Abstract
While photo-identification is an effective tool to monitor individuals in wild populations, it has limitations. Specifically, it cannot be applied to very young animals before their identifying features have stabilized or to dead, decomposed animals. These shortfalls leave gaps in our understanding of survival, parentage, age structure, physical development, and behavioral variability. Here we report on 13 case studies of North Atlantic right whale, Eubalaena glacialis, calves that required genetics to track their life history data. These case studies revealed unexpected variations in mother–calf associations and separation times, as well as calf physical development. Prior to this study, calves were assumed to have died if their mothers were always alone on the feeding ground in the calf’s birth year. Using genetics and photo-identification, four such calves were discovered to be alive; two of the four possibly weaned earlier than expected at 7.5–8.0 months. To put these early separations in context, photo-identification data were queried and revealed that mothers and calves are seen apart from each other on the feeding grounds in 10–40% of all spring/summer sightings; previously, there were no published data on how often pairs are seen apart in the calf’s birth year. Two dead whales initially logged as calves of the year were discovered to be juveniles, thus allowing skewed survival estimates for calves of the year to be corrected. Genetically sampling animals early in their lives before they disperse or separate from their mothers provides an important means of individual identification at a time when photo-identification is not reliable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For decades, photographs of naturally marked individuals have been used to study terrestrial mammals including chimpanzees, Pan troglodytes, (Goodall 1968), red deer, Cervus elaphus, (Clutton-Brock et al. 1982), cheetah, Acinonyx jubatus, (Kelly et al. 1998), giraffe, Giraffa giraffe, (Halloran et al. 2015), and zebra, Equus grevyi, (Zero et al. 2013) to name a few. Clutton-Brock and Sheldon (2010) provided an excellent review of the benefits of these types of individual-based studies and highlighted six advantages over population-level studies. Specifically, they provide data on age structure, life history stages, social structure, derivation of lifetime fitness measures, replications of estimates of selection, and linkages between generations. In short, individual-based studies are crucial to understanding the specific mechanisms driving population level changes.
Beginning in the late 1970s, these photo-identification techniques were applied to cetaceans (e.g. Payne et al. 1983; Hammond et al. 1990). Studies of cetaceans face two unique challenges. First, there is a need to survey a much broader geographic area to capture ocean-wide ranges and second, the opportunities for observation are limited in duration by both the periods when the animal is at the surface to breathe and when marine weather is favorable to document them.
The North Atlantic right whale, Eubalaena glacialis, is one of just two critically endangered large whale species in the world (Cooke 2020) with a current estimate of less than 400 individuals (Pace et al. 2017; Pettis et al. 2021). Individuals of the species are distinguished by photographs of the natural markings on their head, called callosities, and scars on their bodies. Since 1980, the life history parameters of this species have been tracked by an extensive photo-identification catalog, the North Atlantic right whale Catalog (hereafter referred to as the “Catalog”, http://rwcatalog.neaq.org), which contains all known photographed sightings of right whales in the North Atlantic from 1935 to the present (Hamilton et al. 2007; Pace et al. 2017). The data available in the Catalog have provided information on individual movements (Kraus et al. 1986a; Knowlton et al. 1992; Jacobsen et al. 2004; Silva et al. 2012; Gowan et al. 2019), age structure (Hamilton et al. 1998), weaning (Hamilton et al. 1995; Hamilton and Cooper 2010), reproductive success (Knowlton et al. 1994; Kraus et al. 2001; Frasier et al. 2007), mortality (Moore et al. 2004; Sharp et al. 2019), growth rates (Fortune et al. 2012; Stewart et al. 2021), sub-lethal anthropogenic impacts (Kraus 1990; Knowlton et al. 2012) and population dynamics (Pace et al. 2017; Hayes et al. 2018).
While the photo-identification catalog has informed much of what we know about this species, it does have some limitations: it cannot always be applied to dead whales if decomposition has obscured or removed the whale’s identifying features (Sharp et al. 2019) and it cannot be consistently applied to right whale calves. Although the callosity pattern begins to erupt shortly after birth, it is generally not well established until calves are at least 4–5 months old (Hamilton et al. 2007; Patrician et al. 2008). Photographs of additional features (e.g., the pattern of callosities along the jawline, crenulations along the margins of the lower lips) are often needed to identify individuals during their calf year (Hamilton et al. 2007). In some cases, it can take several years after birth before a calf can be confidently photo-identified due to a combination of infrequent sightings and photographs of marginal quality.
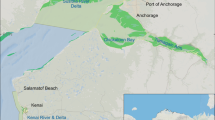
Most North Atlantic right whale calves are first observed during the winter between December and March in waters off the southeast U.S. coast, which is the species’ only known calving ground (Kraus et al. 1986a, b; Patrician et al. 2008; Zani et al. 2008; Foley et al. 2011) (Fig. 1). By April and May/June, mothers and calves have migrated to more northerly feeding grounds around Massachusetts (Kraus et al. 1986a). In June through October, mother/calf pairs are found mostly in the Canadian waters in the Grand Manan Basin in the Bay of Fundy, and more recently in the Gulf of St. Lawrence. There are relatively few right whale sightings in the months of October and November. Calves generally stay with their mothers for 10–12 months, (but ranges from 8 to 18 months) (Hamilton et al. 1995; Hamilton and Cooper 2010) but since sightings of them at that age are uncommon, the specific timing of weaning is unknown.
A map showing the critical habitats and other high use areas of the North Atlantic right whale. The southeast is where they calve during winter months (December through March), Cape Cod Bay and east of Cape Cod is where mothers and calves are first seen on the feeding ground in the spring (April and May), and sightings of mother–calf pairs from June through October are generally north of there in the Bay of Fundy or the Gulf of St. Lawrence
Because their callosities take months to develop, calves are provisionally identified by their close association with their mother (e.g., 2020 calf of right whale #3101). If a calf remains with its mother until its callosity is identifiable and there are adequate photographs of the pair together, the calf can subsequently be added to the Catalog along with its known age and parentage. However, if the calf is separated from its mother early, or if the pair remain together and are simply not adequately photographed, the calf may later be cataloged as a juvenile, but its age and parentage will be unknown (Hamilton et al. 2007). Each year, some calves may not be photo-identified because: (1) they did not survive long enough, or (2) they were not well photographed (or were not photographed with images of sufficiently high quality) due to limited photo-identification surveys (as a result of logistical or financial constraints) or because their mothers migrate to feeding grounds that are not surveyed).
Some of these unknown-age whales added to the Catalog can eventually be retroactively linked to specific uncataloged calves through genetic profiling. The species has been extensively genetically sampled since 1988 (Brown et al. 1991; Frasier et al. 2006), with more than 80% of the cataloged whales genetically sampled (Hamilton et al. 2007; Frasier et al. 2009). Individuals can be uniquely genetically identified based on 36 microsatellite loci (Frasier et al. 2006), which provide very high resolution to discriminate among individuals. For example, given the allele frequencies in this sample set of 760 whales, using these 36 loci results in a probability of identity (PID, Paetkau and Strobeck 1994) of 8.9 × 10–10 that two unrelated whales will have the same genotype, and a probability of identity among siblings (PID-Sib, Evett and Weir 1998; Waits et al. 2001) of 5.8 × 10–6 that two full-siblings will have the same genotype. These equate to expectations of approximately every two in 1 billion whales having the same genotype, and approximately one in every 172,000 pairs of full-siblings having the same genotype. Given that there are fewer than 400 individuals remaining in this species, and that full-siblings are very uncommon (Frasier et al. 2007), these loci provide adequate resolution for individual identification and parentage assignment.
Because they are completely independent identification techniques, the genetic and photo-identification databases serve as excellent quality checks for each other. A comparison of the two methods (Frasier et al. 2009) found extremely low error rates for each approach; 0.0308 errors per photographic identification (as a result of three false positives and two false negatives out of 168 comparisons that contained both photographic and genetics data), 0.00121 genetic errors per locus, and 0.0327 errors per genotype (due to mistakes in 8 out of 245 genotype comparisons). Using genetics, it was also discovered that, in 1987, two mothers swapped their calves on the calving ground and raised each others’ calves throughout the rest of the year (Frasier et al. 2010).
Genetic identifications have become increasingly important for right whales in recent years due to changes in their distribution. Like many baleen whales, North Atlantic right whales show maternally-directed site fidelity to summer feeding grounds (e.g., Baker et al. 1990; Malik et al. 1999; Frasier et al. 2011). In the past, approximately 60% of North Atlantic right whale females used the Bay of Fundy as a summer feeding and nursery area, bringing their calves there in their first year. The other 40% used other, previously unidentified, feeding habitats (Malik et al. 1999). Therefore, these ~ 40% of calves not seen in the summer were difficult to identify photographically because they were only seen with their mothers during the winter months before their callosity pattern developed. Since 2010, relatively few right whales have been sighted in the Bay of Fundy, with an apparent shift into the Gulf of St. Lawrence (Hayes et al. 2018; Record et al. 2019). This change has made genetic identifications more important because now a smaller percentage of calves are seen in the summer months than had been in the past.
While most right whales that are biopsied are identified and matched to cataloged individuals using both photo-identification and genotypes, some individuals can only be linked to their calf sightings genetically. Here, we describe 13 cases of calves that were identified and linked to post-calf sightings through genetics. Most of these calves were first genetically sampled when with their mothers (providing the link to maternity and age) and then again later after their callosities had developed. We investigate how these cases inform our understanding of mother–calf associations and calf physical development. Finally, to provide context for the finding of some apparently early mother–calf separations, we use the photo-identification database to investigate how often mothers and calves were seen apart on the feeding grounds. These represent the first published data on sightings apart in the calf’s birth year.
Materials and methods
Photographic data
Right whale photographs were collected throughout the whales’ migratory range in all months of the year. Broadly speaking, sightings of mothers and calves on the calving grounds (December to March) and the spring feeding grounds (April to June) were collected from planes, while sightings after that, from July to October, were primarily collected from boats. As mentioned above, there were relatively few sightings in the months of October and November. Unlike aerial observations, sightings from boats allow for (1) close up, perpendicular photographs of the head and lip crenulations which are useful for calf photo-identification, (2) provide the opportunity to obtain biopsy samples, and (3) tend to span more time allowing for more thorough behavioral data to be collected. All photographic data were submitted to the New England Aquarium (Boston, MA) for processing. The data were entered, images uploaded, and identifications made and tracked using DIGITS, custom-built software created by the New England Aquarium to manage the Catalog. Data associated with all sightings include: time, date, location, observer, associations, behaviors, and observations. Observations pertinent to this study include those termed ‘calf alone’ and ‘lost calf’. ‘Calf alone’ was applied to any calf not seen swimming next to its mother [a calf without its mother can be distinguished from other small whales by their head shape, incomplete callosity development, and by the color and coverage of the cyamids on their heads (Hamilton et al. 2007; Patrician et al. 2008)]. “Lost calf’ was applied to the last sighting of a mother with her calf if the data indicated that the calf later died. This descriptor was used if: (1) the mother was alone on the calving ground at a time when we expect that a calf cannot be separated from its mother and survive (less than 3 or 4 months), or (2) if the mother was observed alone in the northeast in the year the calf was born for at least two sightings and was never seen with the calf afterwards, or (3) if the mother was alone just once in the northeast in the year/season she gave birth, but had no subsequent sightings with her calf and had a subsequent 2-year calving interval. The last criteria was included because right whales need at least 3 years between calves if they have nursed their previous calf for many months (Knowlton et al. 1994; Kraus et al. 2001), but it is not uncommon for a mother whose calf died on the calving ground to calve again 2 years later. All photo-identification matches were confirmed by at least one other experienced researcher; for difficult matches, three or more researchers were consulted.
Individual right whales were identified using photographs of the pattern of the callosity, patches of rough skin that erupt on the rostrum and mandibles (Payne et al. 1983; Kraus et al. 1986b), scars on their bodies (Kraus 1990), and occasionally by the pattern of white pigmentation on their ventral bodies (Schaeff and Hamilton 1999). The callosities are infested with several species of lightly colored amphipods called cyamids (Kraus et al. 1986a, b; Rowntree 1996) which provide contrast for the outline of the callosities themselves. Calves do not have callosities or cyamids at birth and it can take months for these identifying features to clearly emerge. For a whale to be added to the Catalog, the photographic identification information must be of adequate quality to enable researchers to confidently match subsequent sightings to that individual (Hamilton et al. 2007). The maternity of newborn calves is determined by their association with their mothers. If the calf is well photographed after its distinguishing features have developed and while still accompanied by its mother, then it is cataloged and the maternity of that cataloged whale is determined by that association. A whale that has been genetically sampled but does not have adequate photographic information, such as a whale only seen as a young calf and not in subsequent years, is not added to the Catalog. If it is eventually genetically sampled again and is adequately photographed at that later date, then it will be cataloged and its age and maternity is then determined by the genetic match to the sample of it as a young calf while still associated with its mother.
All calves born between 1988 and 2018 were categorized by whether they had been genetically sampled and whether they were cataloged. These data were tabulated by birth year. Because a single calving season spans two calendar years, any calf born in October through December was given a birth year of the following January so they grouped correctly with that calf cohort. Whales were classified as calves from their date of birth through December 31 of their birth/cohort year. A calf was considered to be weaned only if it was seen alone on at least 3 days with no sightings in between or after with its mother following Hamilton et al. (1995).
Some of the results discovered during this study brought into question how early in a calf’s birth year mothers and calves can separate. While there have been previous analyses on how often a mother and her calf are seen together or alone in the year after the calf’s birth year (Hamilton et al. 1995; Hamilton and Cooper 2010), there are no published data on how often they are seen apart within the year of birth itself. To provide context for the possible early separations of some calves from their mothers, three analyses were conducted using all observations from 1988 to 2018 for the months of April through October. First, to determine how unusual a single sighting apart is, records for all 7 months were assessed to determine the total number of sightings of mothers, the total number of sightings of calves, and then for each of them, the count of those sightings together versus apart. Those numbers, and the percentage of sightings together and apart, were calculated by month. Sightings of mothers whose calves were known to have died were removed from the analysis, as were sightings of dead calves.
The second analysis focused on days rather than sightings and reduced some of the noise in the first analysis by having a stricter definition of being apart. A mother or calf were considered apart only if one or both were seen on a given day but never together. It provides a stronger assessment of separation in cases where one of the two have multiple sightings in a day. For example, if a calf were seen ten times in a day, once with its mother and nine times without her, in this analysis it would count as just a single record with its mother versus in the first analysis (based on sightings rather than days) where it would count as one record with its mother and nine records apart. For the days analysis, the unique days with sightings of the mother or the calf were counted to get the total days sighted for each. Those days were then categorized as either days where they were seen together at least once or days when one was seen but always apart. These data were tabulated in the same way as the sightings data above.
The third analysis focused on potential long-lasting separations in the early spring when we expect mothers and calves to always be together (since the calves are only 4–5 months old). All sightings of mothers seen two or more times without their calves in April and May were investigated. The data were tabulated as follows: a count of the number of days that mother was always seen with her calf, the number of days she had some sightings with her calf and some without, the number of days she was seen but never with her calf, and whether or not the pair were seen together at any point that year after the last April/May sighting of the mother alone. This analysis provides context for the enumerated April/May sightings of the mother from the first analysis and allows for a better assessment of whether sightings alone during this time likely indicated a long-lasting separation from the calf.
Genetic data
Biopsy samples for genetic analysis collected from dead right whales included skin, muscle, or bone, depending on the necropsy case. For live right whales, samples were collected using a crossbow with a modified bolt and bolt tip (Brown et al. 1991; Palsbøll et al. 1991). Once a whale was photographed, the vessel approached and paralleled the whale at a distance of 5–20 m. If a whale was evasive, no more than three approaches were made. To avoid unnecessary harassment caused by a vessel approach and biopsy collection, if a whale was identified with confidence in the field and was known to have been sampled previously, no biopsy attempt was made. Biopsy efforts were focused on calves of the year, other whales recognized in the field that were known to have not been previously sampled, and whales that could not be identified in the field (some of which had in fact been sampled before). In the latter category, juveniles were a priority since some calves are not photographically identified in their calf year and thus need a second genetic sample as a juvenile to link them to the calf sighting. For the majority of the study period, there were no constraints on the age at which a calf could be biopsied, with the exception of a number of years in the middle when research permits restricted biopsy sampling to calves older than 1 month. However, because there are no data or observations to indicate that calves younger than a month are adversely affected by biopsy sampling, that restriction was subsequently removed. Instead of age being the determinant of whether or not to biopsy, researchers assessed a calf’s behavior before sampling; if a young calf was nursing, exhibited extreme avoidance, or was not yet swimming strongly, it was not approached.
Biopsy samples and skin or muscle from dead whales were placed in 20% dimethyl sulfoxide (DMSO), 0.25 M EDTA solution saturated with sodium chloride (Seutin et al. 1991) and stored at room temperature. Dead whale bone tissue was dried and stored at − 20 °C. Genetic samples were sent to the Natural Resources DNA Profiling and Forensic Centre at Trent University in Peterborough, ON (1988–2017) or Saint Mary’s University in Halifax, NS (2018–2019) for processing. DNA from all skin and muscle tissues was extracted following a standard phenol–chloroform extraction protocol followed by ethanol precipitation (after Sambrook and Russell 2001), while DNA from bone tissues was extracted following the protocol of Rastogi et al. (2004). Extracted DNA was re-suspended in TE0.1 buffer.
Genetic profiles for North Atlantic right whales within the genetic databank are comprised of three components: (1) molecular sex (after Gilson et al. 1998), (2) mitochondrial control region haplotype (after Malik et al. 1999), and (3) a multi-locus microsatellite profile with up to 36 loci (after Frasier et al. 2006, 2007). These microsatellites represent a combination of 25 loci originally identified in North Atlantic right whales, and 11 loci originally identified in other whale species but subsequently found to amplify well and be polymorphic in North Atlantic right whales, as described in Frasier et al. (2006). For any dead whale, DNA samples that appeared to be functionally amplifying at < 1 ng/µl (meaning that the DNA is degraded to the point that it amplifies as though it has a concentration < 1 ng/µl) were treated the same as “ancient” samples, where profiles were developed using a smaller subset of five to nine microsatellite loci using an increased quantity of Taq polymerase and an increased number of PCR cycles (Rastogi et al. 2004; McLeod et al. 2010). To verify that these low quantity DNA microsatellite profiles were accurate, samples were also run multiple times (n = 3–7) to verify the profile at each locus.
Genetic identity comparison using CERVUS and random match probability
Whales (and samples thereof) were genetically identified and matched to each other based on the resulting composite profiles comprised of microsatellite genotype, genetic sex, and mitochondrial haplotype. To make these matches, each sample was compared to all other profiles in the genetic database. First, microsatellite genotypes were compared among all individuals in the database using the “Identity Analysis” function in the cervus software v.3.0.7 (Marshall et al. 1998; Kalinowski et al. 2007). A putative match was made if the samples involved were genotyped at five or more loci (due to DNA degradation, some dead individuals can only be genotyped at a few loci) and were identical across all loci compared. Once these potential matches were identified based on the microsatellite data, the sex and mitochondrial haplotype data were compared to ensure they too were consistent. These combined data were used to calculate a case-specific random match probability (RMP). Commonly used in forensics, the RMP is the probability of these characteristics occurring in a random individual in the population, which, for the genotype, is the allele frequency squared (pi2) for a homozygous locus and is two times the frequency of each allele (2pq) for a heterozygous locus. These values were then multiplied across the loci involved in the comparison (e.g., National Research Council 1996). The sex and mitochondrial haplotype data were included by multiplying the frequencies with which that sex and haplotype are found in the sampled population. The RMP calculations were performed using a custom R script in R version 4.0.2 (R Core Team 2020). The RMP calculation is more comprehensive and specific than the standard PID calculation because it includes sex and mitochondrial haplotype data in addition to the genotype data. Moreover, the genotype data used in the RMP are specific to the individuals involved in each case, as opposed to for the entire population, as is the case with the PID. Any comparison between two samples with a probability greater than 1.0 × 10–5 was considered a match. This cut off point is comparable to a genotype appearing only once in every 100,000 individuals and is conservative given there are only hundreds of this species remaining.
Maternity
The genetic data were also used to confirm the relevant maternities initially identified via photo-identification. For each year of these analyses (each year containing one or more putative mother–calf pairs), a list of “candidate” mothers was identified. This list represented all females alive in the year of interest that were either at least 5 years old (the youngest known age of first parturition, Knowlton et al. 1994) or of unknown age in that year. The genotypes of the putative calves were then compared to those of the candidate mothers using cervus v3.0.7. For each comparison, the software produces a percent confidence that the chosen candidate is in fact the true mother.
Link to calf
For this study, all genetic samples from calves collected between 1988 and 2018 were inspected. First, genotyped calves were assessed to determine whether or not they had been linked to later sightings. If a link between the calf sighting and the post-calf sighting (i.e. from subsequent years) was made photographically, even if there was also a subsequent genetic sample of that individual, the link was labeled a ‘photographic’ link. If, on the other hand, that link could only be made genetically (i.e. either the calf was still lacking identifiable features when last photographed or the quality of the photographs was not sufficient for reliable photo-identification), then the match was labeled as ‘genetic.’ The sighting history of the calves that were identified through genetics were investigated to determine the specific circumstances that made photographic identification impossible.
Results
Mother or calf alone (sightings or days apart)
Between 1998 and 2018, there were 3256 sightings of mothers and 3418 sightings of calves in April through October. While mothers and calves were together in most sightings, the two were apart in 26% of all mother sightings and 30% of all calf sightings (Table 1A). The number of days mothers and calves were seen during this same time period is considerably less than the sighting count due to multiple sightings per day. There was a total of 2354 unique mother days (one record per mother per day in each year)—in 80% of the those mothers were observed with their calf at least once that day and in 20% of those days they were not observed with their calf (Table 1B). Calves showed a very similar pattern to mothers. In 81% of the 2331 unique calf days, calves were seen with their mother at least once and in 19% of the days they were not seen with their mothers.
Some interesting patterns emerge when reviewing the sightings and days data by month. In April and May, mothers are more likely to be seen without their calves (11 and 16% of all mother sightings, respectively) than the calves are to be seen without their mothers (4 and 12% of all calf sightings, respectively). That pattern reverses for July through September when as many as 38% of a mother’s sightings are without their calf and as many as 41% of all calf sightings are without their mothers. The percentage of days a mother or calf were only seen alone (Table 1B) showed a similar increasing pattern for June through September with a maximum of 32% of their days spent apart. In October, the proportion of both sightings and days where mothers or calves are alone drops to 21–22 and 17%, respectively.
Early separation (mothers alone in April/May)
There were 17 cases of mothers that had two or more sightings without their calves in April and May (Table 2). These were all potential candidates for mothers that had completely separated from their calves when the calves were just 4 or 5 months old. On average, these mothers were also seen with their calves on 5.9 days over the 2 months. In 14 of the 17 cases, the mother was seen with her calf after her last sighting alone; these cases could be ruled out as long-lasting separations. In two of the remaining three cases, the mothers were seen with their calves in April and May but they were not seen together after the last sighting of the mother alone. While it is possible that these cases could represent early separations, the evidence is weak. The strongest evidence for an early separation is the remaining case, mother #1308 from Case #13 (described below); the mother was not seen with her calf after March and was seen alone twice over a 23 day period in May. Another early separation case is described later in this paper (Case #2), but that mother was not seen at all in April or May and was therefore not captured in this analysis.
Genetic matches
Of the 470 calves observed between 1988 and 2018 (Table 3), 370 (78.7%) were biopsied: 293 when they were calves (267 cataloged, 26 not yet cataloged) and 77 only later in life (all cataloged—these individuals were initially linked to their calf year solely by photographs). Of the 100 that were not biopsied, 32 were cataloged and 68 have not yet been cataloged, or not linked to a whale that was subsequently cataloged. These 68 calves were “identified” solely by their association with their mothers on the calving ground. They are unique individuals (i.e. cannot be any of the other calves). Some of these calves from recent years may yet be photographically matched to subsequent sightings and then added to the Catalog. Others from past years may have already been cataloged as unknown age individuals but the link to their calf year will remain undetected. The remainder will never be added to the Catalog.
Twelve of the 267 calves that were biopsied as calves and cataloged were only able to be linked to the calf sightings using their genetic profiles. After reviewing these cases and discovering a number of apparently early mother–calf separations, a 13th case was found through photo-identification and later confirmed genetically. The identifications of these animals are presented as cases which are summarized in Table 4. Each is categorized by the types of information learned from that case. These categories include: (1) link to parentage and thus age, (2) apparent early separation of the mother and calf, (3) unusual physical development, and (4) calves associating with a different mother. The genetic information is divided into data used to identify the calf and data used to confirm the maternity assignment. Information on the calf include dates of the samples used to make the genetic match, the number of microsatellite loci used for that match, and the probability (RMP) of that same genotype, sex, and mitochondrial haplotype existing in the population. Information for the maternity includes the number of loci used to make the assignment and the confidence in that assignment. The number of loci used in the two analyses differ because the loci scored for both the sample of the calf and the later darting of that individual (Calf ID) will be different than the loci scored for both the calf and mother samples (Maternity). The maternities for all but Case #3 were assigned by photo-identification with the genetics adding additional confirmation. The sighting information is summarized by the dates of the first and last sighting of the pair together, all dates of them seen apart, and whether they were seen together any time after their last observed separation (Table 4). Additional narrative details of each case are provided below where warranted.
Case 1: The sighting of mother #1812 alone on the feeding ground off Massachusetts on May 5, 2004 called into question the survival of her calf. It was only after #3680 was biopsied again as a 3-year-old that we determined the calf had survived. The genotype of the sampled calf of #1812 and that of #3680 are identical at all 31 microsatellite loci at which both were analyzed, as well as for mitochondrial haplotype and sex. The RMP of this profile is extremely low (2.0 × 10–19), suggesting that these do in fact represent the same individual. Confirmation that these samples represent the calf of #1812 was obtained through maternity analyses, which found that #1812 was the only candidate mother to share one allele with #3680 at all 30 microsatellite loci compared, and this maternity was assigned at the 95% confidence level.
Case 2: #3970 was seen alone on the feeding grounds eight times between June 17 and September 17, 2009 and was initially presumed to be a yearling based on his appearance (Fig. 2) and the fact that he was consistently alone. He was first biopsied when he was with #3320 as young calf on the calving ground on January 10, 2009 and again 8 months later in the Bay of Fundy and it was genetically determined that he was #3320’s calf from that year. The genotypes from the two samples of #3970 are identical at all 28 loci at which they were both typed, as well as for mitochondrial haplotype and sex. The RMP of this profile is 8.0 × 10–15, indicating that these represent the same individual. Moreover, #3970 and #3320 share at least one allele at all 26 microsatellite loci compared. Maternity analysis assigned #3320 as the mother of #3970 at the 80% confidence level.
Photographic comparison of the relative size between two calves and a juvenile in September 2009. Ten month old #3970 from Case #2 (a) is closer in appearance to 22 month old #3830 (c) than to 7 month old #3917 (b) based on the head shape, callosity development, and cyamid color and coverage (Patrician et al. 2008). All photographs taken in the Bay of Fundy, Canada by the New England Aquarium under Fisheries and Oceans Canada Section 73 Scientific Research permit #322835
Case 3: three consecutive sightings of #1814 alone on the feeding ground on Jeffreys Ledge in August and September 2007 indicated that her calf from that year likely died. #3790 was seen alone on September 10 and 14, 2007 in the Bay of Fundy. At the time, although the field notes say “large calf alone”, there was some uncertainty whether she was a large calf, or a small yearling. Although #3790 was not biopsy sampled as a calf, a sample collected from her 5 years later on January 25, 2012 was compared to all other whales genotyped to determine parentage. Maternity analysis for #3790 assigned #1814 as the mother, who shared at least one allele at all 23 loci compared, at the 95% confidence level. As this analysis included all adult females, it would have captured any mothers that could have given birth the previous year—ruling out the possibility that #3790 was a small yearling in August 2007. Given the strong genetic evidence and the fact that #1814 was seen with a calf that year, #3790 was assigned as the calf of #1814. Of the 13 cases presented here, this was the only one for which maternity was determined solely by genetics (i.e. without a genetic sample taken when the calf was associated with its mother and thus the maternity determined by association).
Case #4: #3580 was genetically sampled on the feeding ground on May 19, 2005 as a calf while with #1315—a known mother of the year. He was sampled again on October 2, 2005 while with #1970—also a known mother of the year. The two samples from #3580 were identical at all 30 microsatellite loci at which they were compared, as well as sex and haplotype. The RMP of this profile is 8.1 × 10–18, suggesting that they did indeed come from the same individual. Maternity analysis assigned #1970 as the mother at the 95% confidence level, and she was the only candidate to share one allele at every locus with #3580 at all 29 loci compared. It was only through genetics that we could determine that #1970, not #1315, was actually his mother.
Case #5: #3310 was genetically sampled on September 17, 2003 as a calf on the feeding ground with #1608—a known mother of the year. That sample matched a sample from #3310 from September 12, 2003 when he was with #2301- also a known mother of the year. It was only through genetics that we confirmed that #2301 was his mother. Upon further investigation, it was determined there were actually two calves associated with #1608 on September 17, her own and #2301’s, not just one as was assumed in the field. The two samples from #3310 were identical at all 21 microsatellite loci at which they were compared, as well as sex and haplotype. The RMP of this profile is 7.0 × 10–13, confirming that they came from the same individual. Genetic maternity analysis ruled out #1608 and assigned #2301 as the mother at the 95% confidence level, with #2301 and #3310 sharing one allele at all 31 loci compared.
Case #6: #3595 was found dead in the Bay of Fundy on July 25, 2006. She had not been photographically cataloged due to limited photo-identification data. She was classified as a calf of the year in the initial necropsy report because of her small size (9.58 m, case DVS 2006-04745, Sharp et al. 2019) but the genetic sample from the necropsy matched 1604’s calf from the previous year indicating that she was 1.5 years old at the time of her death. The DNA obtained from the carcass was highly degraded. However, the sample could be genotyped at the five most variable microsatellite loci. That genotype and the one from #1604’s calf from the previous year were identical at all five microsatellite loci compared, as well as sex and mitochondrial haplotype. The RMP of this profile is 1.2 × 10–6 suggesting that these do represent the same individual. That genetic match was further supported visually by the portion of callosity that remained on the carcass, and the fact that #3595 has not been seen alive since that carcass was discovered. Genetic maternity analysis also confirmed #1604 as the mother at the 95% confidence level, sharing one allele at each of the 29 loci compared (with the original sample from #3595 when she was alive).
Case #7: #4505 was found dead south of Cape Cod, Massachusetts on August 30, 2018. The initial necropsy report (case IFAW18-244Eg, Sharp et al. 2019) assessed the dead whale as a calf or 1 year old based on an estimated length of 9 m (the flukes were gone preventing an accurate measurement). The DNA from the dead whale was highly degraded however it could be genotype at the five most variable loci. Comparison with the database indicated that the genotype, sex, and mitochondrial haplotype of this dead whale (#4505) were identical to that from whale #2605’s calf from 2015. The RMP of this profile is 8.1 × 10–6, suggesting that these represent the same individual. This result indicates that #4505 was 3.5 years old at the time of death. White ventral pigmentation supported the genetic match, but the photographs alone were not sufficient to make a visual match. Maternity analyses confirmed #2605 as the mother: she shared one allele at each of the 30 loci compared with #4505 (using the original sample from #4505 when he was alive), and maternity was confirmed genetically at the 95% confidence level.
Cases #8–12 all involve whales sampled as calves and again as juveniles and linked to the calf sighting by genetics. No narrative is provided here as all pertinent information is captured in Table 4.
Case #13 was discovered photographically in response to Cases #1–3. Because of the evidence that early mother–calf separations do not necessarily indicate the calf died, we reviewed another case of a calf presumed to be dead because its mother was seen alone on the feeding ground. Whale #4040 and her mother #1308 were first seen together January 24, 2008 and last together March 11, 2008 on the calving ground. #4040 was not seen again that year and #1308 was seen alone on the feeding ground on May 7 and 30. Upon further inspection of the calf photographs from March, we were able to discern enough information from the developing callosity to match those March photographs to #4040, a whale that had been cataloged in 2012 but whose birth year and parentage were unknown. Just months after this photographic link of the calf to #4040, she was biopsied again in August 2019 and the match between her two samples confirmed genetically with a RMP value of 1.8 × 10–15. This closer examination of the photographs would not have happened without the discoveries made with the genetic data. The maternity for this case could not be assigned genetically, as two candidate females, #1308 and #1632, were both genetically consistent as the mother of #4040. However, #1632’s calf survived and is accounted for both photographically and genetically, and #4040 was photographically matched to the young calf with #1308, so the maternity assignment has high confidence based on photo-identification.
Discussion
Age and maternity
The genetic identifications presented here provided age for 12 whales, maternity for one, and supported the maternity assignments for another 10 (Table 4). Knowing the age of individuals allows for more precise age-specific: (1) survival and fecundity to be estimated in population models (Caswell et al. 1999; Pace et al. 2017), (2) growth curves to be developed (Moore et al. 2004; Fortune et al. 2012; Sharp et al. 2019; Stewart et al. 2021), and (3) mortality threats to be assessed (Knowlton et al. 2016). The maternity data are used for female fitness and survival estimates and provide the possibility for paternities to be assigned (Frasier et al. 2007). These familial genetic data are also used to investigate mating patterns and the effects of genetic characteristics on reproductive performance (Frasier et al. 2007, 2013). In a species with less than 400 individuals (Pace et al. 2017; Pettis et al. 2021), the linkage of these data to a dozen individuals has a significant impact on all of the studies above.
Variable mother–calf associations
Sightings apart
The analysis of mothers or calves seen apart from each other (Table 1) provided some important context for the four cases of calves initially thought to have died based on apparently early separations (Cases 1, 2, 3 and 13). In the spring months of April and May, before calves have begun to feed on solid food, one might expect to see mothers and calves staying close by and, if either were to be seen alone, it would more likely be the calf alone at the surface while the mother feeds at depth. The data suggest that the opposite is true; they are seen apart in as much as 16% of the sightings and mothers are more likely to be seen alone than calves during those months (Table 1). This unexpected finding is likely partially explained by a combination of the types of surveys conducted during those months and the feeding behavior of the mother. The majority of sightings in April and May are from planes which have shorter observation periods than those from boats, thus a calf could be easily missed if beneath the mother nursing. Also, most April sightings take place in Cape Cod Bay where surface feeding is common (Mayo and Marx 1990), thus making mothers more detectable. In some of the April/May sightings, the mother may be truly alone, but in others, the calf may be there but simply not detected due to the typically short duration of aerial observations. It is also possible that calves alone are not as detectable from the air because they are smaller and have less visible blows and thus those sightings are underrepresented. Given these caveats, it is difficult to assess the robustness of these association findings. While the existing data are clear that it is not uncommon to see mothers and calves apart in April and May, dedicated vessel-based, behavioral studies would strengthen our understanding of mother/calf associations during these months.
Starting in June, the occurrence of calves without their mothers increases steadily and by August and September, it is common for calves to never be seen with their mothers on a given day (22 and 32% respectively, Table 1B). This is a logical pattern as the calf gains independence and begins to feed on its own (Klumov 1962; Hamilton et al. 1995). The increase in separations may also be due in part to how the mothers are feeding. While surface feeding is common in April and May as stated above, August and September feeding bouts are generally focused at depths of 80 and 175 m (Baumgartner and Mate 2003). Thus, mothers are often at depth and less available to be seen with their calves. However, the potential bias introduced by this feeding behavior is likely offset by the longer observation periods that are typical of the predominant vessel-based surveys during these months. Because those observations often spanned several dive cycles, it is likely that the association data during these months are more reliable than those for April and May.
The pattern of increasing independence through the summer appears to reverses in October. While the sample size is smaller, the percentage of sightings apart drops to 17% (calves) to 21% (mothers), a substantial change from the high of 41–38% the month before (Table 1A). The majority of October sightings were in the Bay of Fundy or on Jefferys Ledge (Fig. 1). Right whale mother–calf pairs typically leave these areas in October and may come together in preparation to travel in the same way that southern right whale (E. australis) and humpback whale (Megaptera novaeangliae) mothers and calves do before departing from the calving grounds (Taber and Thomas 1982, 1984; Zoidis et al. 2014). It should be noted that all but three of the October sightings presented here occurred prior to 2011, before right whales shifted away from the Bay of Fundy and Jefferys Ledge (Hayes et al. 2018; Record et al. 2019). Due to the recent lack of October mother/calf data, it remains unknown whether this association pattern has persisted.
Do sightings apart indicate a lasting mother/calf separation?
We used to infer that any mother that was seen with a calf on the calving ground but always alone later on the feeding ground, had lost her calf. All the mothers in Cases #1, 2, 3, and 13 had such sighting patterns, yet in all four cases, the calf was in fact alive. After reviewing the data in Table 1, the single May separation captured in Case #1 (Table 4) appears to be more common than previously thought; 16% of all sightings of mothers in May are without their calves (Table 1A). Further, all but one mother seen alone more than once in April and May was also seen with her calf at some point during those months (Table 2), indicating that many early spring separations are likely temporary. Therefore, the data in Case #1 do not support the hypothesis of a lasting mother/calf separation; it is quite possible that the calf was nearby and simply not detected.
In contrast to Case #1, Case #2 appears to represent a mother and calf that separated as early as June; the calf seen alone every time during the seven subsequent sightings in every month from June through September. The mother could have been nearby for any of those sightings, but it is unusual to have no detections of the mother with, or near, the calf (Table 2 and Hamilton et al. 1995). This calf was first seen in early December and, based on its physical appearance at the time (head shape and lack of cyamids or callosity), seemed to be just a week or two old (Patrician et al. 2008). Given how well developed that calf was in September [head, shape, apparent size, and cyamid coverage (Fig. 2)], it appears this case is a combination of a relatively early birth, potentially large birth size, and rapid growth. He was the only calf for the mother, #3320, who was a minimum of 12 years old at the time (her year of birth is unknown).
Similar to Case #2, Case #3 appears to represent a lasting separation rather than a short-term one as the mother was seen alone three times over 9 days and the calf was seen alone twice over 4 days (Table 4). Furthermore, those sightings alone were in different areas that are over 200 nm (380 km) apart; the mother was on Jeffreys Ledge and the calf in the Bay of Fundy (Fig. 1).
Finally, it is unclear whether Case #13 represents a lasting separation. While Table 1 indicates that a mother seen alone in May is not uncommon, Table 2 indicates that it is rare to see a mother alone twice and never again with the calf (just three out of 17 examples). Unfortunately, the photographs from her two sightings alone in May spanned just 1 or 2 min each, so it is difficult to determine whether she was truly alone or not.
Does the separation mean a calf has been fully weaned?
While the repeated sightings apart described above indicate increased independence, they do not necessarily prove the calf was fully weaned. Observations of cetaceans are both relatively infrequent and short in duration and therefore do not provide a comprehensive picture of their behavior. For this reason, it is challenging to distinguish between a calf that is spending increased time apart from its mother but still nursing periodically and a calf that is fully weaned. Hamilton et al. (1995) only considered a calf weaned if there were 3 days with the calf alone and no sightings in between or after with the mother. Both Cases #2 and #3 meet these criteria. In Case #2, #3970 may have been weaned as early as June 17. In the June 17 sighting, he was with 13 years old #2640 in the Great South Channel and appeared to be about half the size of the older whale. The sighting spanned just 3 min. His July 5 sighting was off the New Jersey coast close to shore and the sighting lasted 2 min. There is no way to know if his mother was nearby in either of those sightings, but #3970’s large relative size in the June sighting and proximity to the shore in July, in water too shallow for his mother to be feeding below him, suggest that he may in fact have been fully weaned by June 17. Given he was likely 1–2 weeks old on December 4 (as described in the “Genetic matches” of the “Results”), he would have been approximately seven and a half months old in mid-June. While this is not much younger than the previously youngest documented weaning of a right whale at approximately 8 months (Hamilton et al. 1995), the data suggest that weaning at such a young age is rare. Further, the single early weaning example in Hamilton et al. (1995) was the result of the mother’s death, while the early weaning cases presented here and immediately below are likely the result of active, behavioral decisions by the mother (Trivers 1974; Taber and Thomas 1982).
Case #3 (#3790) also meets the Hamilton et al. (1995) criteria for weaning with #3790 weaned by August 30. Although we don’t know when #3790 was born (her first sighting was in April, see Table 4), if we use the birth date of January 5 estimated by Fortune et al. (2012), #3790 would have been 8 months when weaned.
Case #13 (#4040) does not fit the criteria for weaning but is worth noting because of the early separation (Table 4) and the rarity of the separation data for that time of year (Table 2). Case #13 was the only instance of a mother never seen with her calf in April or May even though her calf was still alive. If the two May sightings of #1308 alone represent a complete separation, given #4040 was born between December 20 and January 24, she would have been approximately 5 months old when her mother was first seen alone. Although the possibility that she was weaned by then is bolstered by the data in Table 2 and thus cannot be ruled out, given that the case does not meet the three-sightings-alone criteria, and that it would be a very young weaning, it is more likely that the calf was nearby in one or both sightings and simply not detected.
Calves associated with other mothers
The analysis discovered two cases of calves associating with mothers other than their own. Besides Frasier et al.’s (2010) report of two mothers swapping calves on the calving ground and raising each other’s calves throughout the year, Case #4 is the first time to our knowledge that a mother–calf association observed in May was not a true mother–calf pair. However, the data supporting this association are weak. Calf #3580 and #1315 were together for a short period at the beginning of the 18 min long sighting, then the calf was alone playing at the surface for the remainder of the time before, according to the field notes, he “joined mom and raced away”. There are no photographs of the association at the end of the sighting to confirm it was #1315 he rejoined, so it is possible that he in fact joined his mother #1970. She was photographed nearby less than 2 h earlier so was known to be in the area. Thus, the association with #1315 may have been short-lived.
Case #5 is a particularly interesting case where it was initially thought to be one calf with a mother when in fact that mother associated with two different calves during the observation period. When calves are nursing, they often come up for quick breaths making it difficult to get detailed photographs of their callosity. In this case, #1608 and her calf #3308 were photographed together over a 20-min period. The pair dove and when they resurfaced 10 min later, the research team approached and biopsied the calf assuming, based on the association, that it was the same calf #1608 had been with before. Both the genetics, and subsequent inspection of the photographs, showed this to be incorrect; the darted calf was #3310- the calf of #2301. Whale #3310 had joined #1608 during the dive; it is unclear whether #3308 was also still there and undetected or had swum away. Similar to Case #4 above, #3310’s association with #1608 was likely short-lived as she was seen 80 min later with her own mother, #2301. This case underscores the importance of always taking confirming photographs when biopsying even if the whales were well-photographed just minutes before the sampling event.
Physical development
Two dead whales in this study were incorrectly classified as calves of the year (Table 4). Like all mammals, the length of a right whale at a given point in time is influenced by the fitness of its mother, timing of the calf’s birth, their length at birth, and their growth rate after birth (Moore et al. 2004; Christiansen et al. 2018; Sharp et al. 2019; Stewart et al. 2021). For most right whales, many of these factors are unknown, or only partially known. Given the resulting uncertainties around length, the initial misclassification of the dead whales in Cases #6 and #7 as calves of the year based on their size is understandable. Using measurements from dead, known-age whales, Sharp et al. (2019) classified animals under 9.0 m in length as calves of the year and carcasses between 10.0 and 12.0 m as juveniles. Both of these dead whales had length measurements that fell between the calf and juvenile categories. In Case #6, #3595’s growth was only slightly smaller than average for a 1.5 years old (9.58 m), whereas in Case #7, #4505’s estimated length of 9.0 m was very small for a 3.6 + years old. Some of the shortfall in length in the latter case was likely caused by the need to estimate the length without flukes present. Still, even allowing for error, it seems #4505 was truly small for his age. With limited data, Sharp et al. (2019) found some evidence that whales in the 2010 decade were smaller than whales from previous decades. Those preliminary findings have since been borne out by Stewart et al. (2021), who found decreasing body lengths in right whales over a three-decade period. The authors discovered that a whale born in 2019 is expected to reach a maximum length one meter less than a whale born in 1981. Stunted growth was linked to entanglements in fishing gear, either the whale had been severely entangled previously or its mother had been entangled while nursing. Neither #3593 nor #4505 had evidence of a previous entanglement that would have affected their growth (#4505 died of an entanglement, but had been gear-free just a few months prior to his death) and neither of their mothers were entangled while nursing. Stewart et al. (2021) noted that stunted growth could be the result of cumulative impacts including the additional factors of shifting prey seascapes, vessel strikes, and foraging interference from vessel traffic. Climate change has definitely impacted the prey seascape for right whales since 2012 (Record et al. 2019) and those changes may have, in part, resulted in #3593 and #4505’s stunted growth.
While Cases #6 and #7 represent animals that were smaller than expected, Case #2 is clearly a case of an unusually well-developed calf (Fig. 2). By the age of 10 months, his physical features were more consistent with a 22 month old yearling. Calves can generally be distinguished from 1 or 2-year-old whales based on their size, the shape of their heads (Patrician et al. 2008), and the quantity and color of cyamids on their head. Whereas calves tend to have patches of orange cyamids (Cyamus erraticus), the callosities of whales 1 year old and older have few of this species and instead are colonized by a species that is white (Cyamus ovalis) (Rowntree 1996). Potential reasons for #3970’s advanced development are that his mother #3320 was in good condition and that condition resulted in higher quality milk, his genetics favor robust growth, he was larger than most at birth, he experienced better than average feeding conditions once he began to feed on plankton, or a combination of these factors. Given he was large by June, it seems the first two explanations are more likely as #3970 would have just begun to feed in April.
Genotyped and not cataloged
Since 2011, a relatively large percentage of whales that were biopsied as calves have not been cataloged or biopsied again (Table 3—2011–2017). This increase in unidentified calves compared to previous years is likely caused by fewer calves being well-photographed on the feeding grounds (i.e. they could not be photographically identified) and a delay in genetic re-sampling. The dates between samples in the “Genetics evidence” column in Table 4 show that, on average, it takes almost 5 years for a calf from previous years to be genetically re-sampled. The recent distribution shift, and the fact that most of the surveys in the newly occupied habitats are aerial, has exacerbated this already long delay between re-sampling events because locating and biopsying the unknown juveniles has become more challenging.
As for the biopsied calves from the earlier years (i.e. 1988–2010) that have still not been genetically re-sampled, most represent situations where either (1) the mother was seen 2 years later with a calf indicating that her previous calf likely died (Burnell 2001; Browning et al. 2010) or (2) the mother was an offshore whale and her calf may not have yet returned to inshore waters. In the latter case, the calves were only seen when they were very young before their callosities developed. These whales may still be alive but seen so rarely, if at all, that they have not been biopsied again. The possibility that some of these calves may spend their lives in other habitats is intriguing. We know that other habitats must exist at every time of year and that some whales preferentially use these unknown areas (i.e., mothers that are only seen in their calving years) (Hamilton et al. 2007). Therefore, some of these calves represent a portion of the population whose life history data are poorly tracked.
Implications for other species
While this study is focused on North Atlantic right whales, the results presented here underscore the importance of genetic sampling of young animals of other species as well. There is a tremendous variation in the behavior of wild animals, and that variability is amplified by a rapidly changing climate. Collecting samples from individuals as early as possible without causing harm increases the chances of recognizing them in the future, thus improving our ability to more accurately track the vital rates of a population. While the rationale for early sampling applies to many mammals, it is particularly important for studies of other large cetaceans. Whales tend to have large geographic ranges and only sporadic opportunities for observation due to the challenges inherent in working at sea with long-diving animals. This means that cetologists have to piece together individual whales’ life stories using only partial data from relatively few observations. For this reason, sampling calves on their birthing grounds while they are still associated with their mothers increases the chance that data on age, maternity, and survival, among others, will be as complete as possible.
Conclusion
The 13 cases studies presented here provided insight into right whale calf survival, growth rates, and association patterns. The genetic sampling of these young calves led to the discovery that four calves thought to be dead were actually alive. Two of these calves appear to have been weaned at 7.5 and 8.0 months, 2–4 months earlier than expected. Because we now know these early mother–calf separations do not necessarily mean the calf has died, extra effort should be made to review photographs of right whale calves that were seen only on the calving ground, even if their mother was always alone on the feeding ground later that year. Although the callosity is generally poorly formed during those early months, in some cases it is sufficiently developed to confidently match to later sightings of that individual.
This study shows that it is not uncommon for mothers to be seen without their calves on the feeding ground for short periods as early as April; in fact, more mothers are seen along in April than are calves. The percentage of sightings of mothers and calves apart increases steadily from April through September when it peaks at 41% of all the calf sightings without their mothers. After that, it appears that the two come back together in October. Some calves associate with different mothers for short periods of time as early as April. On average, it took 5 years before the calves in this study were re-sampled, and that delay in linking calves to post-calf sightings has only increased in recent years due to distribution shifts away from known summer habitats, and the fact that there is less biopsy effort in the newly used habitats because they are surveyed primarily from planes. These shifts, and the subsequent delays in sampling, increase the number of gaps in the photo-identification data for calves and juveniles in recent years.
The results presented here underscore the importance of vessel-based surveys for genetic sampling of right whales calves on the calving ground while they are still associated with their mothers, as well as of other “new” whales throughout their range. The study also provides a cautionary note that, because growth rates, weaning times, and mother–calf associations are so variable, genetics should be combined with photo-identification whenever possible to accurately detect this variability.
Change history
22 December 2022
A Change History entry "Supplementary Informaiton was updated." is to be added to the A++ of the publication.
References
Baker CS, Palumbi SR, Lambertsen RH, Weinrich MT, Calambokidis J, O’Brien SJ (1990) Influence of seasonal migration on geographic distribution of mitochondrial DNA haplotypes in humpback whales. Nature 344:238–240. https://doi.org/10.1038/344238a0
Baumgartner MF, Mate BR (2003) Summertime foraging ecology of North Atlantic right whales. Mar Ecol Prog Ser 264:123–135. https://doi.org/10.3354/meps264123
Brown MW, Kraus SD, Gaskin DE, Wong-Brown MW, Gaskin DJ, Gaskin KJ (1991) Reaction of North Atlantic right whales (Eubalaena glacialis) to skin biopsy sampling for genetic and pollutant analysis. Rep Int Whal Comm (Special Issue 13):81–89
Browning CL, Rolland RM, Kraus SD (2010) Estimated calf and perinatal mortality in western North Atlantic right whales (Eubalaena glacialis). Mar Mamm Sci 26(3):648–662. https://doi.org/10.1111/j.1748-7692.2009.00361.x
Burnell SR (2001) Aspects of the reproductive biology, movements and site fidelity of right whales off Australia. J Cetacean Res Manag (Special Issue 2) 2:89–102. https://doi.org/10.47536/jcrm.vi.272
Caswell H, Fujiwara M, Brault S (1999) Declining survival probability threatens the North Atlantic right whale. Proc Natl Acad Sci USA Popul Biol 96:3308–3313. https://doi.org/10.1073/pnas.96.6.3308
Christiansen F, Vivier F, Charlton C, Ward R, Amerson A, Burnell S, Bejder L (2018) Maternal body size and condition determine calf growth rates in Southern right whales. Mar Ecol Prog Ser 592:267–282. https://doi.org/10.3354/meps12522
Clutton-Brock TH, Sheldon BC (2010) Individuals and populations: the role of long-term, individual-based studies of animals in ecology and evolutionary biology. Trends Ecol Evol 25(10):562–573. https://doi.org/10.1016/j.tree.2010.08.002
Clutton-Brock TH, Guinness FE, Albon SD (1982) Red deer: behaviour and ecology of two sexes. Edinburgh University Press, Edinburgh
Cooke JG (2020) Eubalaena glacialis. The IUCN red list of threatened species 2020: e.T41712A162001243. Downloaded on 18 August 2021. https://www.iucnredlist.org/species/41712/162001243. Accessed 1 Aug 2021
Evett IW, Weir BS (1998) Interpreting DNA evidence: statistical genetics for forensic scientists. Sinauer, Sunderland
Foley HJ, Holt RC, Hardee RE, Nilsson PB, Jackson KA, Read AJ, Pabst DA, Mclellan WA (2011) Observations of a western North Atlantic right whale (Eubalaena glacialis) birth offshore of the protected Southeast U.S. critical habitat. Mar Mamm Sci 27:E234–E240. https://doi.org/10.1111/j.1748-7692.2010.00452.x
Fortune SME, Trites AW, Perryman WL, Moore MJ, Pettis HM, Lynn MS (2012) Growth and rapid early development of North Atlantic right whales (Eubalaena glacialis). J Mamm 93:1342–1354. https://doi.org/10.1644/11-mamm-a-297.1
Frasier TR, Rastogi T, Brown MW, Hamilton PK, Kraus SD, White BN (2006) Characterization of tetranucleotide microsatellite loci and development and validation of multiplex reactions for the study of right whale species (Genus Eubalaena): primer note. Mol Ecol Notes 6(4):1025–1029. https://doi.org/10.1111/j.1471-8286.2006.01417.x
Frasier TR, Hamilton PK, Brown MW, Conger LA, Knowlton AR, Marx MK, Slay CK, Kraus SD, White BN (2007) Patterns of male reproductive success in a highly promiscuous whale species: the endangered North Atlantic right whale. Mol Ecol 16:5277–5293. https://doi.org/10.1111/j.1365-294x.2007.03570.x
Frasier TR, Hamilton PK, Brown MW, Kraus SD, White BN (2009) Sources and rates of errors in methods of individual identification in the North Atlantic right whale. J Mamm 90(5):1246–1255. https://doi.org/10.1644/08-mamm-a-328.1
Frasier TR, Hamilton PK, Brown MW, Kraus SD, White BN (2010) Reciprocal exchange and subsequent adoption of calves by two North Atlantic right whales. Aquat Mamm 36(2):115–120. https://doi.org/10.1578/AM.36.2.2010.115
Frasier TR, Koroscil SM, White BN, Darling JD (2011) Assessment of population substructure in relation to summer feeding ground use in the eastern North Pacific gray whale. Endanger Species Res 14:39–48. https://doi.org/10.3354/esr00340
Frasier TR, Gillett RM, Hamilton PK, Brown MW, Kraus SD, White BN (2013) Postcopulatory selection for dissimilar gametes maintains heterozygosity in the endangered North Atlantic right whale. Ecol Evol 3(10):3483–3494. https://doi.org/10.1002/ece3.738
Gilson A, Syvanen M, Levine K, Banks J (1998) Deer gender determination by polymerase chain reaction: validation study and application to tissues, bloodstains, and hair forensic samples from California. Calif Fish Game 84(4):159–169
Goodall J (1968) The behaviour of free-living chimpanzees in the Gombe Stream Reserve. Anim Behav Monogr 1:165–311. https://doi.org/10.1016/s0066-1856(68)80003-2
Gowan TA, Ortega-Ortiz JG, Hostetler JA, Hamilton PK, Knowlton AR, Jackson KA, George RC, Taylor CR, Naessig PJ (2019) Temporal and demographic variation in partial migration of the North Atlantic right whale. Sci Rep 9(1):1–11. https://doi.org/10.1038/s41598-018-36723-3
Halloran KM, Murdoch JD, Becker M (2015) Applying computer-aided photo-identification to messy datasets: a case study of Thornicroft’s giraffe (Giraffa camelopardalis thornicrofti). Afr J Ecol 53:147–155. https://doi.org/10.1111/aje.12145
Hamilton PK, Cooper LA (2010) Changes in North Atlantic right whale (Eubalaena glacialis) cow-calf association times and use of the calving ground: 1993–2005. Mar Mamm Sci 26:896–916. https://doi.org/10.1111/j.1748-7692.2010.00378.x
Hamilton PK, Marx MK, Kraus SD (1995) Weaning in North Atlantic right whales. Mar Mamm Sci 11(3):386–390. https://doi.org/10.3354/meps171285
Hamilton PK, Knowlton AR, Marx MK, Kraus SD (1998) Age structure and longevity in North Atlantic right whales (Eubalaena glacialis) and their relation to reproduction. Mar Ecol Prog Ser 171:285–292. https://doi.org/10.3354/meps171285
Hamilton PK, Knowlton AR, Marx MK (2007) Right whales tell their own stories: the photo-identification catalog. In: Kraus SD, Rolland RM (eds) The urban whale: North Atlantic right whales at the crossroads. Harvard University Press, Cambridge
Hammond PS, Mizroch SA, Donovan GP (eds) (1990) Individual recognition of cetaceans: use of photo-identification and other techniques to estimate population parameters: incorporating the proceedings of the symposium and workshop on individual recognition and the estimation of cetacean population parameters (No. 12). International Whaling Commission, Cambridge
Hayes SA, Gardner S, Garrison LP, Henry A, Leandro L (2018) North Atlantic right whales-evaluating their recovery challenges in 2018. NOAA Technical Memorandum NMFS-NE-247
Jacobsen KO, Marx M, Oien N (2004) Two-way trans-Atlantic migration of a North Atlantic right whale (Eubalaena glacialis). Mar Mamm Sci 200:161–166. https://doi.org/10.1111/j.1748-7692.2004.tb01147.x
Kalinowski ST, Taper ML, Marshall TC (2007) Revising how the computer program cervus accommodates genotyping error increases success in paternity assignment. Mol Ecol 16:1099–1106. https://doi.org/10.1111/j.1365-294x.2007.03089.x
Kelly MJ, Laurenson MK, Fitzgibbon CD, Collins DA, Durant SM, Frame G, Bertram BCB, Caro TM (1998) Demography of the Serengeti cheetah (Acinonyx jubatus) population: the first 25 years. J Zool (lond) 244:473–488. https://doi.org/10.1111/j.1469-7998.1998.tb00053.x
Klumov SK (1962) The right whales in the Pacific Ocean. Biol Mar Stud Trud Inst Okeanogr 58:202–297
Knowlton AR, Sigurjonsson J, Ciano JN, Kraus SD (1992) Long-distance movements of North Atlantic right whales (Eubalaena glacialis). Mar Mamm Sci 8:397–405. https://doi.org/10.1111/j.1748-7692.1992.tb00054.x
Knowlton AR, Kraus SD, Kenney RD (1994) Reproduction in North Atlantic right whales (Eubalaena glacialis). Can J Zool 72(7):1297–1305. https://doi.org/10.1139/z94-173
Knowlton AR, Hamilton PK, Marx MK, Pettis HP, Kraus SD (2012) Monitoring North Atlantic right whale (Eubalaena glacialis) entanglement rates: a 30 yr retrospective. Mar Ecol Prog Ser 466:293–302. https://doi.org/10.3354/meps09923
Knowlton AR, Robbins J, Landry S, McKenna HA, Kraus SD, Werner TB (2016) Effects of fishing rope strength on the severity of large whale entanglements. Conserv Biol 30(2):318–328. https://doi.org/10.1111/cobi.12590
Kraus SD (1990) Rates and potential causes of mortality in North Atlantic right whales (Eubalaena glacialis). Mar Mamm Sci 6:278–291. https://doi.org/10.1111/j.1748-7692.1990.tb00358.x
Kraus SD, Prescott JH, Knowlton AR, Stone GS (1986a) Migration and calving of right whales (Eubalaena glacialis) in the western North Atlantic. Rep Int Whal Commn (Special Issue 10):139–144.
Kraus SD, Moore KE, Price CE, Crone MJ, Watkins WA, Winn HE, Prescott JH (1986b) The use of photographs to identify individual North Atlantic right whales (Eubalaena glacialis). Rep Int Whal Commn (Special Issue 10):145–151
Kraus SD, Hamilton PK, Kenney RD, Knowlton AR, Slay CK (2001) Reproductive parameters of the North Atlantic right whale. J Cetacean ResManage (Special Issue 2):231–236 https://doi.org/10.47536/jcrm.vi.285
Malik S, Brown MW, Kraus SD, Knowlton AR, Hamilton PK, White BN (1999) Assessment of mitochondrial DNA structuring and nursery use in the North Atlantic right whale (Eubalaena glacialis). Can J Zool 77(8):1217–1222. https://doi.org/10.1139/cjz-77-8-1217
Marshall TC, Slate J, Kruuk LEB, Pemberton JM (1998) Statistical confidence for likelihood-based paternity inference in natural populations. Mol Ecol 7:639–655. https://doi.org/10.1046/j.1365-294x.1998.00374.x
Mayo CA, Marx MK (1990) Surface foraging behaviour of the North Atlantic right whale, Eubalaena glacialis, and associated zooplankton characteristics. Can J Zool 68(10):2214–2220. https://doi.org/10.1139/z90-308
McLeod BA, Brown MW, Frasier TR, White BN (2010) DNA profile of a sixteenth century western North Atlantic right whale (Eubalaena glacialis). Conserv Genet 11:339–345. https://doi.org/10.1007/s10592-009-9811-6
Miller CA, Best PB, Perryman WL, Baumgartner MF, Moore MJ (2012) Body shape changes associated with reproductive status, nutritive condition and growth in right whales Eubalaena glacialis and E. australis. Mar Ecol Prog Ser 459:135–156. https://doi.org/10.3354/meps09675
Moore MJ, Knowlton AR, Kraus SD, Mclellan WA, Bonde RK (2004) Morphometry, gross morphology and available histopathology in North Atlantic right whale (Eubalaena glacialis) mortalities (1970–2002). J Cetacean Res Manag 6(3):199–214
National Research Council (1996) The evaluation of forensic DNA evidence. National Academies Press, Washington DC. https://doi.org/10.1017/s0016672397219415
Pace RM III, Corkeron PJ, Kraus SD (2017) State–space mark–recapture estimates reveal a recent decline in abundance of North Atlantic right whales. Ecol Evol 00:1–12. https://doi.org/10.1002/ece3.3406
Paetkau D, Strobeck C (1994) Microsatellite analysis of genetic variation in black bear populations. Mol Ecol 3:489–495. https://doi.org/10.1111/j.1365-294x.1994.tb00127.x
Palsbøll PJ, Larsen F, Sigurd Hansen E (1991) Sampling of skin biopsies from free-ranging large cetaceans in West Greenland: development of new biopsy tips and bolt designs. Rep Int Whal Comm (Special Issue 13):71–79
Patrician MR, Biedron IS, Esch HC, Wenzel FW, Cooper LA, Hamilton PK, Glass AH, Baumgartner MF (2008) Evidence of a North Atlantic right whale calf (Eubalaena glacialis) born in northeastern U.S. waters. Mar Mamm Sci 25(2):462–477. https://doi.org/10.1111/j.1748-7692.2008.00261.x
Payne R, Brazier O, Dorsey EM, Perkins JS, Rowntree VJ, Titus A (1983) External features in southern right whales (Eubalaena australis) and their use in identifying individuals. In: Payne R (ed) Communication and behavior of whales. Westview Press, Boulder, pp 371–445
Pettis HM, Pace RM III, Hamilton PK (2021) North Atlantic right whale consortium 2020 annual report card. Report to the North Atlantic Right Whale Consortium, pp 1–22. http://www.narwc.org. Accessed 1 Aug 2021
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Rastogi T, Brown MW, McLeod BA, Frasier TR, Grenier R, Cumbaa SL, Nadarajah J, White BN (2004) Genetic analysis of 16th-century whale bones prompts a revision of the impact of Basque whaling on right and bowhead whales in the western North Atlantic. Can J Zool 82(10):1647–1654. https://doi.org/10.1139/z04-146
Record NR, Runge JA, Pendleton DE, Balch WM, Davies KTA, Pershing AJ, Johnson CL, Stamieszkin K, Ji R, Feng Z, Kraus SD, Kenney RD, Hudak CA, Mayo CA, Chen C, Salisbury JE, Thompson CRS (2019) Rapid climate-driven circulation changes threaten conservation of endangered North Atlantic right whales. Oceanography 32:162–169. https://doi.org/10.5670/oceanog.2019.201
Rowntree VJ (1996) Feeding, distribution, and reproductive behavior of cyamids (Crustacea: Amphipoda) living on humpback and right whales. Can J Zool 74:103–109. https://doi.org/10.1139/z96-014
Sambrook J, Russell D (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbour Laboratory Press, Cold Spring Harbour
Schaeff CM, Hamilton PK (1999) Genetic basis and evolutionary significance of ventral skin color markings in North Atlantic right whales (Eubalaena glacialis). Mar Mamm Sci 15(3):701–711. https://doi.org/10.1111/j.1748-7692.1999.tb00837.x
Seutin G, White BN, Boag PT (1991) Preservation of avian blood and tissue samples for DNA analyses. Can J Zool 69(1):82–90. https://doi.org/10.1139/z91-013
Sharp SM, McLellan WA, Rotstein DS, Costidis AM, Barco SG, Durham K, Pitchford TD, Jackson KA, Daoust PY, Wimmer T, Couture EL (2019) Gross and histopathologic diagnoses from North Atlantic right whale (Eubalaena glacialis) mortalities between 2003 and 2018. Dis Aquat Org 135:1–31. https://doi.org/10.3354/dao03376
Silva MA, Steiner L, Cascão I, Cruz MJ, Prieto R, Cole T, Hamilton PK, Baumgartner M (2012) Winter sighting of a known western North Atlantic right whale in the Azores. J Cetacean Res Manag 12(1):65–69
Stewart JD, Durban JW, Knowlton AR, Lynn MS, Fearnbach H, Barbaro J, Perryman WL, Miller CA, Moore MJ (2021) Decreasing body lengths in North Atlantic right whales. Curr Biol 31:1–6. https://doi.org/10.1016/j.cub.2021.04.067
Taber SM, Thomas PO (1982) Calf development and mother-calf spatial relationships in southern right whales. Anim Behav 30:1072–1083. https://doi.org/10.1016/s0003-3472(82)80197-8
Taber SM, Thomas PO (1984) Mother-infant interaction and behavioral development in southern right whales, Eubalaena australis. Behaviour 88(1–2):42–60. https://doi.org/10.1163/156853984x00470
Trivers RL (1974) Parent-offspring conflict. Integr Comp Biol 14(1):249–264. https://doi.org/10.1093/icb/14.1.249
Waits LP, Luikart G, Taberlet P (2001) Estimating the probability of identity among genotypes in natural populations: cautions and guidelines. Mol Ecol 10:249–256. https://doi.org/10.1046/j.1365-294x.2001.01185.x
Zani MA, Taylor KD, Kraus SD (2008) Observation of a right whale (Eubalaena glacialis) birth in the coastal waters of the Southeast United States. Aquat Mamm 34:21–24. https://doi.org/10.1578/am.34.1.2008.21
Zero VH, Sundaresan SR, O’Brien TG, Hinnaird MF (2013) Monitoring an endangered savannah ungulate, Grevy’s zebra Equus grevyi: choosing a method for estimating population densities. Oryx 47:410–419. https://doi.org/10.1017/s0030605312000324
Zoidis AM, Lomac-MacNair KS, Chomos-Betz AE, Day AJ, Sasha McFarland A (2014) Effects of sex, seasonal period, and sea state on calf behavior in Hawaiian humpback whales (Megaptera novaeangliae). Aquat Mamm 40(1):44–58. https://doi.org/10.1578/am.40.1.2014.44
Acknowledgements
We’d like to thank the National Marine Fisheries Service for their funding for field sampling efforts as well as for their steadfast support of the North Atlantic Right Whale Catalog. Recent support for the genetic analysis was provided by Fisheries and Oceans Canada (DFO) [Contract no. F5211-180589]. Photographs and genetic samples were collected under the following permits: to Scott Kraus of the New England Aquarium-NOAA Permit #716, #1014, #775-1600-2, #655-1652-01, #14233, and #19674; to Clay George of the Georgia Department of Natural Resources- NOAA permits #15488, #20556; and to the Northeast Fisheries Science Center-NOAA permits #775-1600, #775-1875, #17355, #21371. Photographs and genetic samples were collected in Canadian waters under the following permits: Fisheries and Oceans Canada Section 73 Scientific Research permit MAR-SAR-2007-007, #322835, 325863, DFO-MAR-2016-04. We are indebted to Dr. Moira Brown, who began the genetics program, and Dr. Bradley White, who shepherded it for decades. Many aerial survey teams alerted researchers on the water to potential biopsy targets improving the success of genetic sample collection. In particular, we want to thank the aerial survey teams from Clearwater Marine Aquarium Research Institute and the Florida Fish and Wildlife Conservation Commission on the calving grounds and the Center for Coastal Studies (CCS) and Northeast Fisheries Science Center (NEFSC) on the feeding grounds off Massachusetts. Also, thanks to the team of right whale matchers at the New England Aquarium for their dedicated, detail-oriented efforts that make the photo-identification Catalog such a reliable resource. Thanks to Brooke Hodge for supplying the map. Finally, thanks go to the North Atlantic Right Whale Consortium (http://www.narwc.org) for access to these data, and for fostering collaborations among the many organizations and individuals that work passionately to protect this beleaguered species.
Author information
Authors and Affiliations
Contributions
PKH conceived of the study; PKH, LAC, RCG, and KAJ collected genetic samples; BAF and TRF processed genetic samples (with others); PKH and KAJ processed photographic data (with others), all authors secured funding for different aspects of the work; PKH wrote the manuscript with TRF; BAF, LAC, RCG, and KAJ provided additional edits. All authors approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Handling editors: Leszek Karczmarski and Scott Y.S. Chui.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is a contribution to the special issue on “Individual Identification and Photographic Techniques in Mammalian Ecological and Behavioural Research – Part 2: Field Studies and Applications” – Editors: Leszek Karczmarski, Stephen C.Y. Chan, Scott Y.S. Chui and Elissa Z. Cameron.
Supplementary information
Below is the link to the electronic supplementary material.
Appendix
Appendix
See Fig. A1.
An example of a positive photo-identification of a female-calf pair of the North Atlantic right whales (Eubalaena glacialis). Individuals of this species can be distinguished by the individual-specific pattern of natural markings on their head, called callosities, and scars on their bodies that can be well documented with aerial and shipboard photographs. Here, an individual nicknamed “Mayport” (Catalog #4094), a 6 years old female is shown with her first calf off Ponte Vedra Beach, FL in the Southeast U.S. calving area on February 1, 2016. Photo credit: Florida Fish and Wildlife Conservation Commission, taken under NOAA research permit #15488
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hamilton, P.K., Frasier, B.A., Conger, L.A. et al. Genetic identifications challenge our assumptions of physical development and mother–calf associations and separation times: a case study of the North Atlantic right whale (Eubalaena glacialis). Mamm Biol 102, 1389–1408 (2022). https://doi.org/10.1007/s42991-021-00177-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42991-021-00177-4