Abstract
The coypu, Myocastor coypus, has been introduced worldwide for fur farming and is widely recognized as one of the most invasive alien mammals of the world, affecting natural ecosystems, crops and possibly human health. Here we present a comprehensive up-to-date review of its distribution and status in Asia and Africa. Using a multi-source approach, we collected occurrences from published literature as well as from online biodiversity platforms (e.g. GBIF, iNaturalist), video sharing platforms, and local experts. Additionally, we used an ensemble modelling approach to predict the climatic suitability across Africa and Asia. We present an updated distribution map, including a total of 1506 spatially explicit records from 1973 to 2021, covering 1 African and 16 Asian countries. We find evidence for current populations in Kenya and five new countries since the last review of (Carter and Leonard, Wildl Soc Bull 30:162–175, 2002): Iran, Jordan, Lebanon, Uzbekistan, and Vietnam, and identify main clusters of coypu occurrence in Western (including Transcaucasia) and East Asia. We show that warm temperate and Mediterranean areas on both continents are predicted to be climatically suitable for the coypu and highlight not only areas of possible spread, but also potential data gaps, i.e. with high suitability and low availability of concrete information (e.g. China, Southern Russia). We emphasize the importance of citizen involvement and the urgency for coypu-targeted studies in data-poor regions to obtain a clear picture of the geographical distribution and to better address management strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The coypu, Myocastor coypus is a large semiaquatic rodent native to the subtropical and temperate regions of South America (Woods et al. 1992; Carter and Leonard 2002), and it is considered as one of the worst alien mammals globally (Tricarico et al. 2016). It has been widely introduced outside of its native range for fur farming, and now occurs on all continents except Oceania and Antarctica (Carter and Leonard 2002). When introduced to suitable environments, coypus can quickly reach high population densities and consequently damage natural and human-modified ecosystems by (i) feeding on natural vegetation and crops (e.g. > 11,000,000 € in 5 years in Italy: Panzacchi et al. 2007), (ii) destabilizing riverbanks through burrowing activity (Harvey et al. 2019) and (iii) negatively affecting native animals, e.g. by crashing the eggs of water birds or feeding on mussels (Bertolino et al. 2011; Nagayama et al. 2020). Furthermore, it represents a potential reservoir for several diseases and zoonoses (e.g. infections with Bartonella sp., Leptospira sp., Toxoplasma gondii and Fasciola sp.; Capizzi et al. 2018, Nardoni et al. 2011).
Despite its widespread distribution and invasive potential, studies mostly focused on Europe and Northern America (e.g. Hilts et al. 2019; Schertler et al. 2020). Already two decades ago, Carter and Leonard (2002) pointed out that a comprehensive review on the coypu was not available for Africa and that information for Asian countries was often anecdotal and/or outdated, leading to an unknown status for many countries of introduction in those continents. However, recent studies from South Korea, Iran, Jordan, and Japan do indeed discuss the species’ range expansion (Farashi and Najafabadi 2015; Kawamura et al. 2018; Khoury et al. 2012; Kim et al. 2019a). This highlights the need for an updated overview and redefinition of the current distribution status of the coypu in Afro-Asia, where it poses an environmental and socio-economic risk.
Citizen-science can be a valuable method to map the distribution of invasive alien species, whose large-scale monitoring would otherwise require a well-addressed, time-consuming, and expensive field effort (Johnson et al. 2020). This approach is effective mostly for diurnal, large-sized and unmistakable species (Goldstein et al. 2014; Mori et al. 2017), making the coypu a suitable species (Tsiamis et al. 2017; Milanesi et al. 2020). Although citizen-science in Asia and Africa is a less-common practice compared to Europe, North America, and Oceania (Chandler et al. 2017), citizen-science platforms (e.g. iNaturalist), other social media platforms (e.g. Social Networks, Flickr and YouTube) and virtual communication services constitute an extraordinary opportunity to obtain additional information on species distributions (Mori et al. 2017). Therefore, to review and update the distribution status of the coypu in Africa and Asia, we attempted a multi-source approach, utilizing not only established biodiversity platforms and scientific literature, but also screening social media platforms. We (1) provide an updated distribution map, depicting countries of presence and derived spatially explicit occurrence points, (2) give short summary accounts for the regions of introductions and (3) use an ensemble modelling approach to project the potential current distribution of the coypu in Afro-Asia and (4) compare these findings with its updated known distribution in Afro-Asia, to depict possible areas of spread/monitoring and point out potential data gaps. Finally, we emphasize changes since the last review on the coypu’s distribution (Carter and Leonard 2002).
Methods
Occurrence records of coypus in Asia and Africa were collected between November 2020 and February 2021 through a range of sources: (1) scientific papers; (2) books, technical reports and grey literature (including observations posted on the image hosting website Flickr, on Social Networks and on YouTube); (3) online databases (e.g. iNaturalist “research grade” observations, GBIF, Biodiversity Center of Japan); (4) observations carried out by local experts and wildlife managers.
A detailed literature search was conducted on ISI Web of Science, Scopus and Google Scholar to collect published studies dealing with distribution of coypu in Asia and Africa. We used combinations of the following keywords, in ten languages (English, Italian, French, Spanish, Portuguese, Russian, Japanese and Mandarin): Myocastor, coypu*, Africa, Asia, non-native*, alien*, introduc*, invasi*, allochthonous, naturalized. Articles and books that were retrieved through the resulting reference list were also included. The final literature list is provided in Supplementary Material 1. We cannot rule out that other studies reporting coypu populations in Asia and Africa may occur in different languages (e.g. Arabic, Afrikaans), although we checked all of the main languages used in the scientific literature (Mori et al. 2017). Occurrences of coypus in fur farms and enclosures were not included in our analyses. As to online databases and direct observations, we assessed data reliability by contacting authors, searching for literature confirming species presence or records corroborated by pictures. Occurrence records that did not initially include coordinate information were georeferenced, either by requesting the associated coordinates from data holders or by interpretation of the textual information provided, using the recommendations of Chapman and Wieczorek (2020) as a guideline. We either assigned coordinates and an approximate associated coordinate uncertainty or a geographic shape (e.g., a province/region). Finally, we mapped the country-wise presence and the spatial distribution of derived occurrence records across Africa and Asia.
Additionally, we compared the updated distribution of the coypu with its potential distribution under current climatic conditions by projecting a global species distribution model onto our study region. Therefore, we combined worldwide coypu occurrences (from its native and introduced range) with six bioclimatic predictors to construct ensemble models and predict the suitability of our study region, using the biomod2 framework (see Figures S1–S3 in Supplementary Material 2). For Northern and Southern America, we downloaded GBIF and iNaturalist occurrences, and for Europe we combined the dataset of Schertler et al. (2020) with recently published occurrences from both platforms. Only point occurrences with a coordinate uncertainty of less than 10 km were considered for modelling (if this information was missing, we assumed the uncertainty to be lower than our threshold). Further, we discarded duplicate records (with identical coordinates), old records (pre-1980) and obviously erroneous records (using the country centroid as point occurrence or most likely resulting from misidentification). Removing old records resulted in a more reasonable distribution for Northern America, where the coypu is thought to have never established in a number of central states (Jarnevich et al. 2017). As there was still a notable clustering of records after our cleaning procedure, we spatially thinned the dataset, setting a threshold distance of 50 km to minimize effects of sampling bias, using the spThin package (Aiello-Lammens et al. 2015). We then transferred all remaining point occurrences on a 25 × 25 km grid. As our dataset consists of presences only, we implemented a target-group approach for pseudo-absence data sampling. Here, we used estimates on the completeness of digitally accessible information for mammals (Meyer et al. 2015) as probability surface for the sampling. Their study focused on GBIF, which, as the global most important accessible biodiversity repository, is thought to be representative of general sampling bias patterns. We excluded the Antarctic, as well as biogeographic regions where no introductions had happened in the past (Australia, Pacific islands), as well as grid cells that contained occurrences excluded by the thinning algorithm, from the pseudo-absence sampling procedure (Carter and Leonard 2002). We selected six bioclimatic predictors from CHELSA (Karger et al. 2017a, b), taking into account the biology of the coypu (Woods et al. 1992; Doncaster and Micol 1990; Gosling and Baker 1989) and previous studies (Jarnevich et al. 2017; Schertler et al. 2020): bio06 (minimal temperature of coldest month), bio2 (mean diurnal range), bio7 (temperature annual range), bio12 (mean annual precipitation), bio15 (precipitation seasonality), and bio17 (precipitation of the driest quarter). Predictors were all centred and scaled, and showed reasonable collinearity as they did not exceed a VIF > 6 (Naimi et al. 2014) and pairwise Pearson’s correlations were not larger than |0.73|. To account for inter-variability of different modelling approaches, we used the biomod2 package (Thuiller et al. 2020) to create an ensemble of five different modelling methods, representative for different techniques, i.e., regression-based (GLM; generalized linear model, GAM; generalized additive model), machine-learning (GBM; generalized boosted regression models, RF; random forest) and a profile technique (SRE; Surface-range envelope). To test the robustness of our pseudo-absence selection, we compiled the single models with three random sets of pseudo-absences (n = 10,000). As no differences in model output were found, for computation of ensembles we continued with only one set of pseudo-absences. Only models with a TSS (True Skill Statistic) > 0.7 were included in ensembles. Binary projections were derived by using a threshold that maximises sensitivity (i.e. correctly predicted presences, here 95%), whilst keeping a reasonable specificity, following the recommendation of Jiménez-Valverde et al. (2011). Occurrences were compared with the binary map resulting from the ensemble projection.
All analyses, data manipulation, modelling and visualization were done with R v.4.0.5 (R Core Team 2021) and R Studio (RStudio Team 2020); using the following packages: biomod2 (Thuiller et al. 2020), raster (Hijmans 2020), sf (Pebesma 2018), dplyr (Wickham et al 2021), usdm (Naimi et al. 2014), PresenceAbsence (Freeman and Moisen 2008), ggplot2 (Wickham 2016), spThin (Aiello-Lammens et al. 2015) and cowplot (Wilke 2019).
Results
We collected a total of 1506 occurrences of Myocastor coypus in Asia and Africa, thus comprising the most recent and complete dataset on the distribution of coypu in both continents. Of those, 621 records were derived from biodiversity platforms (416 from GBIF and iNaturalist, and 205 from other national citizen-science platforms), 295 from literature, 562 from personal communications with other researchers and 28 through YouTube and Flickr. The dataset covers the time span from 1973 to 2021, with three quarters of records being from the last two decades.
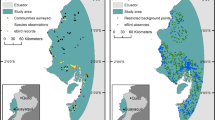
The species was recorded in 17 countries (16 Asian and 1 African), with the Asian occurrences mainly clustered in two macro-areas, namely Western Asia including Transcaucasia, and East Asia (Fig. 1). All, except seven records, for which the textual description named river stretches only, either were originally derived with coordinates or could be georeferenced; 17 records could be assigned to provinces/regions only, as more precise information was missing. The number of occurrences obtained was not balanced per country: ~ 89% of records (1333/1501) were from only 4 countries (Armenia, Israel, Japan, South Korea), where higher efforts have been put by academic researchers or citizens.
Distribution of the coypu across Africa and Asia: a overview, b Eastern Africa, c Western Asia and d East Asia. Countries of coypu presence are colored in orange; dots represent georeferenced records. Note that for areas where available information was restricted to the regional level those regions are depicted in dark orange (e.g. China, Vietnam, North-western Iran)
In the following, short summaries for the different regions are given:
Africa
Current occurrences were restricted to Kenya while old reports of coypu occurrences in other Sub-Saharan countries (Botswana, Tanzania, Zambia, Zimbabwe) mentioned by Carter and Leonard (2002) could not be confirmed (Happold et al. 2013).
Kenya: Coypus were introduced in the 1950s east of the Rift Valley and found in the wild from around 1965 (Gherardi et al. 2011; Happol et al. 2013). Whereas Carter and Leonard (2002) state that the populations disappeared in the 1980s, there are current sightings at Lake Navaisha (Happold et al. 2013), described as localized and ‘ecologically neutral’ by Gherardi et al. (2011). Coypus were also recorded in Laikipia district (Happold et al. 2013) and long-term mammal monitoring data showed increased counts in the Aberdare National Park in the 2000s (Massey 2013).
Western and Central Asia
Coypus were widely introduced for their fur and meat and often raised in a semi-captive way, enabling the spread of the species. Carter and Leonard (2002) point out the unknown status for several countries in those areas.
Israel/Jordan/Lebanon/Palestine: in Israel, after fur farming failure, coypus were released and are nowadays commonly present in the Mediterranean part of the country, along Jordan and Yarmouk rivers, in their tributaries and in the northern mouth of the Dead Sea (Khouri et al. 2012). From Israel the coypu has also conceivably spread to neighboring Lebanon.
Transcaucasia and adjacent countries (Armenia/Azerbaijan/Georgia/Russia and Turkey/Iran): permanent populations are present in the lowlands of Georgia, Azerbaijan and Armenia, where the coypu was introduced already in the 1930/40s, and it is also reported to be widely established in natural habitats of the adjacent Krasnodar Territory in Russia (Carter and Leonard 2002; Bukhnikashvili and Kvavadze 2004). Although apparently still bred, we did not find recent reports of wild populations for Russia, except one video reportage (Supplementary Material 1, Ref 60). In Armenia it is present along the Aras River on the border with Iran and Turkey (Tigran Hayrapetyan, pers. communication). In 1995 the coypu was recorded for the first time in Northern Iran and is nowadays established from the Northwest (Khoi and Maku county) to the Western Caspian Sea (Guilan Province) (Karamii et al. 2008; Farashi and Najafabadi 2015; Dehshiri 2021). From Transcaucasia, the coypu expanded its range to eastern Anatolia in Turkey (Krystufek and Vohralik 2009). In the European part, several populations have existed since the 1980s, most likely originating from Bulgaria (Krystufek and Vohralik 2009).
Central Asia (Turkmenistan/Kazakhstan/Tajikistan/Uzbekistan): introduced coypus are present in Tajikistan and Tashkent region, Uzbekistan (Bykova et al. 2015); we did not find evidence for Turkmenistan or Kazakhstan, which were of unknown status in the review of Carter and Leonard (2002).
East Asia
The coypu was introduced in South Korea, Japan and China, from where it was brought to other countries such as Thailand and Vietnam. Currently it seems to be extinct in Taiwan and Thailand (Saaob Nakasathien, pers. comm. 2020).
China: wild populations are present in at least six provinces (Henan, Hubei, Hunan, Sichuan, Jiangsu, Fujian) and captive populations in five others (Jilin, Liaoning, Inner Mongolia, Hebei, Shanxi: Supplementary Material 1).
Japan: after the abandonment of the fur-farming industry, thousands of animals spread over the main island (Honshu). Despite enormous efforts, with over 50,000 coypus culled between 2008 and 2018, it is still expanding, mainly due to incomplete removals and reinvasions (Kawamura et al. 2018).
South Korea: after the abandonment of farms thousands of coypus escaped and established mostly in the Nakdong River basin area (Kim et al. 2019b). The government established a 9.5-million-USD eradication program in 2014 which is showing the first mild results (Kim et al. 2019a).
Thailand: coypus were imported from China/Taiwan in the 1990s and wild populations occurred a few years later (Carter and Leonard 2002). According to a report of the Mekong Wetland Biodiversity Programme MWBP/RSCP (2006) management included popularising its meat/fur and hunting. Currently it seems to be extinct in Thailand (Saaob Nakasathien, pers. comm. 2020) and no further concrete information could be found.
Vietnam: according to Yen (2021) coypus were intentionally introduced in the 1960s via China and are nowadays present in two regions; the Red River Delta in northern Vietnam and the Central Highlands. It is officially listed as an invasive alien species (MONRE 2018) and management measures include prohibition of culture, import and transport (MWBP/RSCP 2006), as well as hunting (Yen 2021).
Large areas in East Asia (China, Japan, South Korea), coastal and lowland regions along the Mediterranean, Black and Caspian Sea parts of the Kenyan and Ethiopian highlands and South African coastal regions are predicted to be of moderate to high climatic suitability (Fig. 2a). Most parts of the study area that were classified as suitable did coincide with actual occurrences (Fig. 2b).
Potential current distribution of the coypu. a Suitability gradient, with darker colours representing low and bright colours representing high suitability. b Comparison of binary prediction with presence grid cells. Black outlines represent the regional occurrences in China and Vietnam. The binary map was derived by applying a threshold that maximizes sensitivity of the model (95% Sensitivity, i.e. correctly predicted presences). False negatives: absence was predicted but occurrences are reported; true positives: presence was predicted and reported. Predicted presences: climatically suitable, but no report of coypu (this might be due to a lack of data, due to absence because of dispersal limits, or due to absence because of low climatic suitability)
Discussion
Here we showed the most comprehensive summary and update on the distribution of the coypu in Asia and Africa since the detailed review of Carter and Leonard (2002). Firstly, we find evidence for current populations of coypu in Kenya (e.g. Happold et al. 2013; Massey 2013), contrary to Carter and Leonard (2002), who classified them as extinct in the country. On the other side, while we did not find support for its presence in Taiwan, where it was mentioned previously, importantly, we present five new countries (Iran, Jordan, Lebanon, Uzbekistan, and Vietnam) where the coypu is reported to be present. Despite the official listing of the coypu in national invasive alien species lists and governmental control measures (Yen 2021), Vietnam was so far not mentioned in the coypu literature. Additionally, we extend our work beyond sole country-level information and integrate spatially explicit occurrence records. Three quarters of records were derived in the past two decades, hinting towards the increasing mobilization of biodiversity data and the potential of citizen science approaches. Nevertheless, records along water bodies in border areas, as for example in Western Asia, hint towards potential wider spread in data-poor neighboring countries, emphasizing that our findings are probably an underestimation of the species’ actual geographical distribution.
In general, our models showed that only a small part of both continents is suitable for coypu establishment, but many of those suitable areas are not yet known to be colonized (Fig. 2b). This might be the case for several areas in the Caucasus region, adjacent Southern Russia, Turkey, and the coasts along the Mediterranean, Black, and Caspian Sea which are classified as climatically suitable by our model (Fig. 2b), as well Eastern Asia. Here, China is especially predicted to contain large climatically suitable areas in its Southeastern regions, but due to data limitations it was not possible to reliably assess whether the coypu occurs in those areas. The coypu’s predicted potential distribution may be used to define priority regions for further studies, surveillance and management. It may also function as a risk map for ongoing and future spread, and indicate yet unreported occurrences in data-poor regions.
Warm temperate and Mediterranean regions are predicted to be climatically suitable for the coypu, whereas regions characterized by hot arid conditions, cold winter regions, and also hot tropical regions are classified as unsuitable (c.f. Koppen–Geiger climate classification; Kottek et al. 2006), which is in agreement with other studies (e.g. Schertler et al. 2020; Jarnevich et al. 2017; Scheide 2013). In Africa suitable areas are not only predicted in Kenya, where populations are currently known (Happold et al. 2013), but also in the coastal areas of South Africa and Mozambique, and along the Mediterranean Coast, where invasions could take place through range expansions from the Near East. Notably, our predictions also classify the south-central area (Botsuana, Zambia, Zimbabwe), where coypu have been previously reported (Carter and Leonard 2002), as climatically suitable. Large parts of East Asia and the Western Asia seem to be suitable for coypu, whereas the more arid continental environments of Central Asia show overall lower suitability. Although arid environments might be unsuitable for coypu establishment, wetlands and agroecosystems may provide suitable habitat also in these areas, triggering potential conflicts due to feeding damage.
In many regions still ongoing fur farming (e.g. China; Yu et al. 2020) represents a possible source for wild populations. Economic value may drive its spread and impact, as the abandonment of fur farms and low hunting pressure due to marginal market value combined with absence of natural predators, allows for rapid population increase (Schertler et al. 2020). The coypu is known for its high adaptability to a variety of natural and artificial habitats and was found to be strongly associated with rice fields in Northern Italy (Bertolino and Ingegno 2009), emphasizing not only its potential to establish in other rice-growing regions (e.g. East Asia), but also the risk of impacting agriculture, which is an important facet of Asian and African economy (Cervantes-Godoy and Dewbre 2010). The costs resulting from agricultural damage caused by coypu in Japan reach more than one million US$ each year (Yatsuzuka 2018) and damages are also reported from Israel (Khoury et al. 2012) and South Korea (Kim et al. 2019a). Additionally, in Japanese rivers the—usually mainly herbivorous—coypu preys consistently on native unionid mussels, which play various functional roles in freshwater ecosystems (Nagayama et al. 2020). Furthermore, the presence of coypus in anthropized environments poses a potential risk to human health. Indeed, it acts as a reservoir of two zoonotic agents (Strongyloides myopotami and Capillaria hepatica) in Japan (Matsudate et al. 2003), but also in South Korea where several zoonotic agents were isolated and S. myopotami was reported for the first time in the country (Choe et al. 2014; Lim et al. 2019).
Although low coypu densities may result in a low, not-perceived impact (Baroch and Hafner 2002), the time to act is now, to minimise costs. Here prevention of further population increase, and range expansion should be focused, as total eradication in areas of established and continuous populations seems unrealistic, and previous eradication attempts were unsuccessful (e.g. South Korea and Japan). Already conducted management measures in the region comprise the prevention of further introduction (i.e. through trading and farming bans), and population regulation through hunting, which might be coupled with human consumption of the meat and usage of the pelt (MWBP/RSCP 2006; Kim et al. 2019a).
In our work, we have shown that the coypu is present in Eastern Africa and throughout Western and Eastern Asia and present several new countries of occurrence. Despite the frequent focus of studies on North America and Europe, the long introduction history, the many examples of damage and lengthy unfruitful management attempts show that the coypu’s importance in Asia and Africa should not be overlooked. Given that many climatically suitable regions are well connected to existing populations, the coypu’s range could easily increase without appropriate systematic government-directed programs of management and containment. Therefore, it is crucial to clarify its geographical distribution in understudied regions; in this framework, approaches like citizen-science can hold usefulness. Finally, we highlight the need to better monitor the species and implement systematic early intervention strategies. Costs of early intervention have proven to be much smaller than long-term control strategies of established populations (Panzacchi et al. 2007). Once established, eradication seems unlikely in most regions (Carter and Leonard 2002) and lessons should be learnt from the many examples of invasive coypu populations causing ecological and economic damage due to inaction (Table 1).
Availability of data and material
All data are available via the first and the corresponding author.
Code availability
Not applicable.
References
Aiello-Lammens ME, Boria RA, Radosavljevic A, Vilela B (2015) {spThin}: an {R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 38:541–545. https://doi.org/10.1111/ecog.01132
Baroch J, Hafner M (2002) Biology and natural history of the nutria, with special reference to nutria in Louisiana. Nutria (Myocastor coypus) in Louisiana. Genesis Laboratories Inc., Wellington
Bertolino S, Ingegno B (2009) Modelling the distribution of an introduced species: the coypu Myocastor coypus (Mammalia, Rodentia) in Piedmont region, NW Italy. Ital J Zool 76:340–346
Bertolino S, Angelici C, Monaco E, Monaco A, Capizzi D (2011) Interactions between coypu (Myocastor coypus) and bird nests in three Mediterranean wetlands of central Italy. Hystrix 22:333–339. https://doi.org/10.4404/Hystrix-22.2-4595
Bukhnikashvili A, Kvavadze J (2004) On Cadastre of Small Mammals (Insectivora, Chiroptera, Lagomorpha, Rodentia) of Georgia. Tbilisi Statae University Eds, Tbilisi
Bykova EA, Gashev SN, Levykh AY (2015) Natural and historical aspects of the origin and functioning of urban mammals in Western Siberia, Russia and Uzbekistan. Acta Biol Univ Daugavpils 15:47–64
Capizzi D, Monaco A, Genovesi P et al (2018) Impacts of alien mammals on human health. In: Mazza G, Tricarico E (eds) Invasive species and human health. CABI International Edition, New York, pp 130–150
Carter J, Leonard BP (2002) A review of the literature on the worldwide distribution, spread of, and efforts to eradicate the coypu (Myocastor coypus). Wildl Soc Bull 30:162–175
Cervantes-Godoy D, Dewbre J (2010) Economic importance of agriculture for poverty reduction. OECD Food, Agriculture and Fisheries Working Papers 23:1–28.
Chandler M, See L, Copas K et al (2017) Contribution of citizen science towards international biodiversity monitoring. Biol Cons 213:280–294
Chapman AD, Wieczorek JR (2020) Georeferencing Best Practices. https://doi.org/10.15468/doc-gg7h-s853. Accessed 12 Nov 2021
Choe S, Lee D, Park H et al (2014) Strongyloides myopotami (Secernentea: Strongyloididae) from the intestine of feral nutrias (Myocastor coypus) in Korea. Korean J Parasitol 52:531–535
Dehshiri MM (2021) Invasive Alien Species of Iran. In: Pullaiah T, Ielmini MR (eds) Invasive alien species: observations and issues from around the world, volume 2: issues and invasions in Asia and the Pacific Region, 1st edn. Wiley, pp 103–124
Doncaster ACP, Micol T (1990) Response by Coypus to Catastrophic Events of Cold and Flooding. Holarctic Ecology1 13: 98–104. Available from: ttp://www.jstor.org/stable/3682633
Farashi A, Najafabadi MS (2015) Modeling the spread of invasive nutrias (Myocastor coypus) over Iran. Ecol Complex 22:59–64
Freeman EA, Moisen G (2008) {PresenceAbsence}: an R package for presence absence analysis. J Stat Soft 23:1–31
Gherardi F, Britton JR, Mavuti KM et al (2011) A review of allodiversity in Lake Naivasha, Kenya: developing conservation actions to protect East African lakes from the negative impacts of alien species. Biol Cons 144:2585–2596
Goldstein EA, Lawton C, Sheehy E et al (2014) Locating species range frontiers: a cost and efficiency comparison of citizen science and hair-tube survey methods for use in tracking an invasive squirrel. Wildl Res 41:64–75
Gosling LM, Baker SJ (1989) The eradication of muskrats and coypus from Britain. Biol J Linn Soc 38:39–51
Happold D, Kingdon J, Happold M (eds) (2013) Mammals of Africa Vol. 3—Rodents, Hares, and Rabbits. A & C Black, London
Harvey GL, Henshaw AJ, Brasington J, England J (2019) Burrowing invasive species: an unquantified erosion risk at the aquatic-terrestrial interface. Rev Geophys 57:1018–1036. https://doi.org/10.1029/2018RG000635
Hijmans RJ (2020) Raster: Geographic Data Analysis and Modeling. R package version 3.4–5. https://CRAN.R-project.org/package=raster. Accessed 12 May 2021
Hilts DJ, Belitz MW, Gehring TM et al (2019) Climate change and nutria range expansion in the Eastern United States. J Wildl Manage 83:591–598
Jarnevich CS, Young NE, Sheffels TR, Carter J, Sytsma MD, Talbert C (2017) Evaluating simplistic methods to understand current distributions and forecast distribution changes under climate change scenarios: an example with coypu (Myocastor coypus). NeoBiota 32:107–125
Jiménez-Valverde A, Peterson AT, Soberón J, Overton JM, Aragón P, Lobo JM (2011) Use of niche models in invasive species risk assessments. Biol Invasions 13:2785–2797
Johnson BA, Mader AD, Dasgupta R et al (2020) Citizen science and invasive alien species: an analysis of citizen science initiatives using information and communications technology (ICT) to collect invasive alien species observations. Glob Ecol Cons 21:e00812
Karamii M, Hutterer R, Benda P et al (2008) Annotated check-list of the mammals of Iran. Lynx 39:1
Karger DN, Conrad O, Böhner J, Kawohl T, Kreft H, Soria-Auza RW, Zimmermann NE, Linder HP, Kessler M (2017a) Climatologies at high resolution for the earth’s land surface areas. Sci Data 4:1–20
Karger DN, Conrad O, Böhner J, Kawohl T, Kreft H, Soria-Auza RW, Zimmermann NE, Linder HP, Kessler M (2017b) Data from: climatologies at high resolution for the earth’s land surface areas. Dryad Digital Repository. Available at . Accessed on 12 May 2021
Kawamura K, Kaieda S, Kato M et al (2018) Invasion genetics of nutria (Myocastor coypus) in Okayama, Japan, inferred from mitochondrial and microsatellite markers. Eur J Wildl Res 64:1–13
Khoury F, Amr Z, Hamidan N et al (2012) Some introduced vertebrate species to the Hashemite Kingdom of Jordan. Vertebr Zool 62:435–451
Kim YC, Kim A, Lim J et al (2019a) Distribution and Management of Nutria (Myocastor coypus) Populations in South Korea. Sustainability 11:4169
Kim IR, Choi W, Kim A et al (2019b) Genetic diversity and population structure of nutria (Myocastor coypus) in South Korea. Animals 9:1164
Krystufek B, Vohralik V (2009) Mammals of Turkey and Cyprus. In: Rodentia II: Cricetinae, Muridae, Spalacidae, Calomyscidae, Capromyidae, Hystricidae, Castoridae. (ed) Založba Annales Editions, Koper, Slovenia
Kottek M, Grieser J, Beck C, Rudolf B, Rubel F (2006) World map of the Köppen-Geiger climateclassification updated. Meteorologische Zeitschr 15:259–263
Lim SR, Lee DH, Park SY et al (2019) Wild nutria (Myocastor coypus) is a potential reservoir of carbapenem-resistant and zoonotic Aeromonas spp. in Korea. Microorganisms 7:224
Massey A (2013) Ecological assessment of a shifting conservation landscape in Kenya Doctoral dissertation. Nairobi University, Nairobi
Matsudate H, Miyoshi Y, Tamura N et al (2003) A survey of the parasitic helminths of alien rodents (belly-banded squirrel Callosciurus erythraeus and nutria Myocastor coypus) in Japan. Jpn J Zoo Wildl Med 8:63–67
Meyer C, Kreft H, Guralnick R, Jetz W (2015) Global priorities for an effective information basis of biodiversity distributions. Nature Comm 6:8221
Milanesi P, Mori E, Menchetti M (2020) Observer-oriented approach improves species distribution models from citizen science data. Ecol Evol 10:12104–12114
MONRE (Ministry of Natural Resources and Environment (2018) Circular No 35/2018/ TTBTNMT to Assign Criteria of Invasive Alien Species and List of Invasive Alien Species (in Vietnamese), Hanoi, Vietnam
Mori E, Grandi G, Menchetti M et al (2017) Worldwide distribution of non-native Amazon parrots and temporal trends of their global trade. Animal Biodiv Conserv 40:49–62
MWBP/RSCP (2006) Invasive Alien Species in the Lower Mekong Basin: Current State of Play. Mekong Wetland Biodiversity Programme and Regional Species Conservation Programme, The World Conservation Union (IUCN). Asia
Nagayama S, Kume M, Oota M et al (2020) Common coypu predation on unionid mussels and terrestrial plants in an invaded Japanese river. Knowl Manage Aq Ecosyst 421:37
Naimi B, Hamm N, Groen TA, Skidmore AK, Toxopeus AG (2014) Where is positional uncertainty a problem for species distribution modelling. Ecography 37:191–203
Nardoni S, Angelici MC, Mugnaini L, Mancianti F (2011) Prevalence of Toxoplasma gondii infection in Myocastor coypus in a protected Italian wetland. Parasit Vectors 4:240. https://doi.org/10.1186/1756-3305-4-240
Panzacchi M, Bertolino S, Cocchi R et al (2007) Population control of coypu Myocastor coypus in Italy compared to eradication in UK: a cost-benefit analysis. Wildl Biol 13:159–171
Pebesma E (2018) Simple features for R: standardized support for spatial vector data. R J 10:439–446
R Core Team (2021) R: A Language and Environment for Statistical Computing. https://www.r-project.org/ Accessed 20 Mar 2021
RStudio Team (2020) RStudio: Integrated Development Environment for R. http://www.rstudio.com/ Accessed 20 Mar 2021
Scheide D (2013) Die Nutria in Deutschland. Diplomica, p 148
Schertler A, Rabitsch W, Moser D, Wessely J, Essl F (2020) The potential current distribution of the coypu (Myocastor coypus) in Europe and climate change induced shifts in the near future. NeoBiota 58:129
Thuiller W, Georges D, Engler R, Breiner F (2020) Biomod2: Ensemble Platform for Species Distribution Modeling. https://cran.r-project.org/package=biomod2. Accessed 20 Mar 2021
Tricarico E, Junqueira AO, Dudgeon D (2016) Alien species in aquatic environments: a selective comparison of coastal and inland waters in tropical and temperate latitudes. Aq Conserv Mar Freshw Ecosyst 26:872–891
Tsiamis K, Gervasini E, Deriu I et al (2017) Baseline distribution of invasive alien species of union concern. Publications Office of the European Union, Ispra
Wickham H (2016) ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag New York. https://ggplot2.tidyverse.org. Accessed 20 Mar 2021
Wickham H, François R, Henry L, Müller K (2021) dplyr: A Grammar of Data Manipulation. https://cran.r-project.org/package=dplyr. Accessed 20 Mar 2021
Wilke CO, Wickham H, Wilke MCO (2019) Package ‘cowplot’. Streamlined plot theme and plot annotations for ‘ggplot2. Available at http://mirror.lyrahosting.com/CRAN/web/packages/cowplot/cowplot.pdf. Accessed 10 Oct 2021
Woods CA, Contreras L, Willner-Chapman G et al (1992) Myocastor Coypus. Mamm Species 398:1–8
Yatsuzuka K (2018) Species distribution modeling to predict invasion risk of nutria (Myocastor coypus) in Japan. Berkeley University Technical Report, available at: https://nature.berkeley.edu/classes/es196/projects/2018final/YatsuzukaK_2018.pdf. Accessed 30 April 2021
Yen MD (2021) Status and Management of Invasive Alien Species (IAS) in Vietnam. In: Pullaiah T, Ielmini MR (eds) Invasive Alien Species: Observations and Issues from Around the World, Volume 2:Issues and Invasions in Asia and the Pacific Region, vol 2, 1st edn. Wiley, pp 226–243
Yu F, Cao Y, Wang H et al (2020) Host-Adaptation of rare Enterocytozoon bieneusi Genotype CHN4 in coypu (Myocastor coypus) in China. Parasit Vectors 13:578
Acknowledgements
We thank all citizens who provided or uploaded data for this study. Roberto Besso kindly helped us to download data from a Japanese website; Xiaoyu Li provided us with information from China; Tigran Hayrapetyan kindly sent us updated data from Armenia; Yariv Malihi, Yoav Motro and Naumeda Shavangbioh sent us occurrences from the Middle East. Two anonymous reviewers and the Subject Editor kindly took the time to provide us with useful comments.
Funding
AS acknowledges funding by the Austrian Science Foundation FWF (Grant I 3757-B29).
Author information
Authors and Affiliations
Contributions
LP, EM and AS conceived the idea; LP, IL and SG collected most data; LP and AS organised the dataset and analyses; AS created the figures; LP, AS and EM wrote the first draft. LP and AS contributed equally to this work.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Handling editor: Vera Rduch.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pedruzzi, L., Schertler, A., Giuntini, S. et al. An update on the distribution of the coypu, Myocastor coypus, in Asia and Africa through published literature, citizen-science and online platforms. Mamm Biol 102, 109–118 (2022). https://doi.org/10.1007/s42991-021-00207-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42991-021-00207-1