is being increasingly employed to estimate animal distribution and abundance, as well as local species richness (Tobler et al. 2008; O’Connell et al. 2011; Borchers et al. 2014; Kikuchi et al. 2020). This represents a reliable method to assess the temporal behaviour of animal species (Leuchtenberger et al. 2014; Mori et al. 2020a; Rossa et al. 2021). Activity rhythms of the wild cat can hardly be directly estimated by direct observations, as being nocturnal and elusive. Temporal activity patterns of this species have been estimated by camera-trapping at local study sites in Northern Spain (Monterroso et al. 2014), Anatolia (Soyumert, 2020), Central Bulgaria (Tsunoda et al. 2020) and Southern Italy (Mori et al. 2020b; Anile et al. 2021). Nevertheless, studies exploring wildcat activity patterns on a national scale are needed in order to effectively implement conservation strategies over wide areas.
In our study, we aimed at filling this gap, i.e., at determining patterns of the wildcat activity on the Italian national scale, in different bioclimatic regions.
We collected camera-trap data from several national Italian camera-trapping projects, which detected wildcats both as a target species and as a bycatch in monitoring programs of other medium to large-sized mammal species. We assessed the activity of the wildcat with the aim to assess whether the diurnal activity may be influenced by environmental and anthropogenic variables (e.g. distance from paved roads).
Calculating temporal overlap amongst contrasting ecological contexts would provide information on behavioural plasticity of the species in terms of temporal patterns of activity. This would in turn provide researchers with valuable information on the basic ecology of the species, which is pivotal to create successful conservation and management plans (O’Connell et al. 2011). For instance, temporal plasticity of each species should be assessed to determine its ability to cope with local touristic pressure and with global climatic change. Where environmental pressures change (e.g. predation/competition, food and shelter availability, anthropization), the same species may show different adaptations to thrive (e.g. the coypu Myocastor coypus: Mori et al. 2020a; the wild boar Sus scrofa: Brivio et al. 2017; Gordigiani et al. 2021). Following the literature available on the wildcat, we predicted that (1) the European wildcat would have been nocturnal in all study areas; (2) activity of European wildcat would not change in biogeographic regions and at different latitudes; (3) activity of European wildcat would change seasonally; (4) as most carnivores improve hunting abilities in bright moonlight nights, European wildcats might be most active in full moon nights (as in other small felids: Penteriani et al. 2013; Huck et al. 2017; Bhatt et al. 2021); (5) the European wildcat would show some daylight activity with increasing distance from roads (as a proxy of human disturbance).
Materials and methods
Data collection and dataset preparation
We collected data gathered across research and monitoring projects using camera traps specifically centered on the European wildcat in Italy, or relevant to projects mainly targeted on other carnivore species. Analyses obtained through camera-trapping are accurate and precise as radio-tracking data, when events (i.e. photos or videos) of the target species are at least over 30 (Lashley et al. 2018). In contrast, the accuracy of estimates decreases when the sample size is lower than 30 detections (Lashley et al. 2018).
Overall, we obtained 1300 videos and photos of putative wild-living cats from 14 study areas from 2009 to 2021 (Table 1), with altitudes ranging from 0 to 1800 m above sea level, spanning from North-Eastern Italy to the most Southern regions (Fig. 1). Requirements for data to be included in our study were those specified by Lashley et al. (2018): (i) cameras should have been deployed on site according to a sampling design targeted to carnivores; (ii) one-month minimum monitoring time; (iii) records obtained from cameras kept active for the whole 24-h cycle. Furthermore, we identified European wildcats using a blind approach by at least three expert operators that independently analyzed the coat pattern of the species following the specific literature (Ragni and Possenti 1996; Beaumont et al. 2001; Kitchener et al. 2005). Only concordant identifications were included in the analyses. Discrimination between European wildcats and domestic cats has been proved to be achievable by means of morphological features, through ad-hoc keys (e.g. coat color pattern: Ragni and Possenti 1996; Devillard et al. 2014; Migli et al. 2021). We discarded records of domestic cats from our analyses, as well as doubtful F. silvestris/F. catus records (cf. Mattucci et al. 2016), i.e. those not fully respecting typical key pelage characteristics of the European wildcat (Jiménez–Albarral et al. 2021; Migli et al. 2021). We only used detections from the same camera station separated from each other by at least 30 min to limit the autocorrelation bias (Monterroso et al. 2014; Torretta et al. 2016; Mori et al. 2020b; Rossa et al. 2021). After filtering the initial dataset according to the above parameters, 975 nationally independent events from a total of 285 camera locations were included in our analyses with a total survey effort of 65,802 camera days (see the Table 1). In all cases the cameras were deployed on animal trails, footways, or forest roads and they had an average distance from the nearest camera of 1138 m (standard deviation = 1543 m).
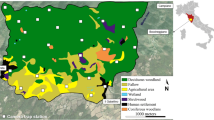
Biogeographic regions (shades of grey), 10 × 10 km cells selected for analyses (in red, N = 85) and latitudinal zones (whose borders are marked by parallels, highlighted in red). Cells have been identified when at least one detection of wildcat was inside the border of cell. Our map excluded Sardinia, where the African wildcat Felis (silvestris) lybica is present. All data were projected on the grid identified by Regulation (EU) No 1089/2010 and the INSPIRE Directive 2007/2/EC (ETRS 89/Laea-EPGS-3035)
For each event, we registered geographical coordinates (EPSG: 3035), latitudinal zones (North, Central and South, see below for definitions), biogeographic areas (categories are: “Continental”, “Mediterranean”, “Alpine”), the solar hour of capture, date, season (categories are: “autumn”: October–December; “winter”: January–March; “spring”: April–June; “summer”: July–September), semester (categories are: “warm months”: April–September; “cold months”: October–March), type of habitat, assessed on the field during camera-trap deployment (deciduous forest; coniferous forest; shrubs; wetland; open land), lunar epact and percentage of the visible moon. The use of season allowed a better total year subdivision for our analyses; however, we also used the “semester” category to allow a reliable comparison with previously published studies which used this categorization (e.g. Mori et al. 2020b).
We used latitudinal zones subdivision to assess the patterns of activity based on similar conditions of light and latitude. We also considered a subdivision of the peninsula with three main latitudinal zones: (i) Northern Italy, above the 44° parallel; (ii) Central Italy, between 44° and 41° parallel; (iii) Southern Italy, under the 41° parallel, without isles (Fig. 1). Moreover, biogeographic regions categorization allowed us to elaborate data according to the main biocenosis distribution and climate conditions. They are described in the Supplemental Material.
Patterns of activity rhythms
We used RStudio version 4.0.3 (RStudio Team 2020; R Core Team 2021) to estimate wildcats’ temporal activity patterns using the non-parametric kernel density estimation (Meredith and Ridout 2014).
Then, we also estimated 95% confidence intervals of activity patterns as percentile intervals from 1000 bootstrap samples (Ridout and Linkie 2009). We used package ‘overlap’ (Meredith and Ridout 2014) to draw the overlap plots between temporal activity patterns assessed in different biogeographic areas, latitudinal zones, seasons and semesters temporal overlap. Considering the similar results between the two temporal scales (seasons and semesters), we have been parsimonious and have considered only “semester”.
Bearing in mind that this work does not follow an homogeneous sampling designs for all study sites, and the events of wildcat have been collected in the framework of different projects, we checked for sampling sites that could weigh more than others due to potential differences in sample size across them, by comparing activity patterns between the full dataset (N = 975 events) and a subsample of randomly selected events homogeneously distributed across sites (N = 312, with 18 random events per site, i.e., the number of events coming from the site with the smallest sample size). We compute the Watson's two-sample test of homogeneity to evaluate the uniformity of the two distributions (Lund et al. 2017). We used the overlap coefficient Δ4 to compare the two samples and to verify their concordance. We calculated the Δ4 estimator coefficient since our sample was > 75 events (Linkie and Ridout 2011; Meredith and Ridout 2014). Overlap was defined as “low” when it was < 0.50, “intermediate” when included between 0.50 ≤ Δ ≤ 0.75, “high” with Δ > 0.75 (Monterroso et al. 2014; Mazza et al. 2020; Mori et al. 2020b). We calculated the 95% confidence intervals for overlap coefficients as percentile intervals from 1000 bootstrap samples (Meredith and Ridout 2017).
Ambiental light and activity
We tested through a chi-square test whether the activity of wildcats was concentrated during night, crepuscular hours, or daylight (Sokal and Rohlf 2012). A Cramer’s V index was calculated to test for the size effect of the variables on wildcat detections. We considered as crepuscular hours the range time between the nautical dawn (sun is 12° below horizon) to sunrise (sun is 0.833° below horizon) and between nautical dusk (sun is 12° below horizon) to sunset (sun is 0.833° below horizon). The nautical dawn and dusk begin ~ 24 min before and after the civil dawn and civil dusk, equal to the time it takes for the earth to rotate 6°. We calculated the times of sunset and sunrise for each camera site using a specific algorithm by Meeus (1991), implemented in a VBA (visual basic for application) script, including coordinates and dates of each wildcat event. In the same way, we also classified surveyed nights following moon phases and epact, to test the effect of night sky brightness on the activity of the wildcat. Nights were classified as it follows: (1) epact days = 0–3, 26–29; (2) epact days = 4–6, 21–25; (3) epact days = 7–9, 17–20; (4) epact days = 10–16 following the approach by Mori et al. (2020b). Then, we conducted a chi-squared test on numbers of nocturnal events (i.e. excluding from this analysis diurnal and crepuscular events) in each of these moon phases, to assess if they were uniform throughout the lunar cycle (Mori et al. 2020a).
Diurnal behaviour
We set a generalized mixed linear model (GLMM) through the package ‘glmmTMB’ (Brooks et al. 2017) and ‘lme4’ (Bates et al. 2015). We created a dichotomous dependent variable using binomial errors (link: logit), labelling daylight events (after sunrise, when the sun is 0.833° below horizon, and before sunset, when the sun is 0.833° below the horizon) as “1” and darkness events (before sunrise and after sunset) as “0”. We chose these sunset and sunrise, because we wanted to include only events with a substantial daylight and not borderline events. Running this model, we aimed at figuring out the diurnal activity of the species based on our detections, outside major peaks of activity during the darkness hours.
First, we used Quantum Geographic Information System (QGIS) version 3.10 ‘A Coruña’ to calculate environmental predictors. We used the MMQGIS-Hub distance plugin and data from Corine Land Cover © European Union, Copernicus Land Monitoring Service, European Environment Agency (EEA)] and open street map (https://www.openstreetmap.org) to calculate minimum distances to woodland (broad-leaved forest, coniferous forest, mixed forest), shrubs (moors and heathland, sclerophyllous vegetation, transitional woodland/shrub), natural and semi-natural open areas (arable land, heterogeneous agricultural areas), discontinuous urban fabric and paved roads. According to the classification of the roads provide from Open Street Map, we excluded to our analysis the pathways and “minor road” (forest roads, residential roads, pedestrian roads) and we included only principal paved roads (“major roads”: motorway and highway). In the analyses we only considered environmental predictors that had a distance within 2500 m (hence our sample is reduced to N = 729) using as reference the approximate average radius of largest home-ranges of European wildcats in Italy (males and females) in literature (Anile et al. 2017). Moreover, the average daily home-range from radio-tracking data was similar, i.e., 2.26 km (Sarmento et al. 2006; Monterroso et al. 2009). Since more of 50% of our camera-trapping stations (N = 285) were farther than our limit distance to natural and semi-natural open areas and discontinuous urban fabric we did not consider these land use categories as predictors in our model. We used the ‘raster sampling’ plugin and DEM at an accuracy of 20 m (data from National Environmental Information System Network of the Italian National Institute for Environmental Protection and Research: ‘SinaNet-ISPRA’, www.mais.sinanet.isprambiente.it/ost/) to calculate the elevations of each camera-trapping station.
Overall, in our global model, we considered as predictors: (i) season, (ii) % of visible moon, (iii) elevation, (iv) distance to the nearest paved road, (v) distance to the nearest shrubs, (vi) distance to the nearest woodland, (vii) type of habitat at each camera-trap site. As random effects, we selected the year and the study areas. We assessed collinearity among predictors through correlations using the Pearson and Spearman correlation coefficient for each possible couple of predictors, using a threshold of |0.5| (Crawley 2007).
A global model was initially evaluated for this analysis with all predictors. Subsequently, all possible models were calculated with the different combinations of considered predictors, evaluated through model selection procedure based on comparison of AIC scores (Akaike Information Criterion). We identified as the best model the most parsimonious one, i.e., the one having the lowest AICc (Burnham and Anderson 2002; Richards et al. 2011). Moreover, we selected for inference all models with AICc ≤ 2 (Burnham and Anderson 2002; Harrison et al. 2018) and among these, those which were not more complex versions of the simpler model (Richards et al. 2011); we used the selection model with nesting rule to avoid retaining overly complex models (Richards et al. 2011; Harrison et al. 2018). Model selection was conducted through the R package ‘MuMIn’ (Barton 2012). We estimated parameters (95% confidence intervals and B coefficients, which is the degree of change in the response variable for every 1-unit of change in the predictor variable) of the best model by using the R packages ‘glmmTMB’ (Brooks et al. 2017) and ‘lme4’ (Bates et al. 2015). Then, the best model was validated by visual inspection of the distribution of residuals (Zuur et al. 2009) through the ‘DHARMa’ package (Hartig 2021). Model weight was standardized within the subset of selected models.
For our best model we also performed a post-hoc analysis for the categorical season predictor.
Results
Activity rhythm patterns on the national scale
We included in our analyses a total of 975 events (warm, N = 524; N = 451). The comparison between the random sample generated and our total sample underlined high overlap between two distributions (Fig. 2a–b). The Watson two test was not significant (Fig. 2c); thus, we used the total sample for the followed analyses. The European wildcat activity peaked at night on an annual level, with two main peaks around 10:00 pm and 04:00 am (Fig. 2a–c).
a Total activity rhythms of the wildcat in Italy; b activity rhythms estimated through a random subset of wildcat records; c overlap between a and b Δ4 = 0.96; 95% confidence intervals = 0 .89–0.96; Watson test: W < 0.001, P > 0.10. Coloured lines represent bootstrapped estimates of activity patterns; dashed black lines represent 95% confidence intervals
In the warm months, the wildcat activity increased from 07:00 pm, with a maximum peak between 02:00 and 04:00 am. In the cold months, the greatest activity was recorded between 05:00 pm and midnight, with a second peak at about 05:00 am (Fig. S1 in Supplementary Material).
Patterns of activity rhythms at the biogeographic scale
The overlap in all cases (Fig. S2–3 in Supplementary Material), between the activity of the wildcats in the three different biogeographic contexts, on a yearly scale was high. Nonetheless, some minor differences could be observed (Fig. 3a–c). The highest peak in the Continental area was at 10:00 pm with a second lowest peak at 05:00 am, whereas, in the Mediterranean area, the maximum peak was at 05:00 am and a second one after 11:00 pm. The wildcat had a little activity also during the daylight, with two small peaks at midday and at 04:00 pm. In the Alpine area, the activity began to increase around 04:00 pm with a peak around 11:00 pm with a plateau until 03:00 am. As to the analyses of two semesters (cold and warm), the intra-area overlap was high for the Alpine and Mediterranean areas and intermediate for the Continental area (Fig. S3 in Supplementary Material). There was a slight peak of activity in diurnal hours for the Alpine and Mediterranean areas in the warm period but not in the cold one.
Patterns of activity rhythms at the latitudinal zones scale
In the northern area, the major peak was recorded around 10:00 pm, but the activity kept being high until 05:00 am. In central Italy the wildcat had two highest peaks of activity at 10:00 pm and 04:00 am. In southern Italy, the activity of wildcat began to increase around 06:00 pm reaching the highest peak at about 03:00 am (Fig. 4a–c).
The overlap was high in all cases (Fig. S4–5 in Supplementary Material), on a yearly scale (Fig. S4 in Supplementary Material), at the semester scale (warm and cold months), at the intra-area and the inter-area levels (Fig. S5 in Supplementary Material), underlining a substantial activity overlap among the three different latitudinal zones. The peaks of activities during cold months were anticipated with respect to warm months in all latitudinal zones. Moreover, a slight increment of wildcat diurnal activity in the warm months was observed, with respect to the cold ones.
Ambiental light and activity
The wildcat activity resulted significantly dependent from night-day phases being recorded for 70.2% of our events in night ours, for 20% during daylight and for the remaining 9.8% during the twilight (χ2 = 914.87, df = 2, P < 0.001; Cramer’s V = 0.56). Nocturnal activity of the wildcats was not constant in different moon phase nights (year: χ2 = 66.54, df = 3, P < 0.01; warm months: χ2 = 30.83, df = 3, P < 0.01; cold months, χ2 = 86.85, df = 3, P < 0.01; Cramer’s V = 0.57), decreasing from darkest nights (78.25% nocturnal records) to full moon nights (21.75% nocturnal records).
Diurnal behaviour
In the global model none of the predictors were correlated and they could all be included.
In the best model we considered the follow predictors: (i) distance to the nearest shrub woods; (ii) elevation; (iii) season.
The probability of wildcat detection in daylight was favoured by a minor distance to shrubs and low altitudes (Table 2; Fig. S6 in Supplementary Material). Moreover, the model underlines that the probability of wildcat detection in daylight is higher in spring and summer compared to the autumn. Conversely, the human structures, i.e., paved roads—seem not to influence the diurnal activity of the species, since this variable was not selected to be part of the best model (Table S1 in Supplementary Material).
Discussion
In all our outcomes and pairwise comparisons, the European wildcat confirmed a predominantly nocturnal habit as also reported in other studies (Daniels et al. 2001; Germain et al. 2008; Soyumert 2020; Anile et al. 2021; Migli et al. 2021), with over 70% events falling in dark hours. Furthermore, the wildcat seemed not to select the transition period between night and daytime (only less than 10% of events were in crepuscular hours). Surprisingly, we found a 20% of our detections fell during daylight. One reasons could lie in the sample size of our study being significantly larger with respect to most of other works, carried out mostly on local or regional scale (Germain et al. 2008; Can et al. 2011; Anile et al. 2021; Migli et al. 2021) and that might have better detected this phenomenon. Moreover, our data come from a more representative geographic range that could have intercepted hidden differences in prey typology and abundance with a consequent shift in feeding habits for some individuals. Indeed, in areas where some wildcat preys are diurnal (e.g. voles), wildcats may show some diurnal activity (cf. Jiménez–Albarral et al. 2021). Inter-season activity differences showed shifts in the peaks, in line with differences in sunrise and sunset time, suggesting a preference toward total darkness.
Wildcats show several physical and physiological adaptations to nocturnal or crepuscular activity, mainly involving hunting and courtship behaviour. These adaptations include an acute auditory sense, an improved tactile sense from vibrissae and other hair tufts, and an acute sense of smell for maximizing the activity at dark (Tabor 1983), which may explain why they are mostly reported as nocturnal species. Furthermore, wildcats have large eyes with a high proportion of rods in the retina for better vision in poor low-light vision (Tabor 1983).
Amongst mammals, the activity of predators is often synchronized with the activity of their prey (Daan and Aschoff 1981; Zielinski et al. 1983; Monterroso et al. 2013), or shaped by the need of avoidance of humans or other competitors (Wang et al. 2015; Mori et al. 2020b; Murphy et al. 2021). As a matter of fact, nocturnal activity is one of the strategies that wildlife adopts to avoid encounters with humans (Gaynor et al. 2018; Nickel et al. 2020). Differently from our hypotheses, our results suggested that nocturnal activity of the wildcat was the lowest in bright moonlight nights. This behaviour might be explained in relation to the temporal behaviour of its main prey. Rodents and lagomorphs tend to avoid bright moonlight nights (Mori et al. 2014; Penteriani et al. 2013; Pratas-Santiago et al. 2016; Viviano et al. 2021), with ranging movements mostly concentrated in concealed habitats or during the darkest nights. Therefore, it is likely that the wildcat synchronized its movements with those of its main prey, e.g., by decreasing its activity in bright moonlight nights. Another reason for which the wildcat avoids bright moonlight nights could be related to the fact that increased visibility would make it less effective in predatory activity (Prugh and Golden 2014). Moreover, the avoidance of bright moonlight could also be related to the presence of apex predators, i.e., the grey wolf Canis lupus, which is present and abundant throughout Italy, and, the lynx Lynx lynx, only present with few individuals in the Alps (Loy et al. 2019). Accordingly, apex predators are mostly active in bright moonlight nights, potentially forcing mesocarnivores to be active mostly in the darkest nights (e.g. Theuerkauf et al. 2003; Penteriani et al. 2013). Several other small-sized carnivores coexist with the wildcat and may compete with this species for diet and/or spatiotemporal behaviour (cf. Mori et al. 2020b). Our result seems to agree with Di Bitetti et al. (2006), as they examined feline activity on the trails in Argentina and found that ocelots Leopardus pardalis were predominantly nocturnal with no significant differences between males and females and more active during dark sky periods (new moon near periods). However, ocelots are adapted to thrive in environments with abundance of larger carnivores (e.g. pumas Puma concolor and jaguars Panthera onca). Conversely, where the mesocarnivore guild is composed by a lower number of species or in areas where wolves are a rare occurrence, no effect of moonlight is observed in wild cats (Migli et al. 2021).
Diurnal activity occurred in about 20% of our events and was reported especially in the warm season (spring and summer) perhaps due to the activity of diurnal prey such as arthropods, reptiles, squirrels, birds (Apostolico 2003; Apostolico et al. 2005; Ragni et al. 2014), or due to, most likely, the reduced prey availability during summer which, in the Mediterranean climate, represents the limiting season. Therefore, the wildcat may switch prey to maximize hunting opportunities, considering also that during summer the daylight hours are more represented during the day. This behaviour has never been reported for the wildcat. A similar behaviour has been observed in the jaguar, which exploit diurnal hours to search for peccaries when nocturnal turtle abundance was the lowest (Carrillo et al. 2009). Furthermore, the warm period coincides with the weaning of offspring and consequently with an increase in the physiological demand for additional food resources. Accordingly, Migli et al. (2021) confirmed a peak of activity in night hours in radio-tracked wildcats in Greece, with a peak in diurnal activity of reproductive breeding females in warm months.
Diurnal movements are negatively correlated with increasing distance from shrubs, but not with forests. Thus, we suggest the importance of shrubs for the species probably due to a greater preference for protection, shelter, and the abundance of prey (Monterroso et al. 2009; Lozano et al. 2010; Ferretti et al. unpublished data). A preference for these habitats has also been revealed by wildcat monitoring in the Polish Carpathians (Okarma et al. 2002). Conversely, Anile et al. (2019) reported a preference for mixed forests in a volcanic environment of Southern Italy. In this area, the shelter provided by shrub is largely overwhelmed by the local abundance of natural cavities typical of the volcanic soil. This particular situation is related to the volcanic area and to a wildcat population which is genetically isolated from the peninsular population. Our study, including data from the whole of the Italian peninsula, stresses that shrublands are important for the species, in agreement with other studies carried out in the Mediterranean.
Wildcat occupancy has been reported as negatively affected by altitude (Anile et al. 2019). Our results hence showed that diurnal activity decreases with increasing altitude (i.e., up to 1800 m a.s.l). Nevertheless, the interpretation of this result requires further investigation.
The presence of paved roads seemed not to affect the diurnal activity of wildcats, even though the transit of vehicles generally increases during the day. This result suggests that in presence of natural shelters, such as shrubs and other protection elements, which sometimes occur on paved road sides, this species can tolerate these anthropogenic infrastructures (Jerosch et al. 2009; Wening et al. 2019). Klar et al. (2008) highlighted how human infrastructures such as roads and villages are usually avoided by the wildcat although over a certain distance (i.e., ca. 200 m for single streets and houses, ca. 900 m for villages). Thus, human infrastructures do not seem to influence the wildcat ranging movement patterns, further suggesting that a small number of main roads can be tolerated within the home-range of a wildcat, despite being an important mortality factor (Klar et al. 2009). Many of our study areas are protected areas that are in natural and rural zones with roads usually not too busy, hence further ad hoc study could be required to confirm the result. Moreover, this result could be affected by a gender factor as highlighted in Jerosch et al. (2018) suggesting a gender difference, with females avoiding the areas near roads more than males.
In conclusion, our insights shed lights on some basic ecology elements of wildcat behaviour in Italy, for the first time with a national-scale perspective and at different latitudes, including some novel information about its diurnal movements. These aspects are pivotal for the conservation and effective management of this endangered species that has been poorly studied on a large scale. Nonetheless, we are aware that gathering data from different monitoring projects, carried out with heterogeneous designs, even though complying with fundamental requirements, could lead to some inaccuracies that could be taken into consideration. We, therefore, think it should be advisable to standardize as much as possible the camera-trapping protocols and to tend to national-scale coordination in wildcat monitoring in Italy. Several in-depth analyses will be necessary for the future, holding into consideration the behaviour of wildcat prey and/or direct potential competitors, including Martes spp., red foxes Vulpes vulpes, apex predators (wolves and lynxes), golden jackals Canis aureus and, locally, alien species too (e.g., the racoon Procyon lotor and the genet Genetta genetta). Moreover, another element to investigate is the spatio-temporal behaviour of wildcats in relation to climate change scenarios, particularly in the context of the Mediterranean region, with drought currently lasting longer than in the past, and potentially leading to behavioural modulation changes.
To conclude, the data collected during this study made it possible to highlight that the currently known distribution of the species is lacking and needs further nationwide studies, essential to properly describe the range of the European wildcat in the Italian territory.
References
Anile S, Ragni B, Randi E, Mattucci F, Rovero F (2014) Wildcat population density on the Etna volcano, Italy: a comparison of density estimation methods. J Zool 293:252–261
Anile S, Bizzarri L, Lacrimini M, Sforzi A, Ragni B, Devillard S (2017) Home-range size of the European wildcat (Felis silvestris silvestris): a report from two areas in central Italy. Mammalia 8:1–11
Anile S, Devillard S, Ragni B, Rovero F, Mattucci F, Lo Valvo M (2019) Habitat fragmentation and anthropogenic factors affect wildcat Felis silvestris silvestris occupancy and detectability on Mt. Etna Wildl Biol 1:1–13
Anile S, Devillard S, Nielsen CK, Lo Valvo M (2021) Anthropogenic threats drive spatio-temporal responses of wildcat on Mt. Etna Eur J Wildl Res 67:50
Apostolico F (2003) Feeding behaviour of European wildcat Felis silvestris silvestris Schreber, 1777 in Italy. Degree thesis. University of Perugia, Italy.
Apostolico F, Vercillo F, Ragni B (2005) Variations of prey species for Felis silvestris silvestris in Italy in the last 30 years. Hystrix 1:94
Ashby KR (1972) Patterns of daily activity in mammals. Mammal Rev 1:171–185
Barton K (2012) MuMIn: multi-model inference. R package version 1.15.6. Available at: https://cran.r-project.org/web/packages/MuMIn. Accessed 27 June 2021.
Bastianelli ML, Premier J, Herrmann M, Anile S, Monterroso P, Kuemmerle T, Dormann CF, Streif S, Jerosch S, Götz M, Simon O, Moleón M, Gil-Sánchez JM, Biró Z, Dekker J, Severon A, Krannich A, Hupe K, Germain E, Pontier D, Janssen R, Ferreras P, Díaz-Ruiz F, López-Martín JM, Urra F, Bizzarri L, Bertos-Martín E, Dietz M, Trinzen M, Ballesteros-Duperón E, Barea-Azcón JM, Sforzi A, Poulle M-L, Heurich M (2021) Survival and cause-specific mortality of European wildcat (Felis silvestris) across Europe. Biol Cons 261:109239
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Soft 1:67
Beaumont M, Barratt EM, Gottelli D, Kitchener AC, Daniels MJ, Pritchard JK, Bruford MW (2001) Genetic diversity and introgression in the Scottish wildcat. Mol Ecol 10:319–336
Bhatt U, Singh Adhikari B, Habib B, Lyngdoh S (2021) Temporal interactions and moon illumination effect on mammals in a tropical semi evergreen forest of Manas National Park, Assam, India. Biotropica 53:831–845
Borchers D, Distiller G, Foster R, Harmsen B, Milazzo L (2014) Continuous-time spatially explicit capture-recapture models, with an application to a jaguar camera-trap survey. Meth Ecol Evol 5:656–665
Brivio F, Grignolio S, Brogi R, Benazzi M, Bertolucci C, Apollonio M (2017) An analysis of intrinsic and extrinsic factors affecting the activity of a nocturnal species: the wild boar. Mamm Biol 84:73–81
Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Mächler MM, Bolker BM (2017) GlmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. The R J 9:378–400
Can Ö, Kandemi̇r İ, Toga İ (2011) The wildcat Felis silvestris in northern Turkey: assessment of status using camera trapping. Oryx 45(1):112–118. https://doi.org/10.1017/S0030605310001328
Carrillo E, Fuller TK, Saenz JC (2009) Jaguar (Panthera onca) hunting activity: effects of prey distribution and availability. J Trop Ecol 25:563–567
Crawley M (2007) The R Book. Wiley, Chichester
Daan S, Aschoff J (1981) Short-term rhythms in activity. In: Aschoff J (ed) Biological rhythms. Springer, Boston, pp 491–498
Daniels MJ, Beaumont MA, Johnson PJ, Balharry D, Macdonald DW, Barratt E (2001) Ecology and genetics of wild-living cats in the north-east of Scotland and the implications for the conservation of the wildcat. J Appl Ecol 38:146–161
Devillard S, Jombart T, Léger F, Pontier D, Say L, Ruette S (2014) How reliable are morphological and anatomical characters to distinguish European wildcats, domestic cats and their hybrids in France? J Zool System Evol Res 52:154–162
Di Bitetti MS, Paviolo A, De Angelo C (2006) Density, habitat use and activity patterns of ocelots (Leopardus pardalis) in the Atlantic forest of Misiones, Argentina. J Zool 270:153–163
Fonda F, Bacaro G, Battistella S, Chiatante GP, Pecorella S, Pavanello M (2022) Population density of European wildcats in a pre-alpine area (northeast Italy) and an assessment of estimate robustness. Mamm Res 67:9–20
Gavagnin P (2021) The European wildcat in the Italian western range: something new? Atti Mus Sto Nat Maremma 25:63–72
Gaynor KM, Hojnowski CE, Carter NH, Brashares JS (2018) The influence of human disturbance on wildlife nocturnality. Science 360:1232–1235
Germain E, Benhamou S, Poulle ML (2008) Spatio-temporal sharing between the European wildcat, the domestic cat and their hybrids. J Zool 276:195–203
Gese EM (2001) Monitoring of terrestrial carnivore populations. In: Gittleman JL, Funk SM, Macdonald DW, Wayne RL (eds) Carnivore conservation. Cambridge University Press, Cambridge, pp 372–396
Gordigiani L, Viviano A, Brivio F, Grignolio S, Lazzeri L, Marcon A, Mori E (2021) Carried away by a moonlight shadow: activity of wild boar in relation to nocturnal light intensity. Mamm Res. https://doi.org/10.1007/s13364-021-00610-6
Harrison XA, Donaldson L, Correa-Cano ME, Evans J, Fisher DN, Goodwin CED, Robinson BS, Hodgson DJ, Inger R (2018) A brief introduction to mixed effects modelling and multi-model inference in ecology. PeerJ 6:e4794
Hartig F (2021) DHARMa: residual diagnostics for hierarchical (multi-level/mixed) regression models. Theoretical ecology. University of Regensburg, Regensburg
Huck M, Juárez CP, Fernández-Duque E (2017) Relationship between moonlight and nightly activity patterns of the ocelot (Leopardus pardalis) and some of its prey species in Formosa, Northern Argentina. Mamm Biol 82:57–64
Jerosch S, Götz M, Klar N, Roth M (2009) Characteristics of diurnal resting sites of the endangered European wildcat (Felis silvestris silvestris): Implications for its conservation. J Nat Cons 18:45–54
Jiménez-Albarral JJ, Urra F, Jubete F, Roman J, Revilla E, Palomares F (2021) Abundance and use pattern of wildcats of ancient human-modified cattle pastures in northern Iberian pensinsula. Eur J Wildl Res 67:94
Jordan NR, Cherry MI, Manser MB (2007) Latrine distribution and patterns of use by wild meerkats: implications for territory and mate defence. Anim Behav 73:613–622
Karanth KU, Srivathsa A, Vasudev D, Puri M, Parameshwaran R, Kumar NS (2017) Spatio-temporal interactions facilitate large carnivore sympatry across a resource gradient. Proc R Soc B 284:20161860
Kerr JT (1997) Species richness, endemism, and the choice of areas for conservation. Conserv Biol 11:1094–1100
Kikuchi DM, Zhumabai Uulu K, Sharma K, Soma T, Kinoshita K (2020) Is water an important resource for the snow leopard (Panthera uncia) in periods when terrain is covered with snow? Arc Ant Alp Res 52:105–108
Kilshaw K, Johnson PJ, Kitchener AC, Macdonald DW (2015) Detecting the elusive Scottish wildcat Felis silvestris silvestris using camera trapping. Oryx 49:207–215
Kitchener AC, Yamaguchi N, Ward JM, Macdonald DW (2005) A diagnosis for the Scottish wildcat (Felis silvestris): a tool for conservation action for a critically-endangered felid. Anim Cons 8:223–237
Klar N, Fernández N, Kramer-Schadt S, Herrmann M, Trinzen M, Büttner I, Niemitz C (2008) Habitat selection models for European wildcat conservation. Biol Cons 141:308–319
Klar N, Herrmann M, Kramer‐Schadt S (2009) Effects and mitigation of road impacts on individual movement behavior of wildcats. J Wildl Manage 73:631–638
Lashley MA, Cove MV, Chitwood MC, Penido G, Gardner B, DePerno CS, Moorman CE (2018) Estimating wildlife activity curves: comparison of methods and sample size. Sci Rep 8:1–11
Leuchtenberger C, Zucco CA, Ribas C, Magnusson W, Mourão G (2014) Activity patterns of giant otters recorded by telemetry and camera traps. Ethol Ecol Evol 26:19–28
Linkie M, Ridout MS (2011) Assessing tiger-prey interactions in Sumatran rainforests. J Zool 284:224–229
Loy A, Aloise G, Ancillotto L, Angelici FM, Bertolino S, Capizzi D, Castiglia R, Colangelo P, Contoli L, Cozzi B, Fontaneto D, Lapini L, Maio N, Monaco A, Mori E, Nappi A, Podestà M, Russo D, Sarà M, Scandura M, Amori G (2019) Mammals of Italy: an annotated checklist. Hystrix 30:87–106
Lozano J (2010) Habitat use by European wildcats (Felis silvestris) in central Spain: what is the relative importance of forest variables? Anim Biodivers Conserv 33:143–150
Lund U, Agostinelli C, Arai H, Gagliardi A, Portugues EG, Giunchi D, Irisson JO, Pocernich M, Rotolo F (2017) Circular statistics. https:// cran.r-project.org/web/packages/circular/circular.pdf. Accessed on 10.10.2021.
Mattucci F, Oliveira R, Bizzarri L, Vercillo F, Anile S, Ragni B, Lapini L, Sforzi A, Alves PC, Lyons LA, Randi E (2013) Genetic structure of wildcat (Felis silvestris) populations in Italy. Ecol Evol 3:2443–2458
Mattucci F, Oliveira R, Lyons LA, Alves PC, Randi E (2016) European wildcat populations are subdivided into five main biogeographic groups: consequences of Pleistocene climate changes or recent anthropogenic fragmentation? Ecol Evol 6:3–22
Mazza G, Marraccini D, Mori E, Priori S, Marianelli L, Roversi PF, Gargani E (2020) Assessment of color response and activity rhythms of the invasive black planthopper Ricania speculum (Walker, 1851) using sticky traps. Bull Entomol Res 110:480–486
Meredith M, Ridout M (2014) Overview of the overlap package. https://cran.cs.wwu.edu/web/packages/overlap/vignettes/overl ap.pdf. Accessed on 19.08.2021.
Meredith M, Ridout M (2017) Overlap: estimates of coefficient of overlapping for animal activity patterns, https://cran.r-project.org/web/packages/overlap/overlap.pdf. Accessed on 10.10.2021.
Migli D, Astaras C, Boutsis G, Diakou A, Karantanis NE, Youlatos D (2021) Spatial ecology and diel activity of European wildcat (Felis silvestris silvestris) in a protected lowland area in Northern Greece. Animals 11:3030
Monterroso P, Brito JC, Ferreras P, Alves PC (2009) Spatial ecology of the European wildcat in a Mediterranean ecosystem: dealing with small radio-tracking datasets in species conservation. J Zool 279:27–35
Monterroso P, Alves PC, Ferreras P (2013) Catch me if you can: diel activity patterns of mammalian prey and predators. Ethol 119:1044–1056
Monterroso P, Alves PC, Ferreras P (2014) Plasticity in circadian activity patterns of mesocarnivores in southwestern Europe: implications for species coexistence. Behav Ecol Sociobiol 68:1403–1417
Mori E, Nourisson DH, Lovari S, Romeo G, Sforzi A (2014) Self-defence may not be enough: moonlight avoidance in a large, spiny rodent. J Zool 294:31–40
Mori E, Andreoni A, Cecere F, Magi M, Lazzeri L (2020a) Patterns of activity rhythms of invasive coypus Myocastor coypus inferred through camera-trapping. Mammal Biol 100:591–599
Mori E, Bagnato S, Serroni P, Sangiuliano A, Rotondaro F, Marchianò V, Cascini V, Poerio L, Ferretti F (2020b) Spatiotemporal mechanisms of coexistence in an European mammal community in a protected area of southern Italy. J Zool 310:232–245
Murphy A, Diefenbach DR, Ternent M, Lovallo M, Miller D (2021) Threading the needle: how humans influence predator–prey spatiotemporal interactions in a multiple-predator system. J Anim Ecol 90:2377–2390
Nickel BA, Suraci JP, Allen ML, Wilmers CC (2020) Human presence and human footprint have non-equivalent effects on wildlife spatiotemporal habitat use. Biol Cons 241:108383
O’Connell AF, Nichols JD, Karanth KU (2011) Camera traps in animal ecology. Methods and analyses. Springer, New York
Okarma H, Śnieżko S, Olszańska A (2002) The occurrence of wildcat in the Polish Carpathian Mountains. Acta Theriol 47:499–504
Pearman PB, Guisan A, Broennimann O, Randin CF (2008) Niche dynamics in space and time. Trends Ecol Evol 23:149–158
Penteriani V, Kuparinen A, del Mar DM, Palomares F, Lopez-Bao JV, Fedriani JM, Calzada J, Moreno S, Villafuerte R, Campioni L, Lourenço R (2013) Responses of a top and a meso predator and their prey to moon phases. Oecol 173:753–766
Pratas-Santiago LP, Gonçalves ALS, da Maia Soares AMV, Spironello WR (2016) The moon cycle effect on the activity patterns of ocelots and their prey. J Zool 299:275–283
Prugh LR, Golden CD (2014) Does moonlight increase predation risk? Meta-analysis reveals divergent responses of nocturnal mammals to lunar cycles. J Anim Ecol 83:504–514
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/ Accessed on 19.02.2022.
Ragni B, Mandrici A (2003) L’areale italiano del gatto selvatico europeo (Felis silvestris silvestris): ancora un dilemma? IV National Congress of Teriology-Scientific Research and Conservation of Mammals in Italy. 6–8 November 2003, Riccione, Italy.
Ragni B, Possenti M (1996) Variability of coat-colour and markings system in Felis silvestris. Ital J Zool 63:285–292
Ragni B, Lucchesi M, Tedaldi G, Vercillo F, Fazzi P, Bottacci A, Quilghini G (2014) European Wildcat in Casentinesi Natural Reserves. Graphic Arts Cianferoni Editions, Stia
Richards SA, Whittingham MJ, Stephens PA (2011) Model selection and model averaging in behavioural ecology: the utility of the IT-AIC framework. Behav Ecol Sociobiol 65:77–89
Ridout MS, Linkie M (2009) Estimating overlap of daily activity patterns from camera trap data. J Agric Biol Environm Stat 14:322–337
Roedenbeck IA, Fahrig L, Findlay C, Houlahan JE, Jaeger JAG, Klar N, Kramer-Schadt S, Van der Grift EA (2007) The Rauischholzhausen agenda for road ecology. Ecology and Society 12:11. https://www.ecologyandsociety.org/vol12/iss1/art11/. Accessed on 7 January 2021.
Rossa M, Lovari S, Ferretti F (2021) Spatiotemporal patterns of wolf, mesocarnivores and prey in a Mediterranean area. Behav Ecol Sociobiol 75:1–13
RStudio Team (2020) RStudio: Integrated Development for R. RStudio, PBC, Boston, MA URL http://www.rstudio.com/ Accessed on 19.02.2022.
Sarmento P, Cruz J, Tarroso P, Fonseca C (2006) Space and habitat selection by female European wildcats (Felis silvestris silvestris). Wildl Biol Pract 2:79–89
Sokal RR, Rohlf FJ (2012) Biometry, 3rd edn. W.H. Freeman and Company, New York
Soyumert A (2020) Camera-trapping two felid species: monitoring Eurasian lynx (Lynx lynx) and wildcat (Felis silvestris) populations in mixed temperate forest ecosystems. Mamm Study 45:41–48
Spellerberg IF (2002) Ecological effects of roads. Science, Enfield
Steyer K, Kraus RH, Mölich T, Anders O, Cocchiararo B, Frosch C, Geib A, Gotz M, Herrmann M, Hupe K, Kohnen A, Kruger M, Muller F, Pir JB, Reiners TE, Roch S, Schade U, Schiefenhovel P, Siemund M, Simon O, Steeb S, Streif S, Streit B, Thein J, Tiesmeyer A, Trinzen M, Vogel B, Nowak C (2016) Large-scale genetic census of an elusive carnivore, the European wildcat (Felis s. silvestris). Cons Gen 17:1183–1199
Tabor RK (1983) The wildlife of the domestic cat. Arrow Books Editions, London
Theuerkauf J, Jȩdrzejewski W, Schmidt K, Okarma H, Ruczyński I, Śniezko S, Gula R (2003) Daily patterns and duration of wolf activity in the Białowieza Forest, Poland. J Mammal 84:243–253
Tobler MW, Carrillo-Percastegui SE, Leite Pitman R, Mares R, Powell G (2008) An evaluation of camera traps for inventorying large- and medium-sized terrestrial rainforest mammals. Anim Cons 11:169–178
Tormen G, Catello M, Deon R, Galletti A (2020) Il gatto selvatico europeo (Felis silvestris silvestris Schreber, 1977) in Veneto. Notiziario Anno 2020. Gruppo Natura Bellunese 1:27–38
Torretta E, Serafini M, Puopolo F, Schenone L (2016) Spatial and temporal adjustments allowing the coexistence among carnivores in Liguria (NW Italy). Acta Ethol 19:123–132
Tsunoda H, Newman C, Peeva S, Raichev E, Buesching CD, Kaneko Y (2020) Spatio-temporal partitioning facilitates mesocarnivore sympatry in the Stara Planina Mountains. Bulgaria Zoology 141:125801
Velli E, Bologna MA, Castelli S, Ragni B, Randi E (2015) Non-invasive monitoring of the European wildcat (Felis silvestris silvestris Schreber, 1777): comparative analysis of three different monitoring techniques and evaluation of their integration. Eur J Wildl Res 61:657–668
Viviano A, Mori E, Fattorini N, Mazza G, Lazzeri L, Panichi A, Strianese L, Mohamed WF (2021) Spatiotemporal overlap between the European brown hare and its potential predators and competitors. Animals 11:562
Wang Y, Allen ML, Wilmers CC (2015) Mesopredator spatial and temporal responses to large predators and human development in the Santa Cruz Mountains of California. Biol Conserv 190:23–33
Wening H, Werner L, Waltert M, Port M (2019) Using camera traps to study the elusive European Wildcat Felis silvestris silvestris Schreber, 1777 (Carnivora: Felidae) in central Germany: what makes a good camera trapping site? J Threaten Taxa 11:13421–13431
Yamaguchi N, Kitchener A, Driscoll C, Nussberger B (2015) Felis silvestris. The IUCN Red List of Threatened Species 2015: e.T60354712A506523
Zielinski WJ, Spencer WD, Barrett RH (1983) Relationship between food habits and activity patterns of pine martens. J Mammal 64:387–396
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer Editions, New York
Acknowledgements
The authors thank: Giacomo Gervasio, Francesca Crispino and Salvatore Urso for the data collected as part of the Aspromonte National Park Authority project: “Studio su mesocarnivori del Parco Nazionale dell’Aspromonte mediante l’utilizzo di fototrappole”; Milena Provenzano and Vincenzina Fava for the data collected as part of the Aspromonte National Park Authority, project: “Convivere con il lupo: conoscere per preservare”; Coordinator Eng. Francesco Mordente for the data collected as part of the "Monitoraggio ambientale per il completamento dello schema idrico del torrente Menta" carried out by So.Ri.Cal. S.p.A. (Reggio Calabria); Enrico Vettorazzo (Dolomiti Bellunesi National Park), Mauro Bon (Museo di Storia Naturale G. Ligabue) and Fabio Dartora for the data collected as part of the project “Progetto di fototrappolaggio dei mustelidi e del gatto selvatico (Felis s. silvestris) nel Parco Nazionale delle Dolomiti Bellunesi”, Ivan Mazzon for data collected from Dolomiti Bellunesi; Unione Montana Alta Val di Cecina and Marco Pini for the data collected in Montioni Regional Park; Claudia Iozzelli and Luca Cecconi for the data gathered in Acquerino Cantagallo Regional Reserve; Alex Nardone for the records around Cassino (FR); the Maremma Regional Park Agency and its staff; students and collaborators of Department of Life Sciences—University of Siena—for support in data collection and entry; Arianna Dissegna, (University of Padova) for data collected as part of the phd project “Research and implementation of an integrated monitoring system of the wolf population on Foreste Casentinesi National Park”; Giulia Bianchi, Lorena di Benedetto for data organization in Foreste Casentinesi National Park; Carabinieri Foreste Casentinesi National Park Department and Carabinieri Biodiversity Department of Pratovecchio for data collection; Dr. Niccolò Fattorini for the advice about statistical analysis. Moreover, we thank Tommaso Nuti, Silvia Mascagni, Renato Cottalasso, Luca Cherubini. We would like to thank the Carabinieri Biodiversity Department in Castel di Sangro, Monte Genzana Alto Gizio Nature Reserve, Salviamo l'Orso and Rewilding Apennine for providing data from the Abruzzo region. The people involved in the activities including Col. Luciano Sammarone, Lt. Col. Bruno Petriccione, Antonio di Croce and Mario Cipollone for project coordination while Mario Romano, Mario Posillico, Filippo La Civita, Paula Mayer, Antonio Monaco, Fabrizio Cordischi, Julien Leboucher, Simone Giovacchini, Irene Shivji, and Jan-Niklas Trei for collecting data in the field. The data collection for the Abruzzo region was partially supported by the LIFEESC360/LIFE17 ESC IT 001 project. Data from Prealpi Carniche were provided by ‘Canislupus Italia ONLUS’, ‘Alka Wildlife ops’, Leandro Dreon, Luca Dorigo, Andrea Caboni, Stefano Pesaro, Cristina Rieppi, Marko Zupan, Fabio Marcolin, and the ‘Corpo Forestale Regionale’ of Friuli Venezia Giulia. Finally, we would also thank Alessio Ottogalli and Adriana Isolani for English revision of the manuscript. This study was not intended to update the wildcat distribution in Italy. A national project is already underway on this topic (www.gattoselvatico.it). Nevertheless, we added a map of the distribution (Fig. S8 in Supplementary Material) since, among all data collected by us or belonging to other unpublished personal archives (including detections not comprised in the above analyses), some were interesting for their collocation in areas not yet included in the currently formal national distribution of the species. Authors acknowledge Prof. Marco Bologna (University of Roma Tre), Silvia Castelli and Nicole Marini for their support in the surveys in the Foreste Casentinesi National Park, and Federica Mattucci, Romolo Caniglia and Nadia Mucci (ISPRA), for their advice and experience. Authors would like to thank three anonymous reviewers for their useful comments on our manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit. This work was partially supported by Italian MIUR (Italian Ministry of University and Public Instructions) and Dolomiti Bellunesi National Park for the data collected in Belluno area (NE Italy).
Author information
Authors and Affiliations
Contributions
LL, PF, ML, EM, EV and Asp: conceived, planned the study and requested the data of the study areas. LL: collected data for Val di Cecina; PF and ML: collected data for Casentinesi State Natural Reserve; EM: collected data for Prata’s hills; EV and NC: collected data for Foreste Casentinesi National Park; FC: collected data for Pistoia’s Apennine; ASi: coordinated the projects in Aspromonte National Park; FFo, MP and SP: collected data for Friuli Venezia Giulia. ASa: collected data for Pollino National Park; FFe: collected data in Maremma Regional Park; ASf: participated in data validation for Maremma Regional Park; Asp: collected the data for Dolomiti Bellunesi National Park and Torre and Malina municipal park; CP: collected the data for Abruzzo region. LL and EM: conducted statistical analyses. PF and Asp: conducted the GIS analyses. LL, PF, ML, EM, EV and Asp: wrote the first draft; all authors participated in writing the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors certify that they have no affiliation with or involvement in any organization or entity with any financial or non-financial interest in the subject matter or materials discussed in this manuscript. Therefore, they have no conflict of interest to declare.
Additional information
Handling editor: Adriano Martinoli.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lazzeri, L., Fazzi, P., Lucchesi, M. et al. The rhythm of the night: patterns of activity of the European wildcat in the Italian peninsula. Mamm Biol 102, 1769–1782 (2022). https://doi.org/10.1007/s42991-022-00276-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42991-022-00276-w