Abstract
Exercise has well-documented effects on immune function with both positive and negative sequelae dependent on intensity, volume, and duration. The immunosuppressive effects of exercise are mediated, in part, by the complex interaction of cytokines, catecholamines, and nutrition. Caffeine has been reported to modulate immune function; however, the interaction with brief exhaustive exercise is not well characterized. It was hypothesized that caffeine would upregulate the cytokine response to intense exercise. Seven male students volunteered to participate in a randomized, double-blinded crossover study where they ingested either caffeine (6 mg/kg) or a maltodextrin placebo (6 mg/kg) 1 h prior to a cycling exercise. The exercise protocol started at 100 W and the intensity was increased by 50 W every two minutes until exhaustion. Serum samples were collected pre-, post-, and 1-h post-exercise and analyzed for IL-4, IL-10, and TGF-β1. Caffeine ingestion increased time to exhaustion (P = 0.005; Effect Size [ES] = 1.33), IL-4 (P = 0.004; ES = 2.34), IL-10 (P = 0.047; ES = 0.41 ± 0.57), and TGF-β1 (P = 0.013; ES = 0.76). The accentuated response of the cytokines may have important ramifications due to their potent anti-inflammatory and immunoregulatory properties. Specifically, the 6 mg/kg caffeine dose not only improved exercise performance but the cytokine data is indicative of an upregulated inflammatory response and an enhancement of the anti-inflammatory benefits of exercise.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exercise is an immunomodulatory stimulus with both acute and chronic exercise modulating aspects of the innate and adaptive immune system [40]. Notably, moderate exercise has been associated with improved immune function [22], whereas high volumes of training and competing in endurance events can negatively affect the risk of infection [26]. Prolonged exercise has been linked to an increased risk of upper respiratory symptoms [7] and decreased salivary immunoglobulin A (IgA) secretion interpreted as acute immunosuppression [25].
Intense exercises such as wrestling [21], resistance exercise [23], or cycling [20] can also have a significant impact on the expression and quantity of immune cells. Even though many athletes adopt a polarized training model and dedicate a significant amount of time to high-intensity training [31, 35], less research effort has focused on the immunosuppressive effects of intense or exhaustive training modalities. The impact on immune function resultant from high-intensity exercise has been suggested to be due to the release of catecholamines [29] and stress hormones that are known to have potent immunosuppressive effects [6].
Nutritional adjuncts to counter the negative sequelae of exercise-induced fluctuations in immune functioning include carbohydrate, whey protein, vitamin, and caffeine supplementation [11, 24]. While caffeine does demonstrate antioxidant properties [1], it is also known to elevate post-exercise cortisol in a dose-dependent manner [2]. Thus, there is the potential for immunosuppressive effects either directly via adenosine receptor antagonism, or indirectly via an enhanced catecholamine and/or cortisol release [9, 16]. Direct increases in activation of natural-killer at caffeine doses of 6 mg kg−1 have also been reported [10]. Currently, an interaction between caffeine and high-intensity exercise involving incremental exercise until exhaustion on immune function is not well characterized. Here we tested the hypothesis that caffeine would upregulate anti-inflammatory and immunoregulatory cytokine release (IL-4, IL-10, and TGF-β1) during short incremental exercise until exhaustion.
Methods and Materials
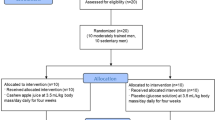
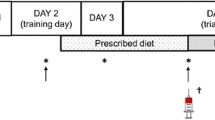
Seven healthy male physical education students (age 22.1 ± 0.6; height: 1.83 ± 0.08 m; body mass: 72.8 ± 13.0 kg) volunteered to participate in this randomized, double-blinded crossover study with at least seven days between trials. The only inclusion criteria were that the participants were free from injury or any contraindications to performing exhaustive exercise. All participants reported being low caffeine consumers with daily dietary consumption of < 60 mg per day. After signing to acknowledge informed consent and refraining from strenuous exercise and all forms of caffeine intake for 48 h, each participant performed an exhaustive exercise protocol on a cycle ergometer. The initial workload for the exercise protocol was set at 100 W and the intensity was increased by 50 W every two minutes until either volitional exhaustion or the time point at which the participant was unable to maintain a pedaling cadence of 60 rpm. Sixty minutes before each exercise protocol the participants were randomly assigned to ingest either caffeine (6 mg/kg) or a maltodextrin placebo (6 mg/kg) administered in an indistinguishable gelatin capsule via simple random allocation using a computer software program to generate the random sequence. Blood samples were collected pre-, post-, and 1-h post-exercise and analyzed for serum levels of IL-4, IL-10, and TGF-β1 following the manufacturer’s instructions (Immunoready, Kampenhout, Belgium) and reported in pg/mL. Seated venous blood samples were collected in suitable vacutainers. Within 30 min of blood collection, plasma was obtained via centrifugation (15 min, 1000 g, 4 °C) and all samples were stored at − 80 °C until measurements were performed. The experimental protocol was approved by the University of Kurdistan Review Board in accordance with the latest version of the Declaration of Helsinki.
Changes in the mean of each measure with and without caffeine treatment were used to assess magnitudes of effects by dividing the changes by the appropriate between-participant standard deviations. Pairwise comparisons were made between conditions, and differences were interpreted in relation to the likelihood of exceeding the smallest worthwhile effects with individual change thresholds for each variable. Hormonal data were log-transformed to reduce non-uniformity of error, with effects derived by back transformation as percentage changes Magnitudes of the standardized effects were interpreted using thresholds of 0.2, 0.6 and 1.2 for small, moderate, and large, respectively [15]. Standardized effects of between − 0.19 and 0.19 were termed trivial. To make inferences about the large-sample value of an effect, the uncertainty in the effect was expressed as 90% confidence limits. An effect was deemed unclear if the confidence interval overlapped the thresholds for both small positive and negative effects; otherwise, the effect was deemed clear and substantial. The significance level was set at P < 0.05.
Results
Caffeine ingestion increased time to exhaustion by 14.1% ± 6.1% (P = 0.005; Effect Size [ES] = 1.33; Fig. 1; Mean ± SD). Specifically, caffeine ingestion improved the time to exhaustion from 413 ± 25.5 s to 472 ± 41.4 s. IL-4 increased during exercise with a 22.3% ± 10.5% greater increase following caffeine ingestion (P = 0.004; ES = 2.34). A substantial difference in IL-4 concentration persisted between the caffeine and placebo interventions one hour after exercise cessation (P = 0.027; 10.1% ± 7.1%; ES = 1.11; Fig. 2A). Very large increases in IL-10 were observed following exercise and again these increases were 10% ± 15.1% greater following caffeine ingestion (P = 0.047; ES = 0.41). A substantial difference persisted in IL-10 concentration between the caffeine and placebo interventions one hour after exercise cessation (13.9% ± 13.3%; ES = 0.81; Fig. 2B). TGF-β1 increased to a greater extent following exercise in the caffeine condition (16.9% ± 16.7%; P = 0.013; ES = 0.76); however, there were no substantial differences between the conditions in TGF-β1 concentration at the post-exercise or 1-h post-exercise time point. Specifically, the exercise-induced increase in TGF-β1 was from 525 ± 174 to 556 ± 198 pg/mL in the control condition; whereas, it increased from 485 ± 38 to 600 ± 89 pg/mL in the caffeine condition.
Discussion
Here we present data that caffeine demonstrates both an acute exercise performance enhancement and an immunoregulatory effect with an amplified response of the cytokines IL-4 and IL-10. This modulation may have important ramifications as these cytokines have potent anti-inflammatory and immunoregulatory properties, and can enhance the production of salivary immunoglobulin A [32, 36]. Thus, caffeine may enhance the protective effect of intense regular exercise on diseases associated with chronic inflammation [40].
The large improvement in time-to-exhaustion performance observed in the current study is consistent with other research demonstrating improved cycling performance [12]. The mechanism behind the impact of caffeine on performance is likely related to a decrease in perceived exertion, a decrease in the neuronal activation threshold of motor neurons, and/or an enhanced energy contribution from anaerobic metabolism [3, 8, 33]. The results of the current study confirm previous work demonstrating that caffeine can have a positive effect on incremental, time to exhaustion exercise protocols [34].
IL-4 is a classic Th-2 (T lymphocyte helper cells expressing cell surface molecule CD4) type cytokine and has been labeled as a “prototypic immunoregulatory cytokine” [32]. While in vitro studies have reported that methylxanthines such as caffeine inhibit the Th-2 immune response [28], in vivo human research has shown an increase in lymphocyte count and activation of CD4+ cells following exercise combined with 6 mg/kg caffeine ingestion [4]. These contrasting results demonstrate that the immune response to exercise is part of a complex network, under the control of a range of interactive regulatory and feedback mechanisms not easily recapitulated in an in vitro environment. The accentuated response of IL-4 resultant from our incremental exercise until exhaustion protocol is consistent with prior work demonstrating an enhanced anti-inflammatory physiological milieu. Given the large magnitude of the accentuated response, we believe it is reasonable to assume that caffeine could have a meaningful impact on the overall anti-inflammatory response.
Similar to IL-4, IL-10 is produced by Th-2 cells and is a potent anti-inflammatory agent involved in the production of IgA, the main immunoglobulin in salivary immunity [32]. IL-10 is a “key immunoregulator” during infection [5], and a depressed post-exercise level of IgA has been associated with upper respiratory tract infections in endurance runners [25]. While an appropriate pro-inflammatory response is required upon immune challenge, a positive feedback loop coordinated by IL-10 allows the resolution of infection. Of note, Northoff and colleagues [27] compared the immune response to an acute bout of exercise to that induced by infection. In a previous exercise study that assessed an interaction between caffeine and IL-10, accentuated responses consistent with our data have been observed following a 15 km run of ~ 68 min duration [39] using an identical dose to our protocol. These authors had earlier suggested that the higher IL-10 response following caffeine ingestion was mediated by inhibition of cAMP-phosphodiesterase [38]. While small, the significant and clear amplification of the IL-10 response in the caffeine condition is again indicative of an enhanced anti-inflammatory response.
TGF-β is a pleiotropic cytokine with immunosuppressive effects that works in concert with IL-10 and the production and function of these two cytokines may be interdependent [18, 30]. The resultant activity ensures a controlled inflammatory response that inhibits T-cell-mediated immunopathology [19]. As the IL-10 response to exercise was accentuated by caffeine in the current exhaustive protocol, it is unsurprising that a similar, if somewhat shorter time course of elevation was observed in TGF-β1. It is worth noting though that this accentuated response contrasts that observed in in vitro work [14, 37].
Here we present novel data that addresses the interaction between caffeine and incremental cycling exercise until exhaustion on specific aspects of immune function. We acknowledge that a distinction must be made between acute and chronic cytokine responses and that the “contextual dependence” of cytokines must be taken into consideration [40]. While we did not directly assess caffeine or catecholamine levels post-ingestion, we did use an identical dosage to previous work that elicited increases in these measures; albeit in longer [4, 38] and shorter [13, 17] exercise protocols. Additionally, other biomarkers, such as immunoglobulin A would add insight into the immune response. We also note that the relative contribution of the bioactive caffeine metabolites paraxanthine, theobromine, and theophylline to the observed results is not elucidated in the current work. It is also worth acknowledging that there is no sport or competition where the outcome is determined by time to exhaustion; thus, the physiological responses to the exercise protocol adopted herein is reflective only of a brief incremental exercise until exhaustion. The large variability in responses is also noteworthy, and previous research has identified individual responsiveness to caffeine intake [14]. Regardless, the 6 mg/kg caffeine dose protocol improved exercise performance, and the cytokine data are indicative of an enhancement of the anti-inflammatory properties of exercise proposed earlier [39].
Data Availability
All data utilised in the preparation of this manuscript will be made available upon reasonable request.
Abbreviations
- IL-4:
-
Interleukin-4
- IL-10:
-
Interleukin-10
- TGF-β1:
-
Transforming growth factor β1
- ES:
-
Effect size
- IgA:
-
Salivary immunoglobulin A
- Rpm:
-
Revolutions per minute
- SD:
-
Standard deviation
- Th-2:
-
Helper T cells-2
- CD4:
-
Cluster of differentiation 4
- cAMP:
-
Cyclic adenosine monophosphate
References
Arauz J, Zarco N, Segovia J, Shibayama M, Tsutsumi V, Muriel P. Caffeine prevents experimental liver fibrosis by blocking the expression of TGF-beta. Eur J Gastroenterol Hepatol. 2014;26(2):164–73. https://doi.org/10.1097/MEG.0b013e3283644e26.
Beaven CM, Hopkins WG, Hansen KT, Wood MR, Cronin JB, Lowe TE. Dose effect of caffeine on testosterone and cortisol responses to resistance exercise. Int J Sport Nutr Exerc Metab. 2008;18(2):131–41.
Bell DG, Jacobs I, Ellerington K. Effect of caffeine and ephedrine ingestion on anaerobic exercise performance. Med Sci Sports Exerc. 2001;33(8):1399–403.
Bishop NC, Fitzgerald C, Porter PJ, Scanlon GA, Smith AC. Effect of caffeine ingestion on lymphocyte counts and subset activation in vivo following strenuous cycling. Eur J Appl Physiol. 2005;93(5–6):606–13. https://doi.org/10.1007/s00421-004-1271-6.
Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol. 2008;180(9):5771–7. https://doi.org/10.4049/jimmunol.180.9.5771.
Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;335(1):2–13. https://doi.org/10.1016/j.mce.2010.04.005.
Cullen T, Thomas AW, Webb R, Phillips T, Hughes MG. sIL-6R is related to weekly training mileage and psychological well-being in athletes. Med Sci Sports Exerc. 2017;49(6):1176–83. https://doi.org/10.1249/MSS.0000000000001210.
Doherty M, Smith PM, Hughes MG, Davison RCR. Caffeine lowers perceptual response and increases power output during high-intensity cycling. J Sports Sci. 2004;22(7):637–43. https://doi.org/10.1080/02640410310001655741.
Dulson DK, Bishop NC. Effect of a high and low dose of caffeine on human lymphocyte activation in response to antigen stimulation. Appl Physiol Nutr Metab. 2016;41(2):224–7. https://doi.org/10.1139/apnm-2015-0456.
Fletcher DK, Bishop NC. Effect of a high and low dose of caffeine on antigen-stimulated activation of human natural killer cells after prolonged cycling. Int J Sport Nutr Exerc Metab. 2011;21(2):155–65. https://doi.org/10.1123/ijsnem.21.2.155.
Freidenreich DJ, Volek JS. Immune responses to resistance exercise. Exerc Immunol Rev. 2012;18:8–41.
Glaister M, Pattison JR, Muniz-Pumares D, Patterson SD, Foley P. Effects of dietary nitrate, caffeine, and their combination on 20-km cycling time trial performance. J Str Cond Res. 2015;29(1):165–74. https://doi.org/10.1519/JSC.0000000000000596.
Greer F, McLean C, Graham TE. Caffeine, performance, and metabolism during repeated Wingate exercise tests. J Appl Physiol. 1998;85(4):1502–8.
Gressner OA, Lahme B, Rehbein K, Siluschek M, Weiskirchen R, Gressner AM. Pharmacological application of caffeine inhibits TGF-beta-stimulated connective tissue growth factor expression in hepatocytes via PPARgamma and SMAD2/3-dependent pathways. J Hepatol. 2008;49(5):758–67. https://doi.org/10.1016/j.jhep.2008.03.029.
Hopkins WG, Marshall SW, Batterham AM, Hanin J. Progressive statistics for studies in sports medicine and exercise science. Med Sci Sports Exerc. 2009;41(1):3–13. https://doi.org/10.1249/MSS.0b013e31818cb278.
Horrigan LA, Kelly JP, Connor TJ. Immunomodulatory effects of caffeine: friend or foe? Pharmacol Ther. 2006;111(3):877–92. https://doi.org/10.1016/j.pharmthera.2006.02.002.
Karayigit R, Forbes SC, Osmanov Z, Yilmaz C, Yasli BC, Naderi A, Buyukcelebi H, Benesova D, Gabrys T, Esen O. Low and moderate doses of caffeinated coffee improve repeated sprint performance in female team sport athletes. Biology. 2022;11(10):1498.
Levings MK, Bacchetta R, Schulz U, Roncarolo MG. The role of IL-10 and TGF-beta in the differentiation and effector function of T regulatory cells. Int Arch Allergy Immunol. 2002;129(4):263–76. https://doi.org/10.1159/000067596.
Li MO, Flavell RA. Contextual regulation of inflammation: a duet by transforming growth factor-beta and interleukin-10. Immunity. 2008;28(4):468–76. https://doi.org/10.1016/j.immuni.2008.03.003.
Natale VM, Brenner IK, Moldoveanu AI, Vasiliou P, Shek P, Shephard RJ. Effects of three different types of exercise on blood leukocyte count during and following exercise. Sao Paulo Med J. 2003;121(1):9–14.
Nemet D, Mills PJ, Cooper DM. Effect of intense wrestling exercise on leucocytes and adhesion molecules in adolescent boys. Br J Sports Med. 2004;38(2):154–8.
Nieman DC, Nehlsen-Cannarella SL, Markoff PA, Balk-Lamberton AJ, Yang H, Chritton DB, Lee JW, Arabatzis K. The effects of moderate exercise training on natural killer cells and acute upper respiratory tract infections. Int J Sports Med. 1990;11(6):467–73. https://doi.org/10.1055/s-2007-1024839.
Nieman DC, Henson DA, Sampson CS, Herring JL, Suttles J, Conley M, Stone MH, Butterworth DE, Davis JM. The acute immune response to exhaustive resistance exercise. Int J Sports Med. 1995;16(5):322–8. https://doi.org/10.1055/s-2007-973013.
Nieman DC, Henson DA, McAnulty SR, McAnulty LS, Morrow JD, Ahmed A, Geward CB. Vitamin E and immunity after the Kona triathlon world championship. Med Sci Sports Exerc. 2004;36(8):1328–35.
Nieman DC, Henson DA, Dumke CL, Lind RH, Shooter LR, Gross SJ. Relationship between salivary IgA secretion and upper respiratory tract infection following a 160-km race. J Sports Med Phys Fitness. 2006;46(1):158–62.
Nieman DC, Johanssen LM, Lee JW, Arabatzis K. Infectious episodes in runners before and after the Los Angeles Marathon. J Sports Med Phys Fitness. 1990;30(3):316–28.
Northoff H, Berg A, Weinstock C. Similarities and differences of the immune response to exercise and trauma: the IFN-gamma concept. Can J Physiol Pharmacol. 1998;76(5):497–504.
Rosenthal LA, Taub DD, Moors MA, Blank KJ. Methylxanthine-induced inhibition of the antigen- and superantigen-specific activation of T and B lymphocytes. Immunopharmacology. 1992;24(3):203–17. https://doi.org/10.1016/0162-3109(92)90076-O.
Sand KL, Flatebo T, Andersen MB, Maghazachi AA. Effects of exercise on leukocytosis and blood hemostasis in 800 healthy young females and males. World J Exp Med. 2013;3(1):11–20. https://doi.org/10.5493/wjem.v3.i1.11.
Sanjabi S, Zenewicz LA, Kamanaka M, Flavell RA. Anti-inflammatory and pro-inflammatory roles of TGF-beta, IL-10, and IL-22 in immunity and autoimmunity. Curr Opin Pharmacol. 2009;9(4):447–53. https://doi.org/10.1016/j.coph.2009.04.008.
Seiler KS, Kjerland GØ. Quantifying training intensity distribution in elite endurance athletes: is there evidence for an “optimal” distribution? Scand J Med Sci Sports. 2006;16(1):49–56.
Slavish DC, Graham-Engeland JE, Smyth JM, Engeland CG. Salivary markers of inflammation in response to acute stress. Brain Behav Immun. 2015;44:253–69. https://doi.org/10.1016/j.bbi.2014.08.008.
Spriet LL, Howlett RA. In: Maughan RJ, editor. Nutrition in sport. Massachusetts: Blackwell Science Ltd; 2000. pp. 379–92.
Stadheim H, Stensrud T, Brage S, Jensen J. Caffeine increases exercise performance, maximal oxygen uptake and oxygen deficit in elite male endurance athletes. Med Sci Sports Exerc. 2021;53(11):2264–73.
Stöggl T, Sperlich B. Polarized training has greater impact on key endurance variables than threshold, high intensity, or high volume training. Front Physiol. 2014;5:33. https://doi.org/10.3389/fphys.2014.00033.
Stoner L, Lucero AA, Palmer BR, Jones LM, Young JM, Faulkner J. Inflammatory biomarkers for predicting cardiovascular disease. Clin Biochem. 2013;46(15):1353–71. https://doi.org/10.1016/j.clinbiochem.2013.05.070.
Tatler AL, Barnes J, Habgood A, Goodwin A, McAnulty RJ, Jenkins G. Caffeine inhibits TGFβ activation in epithelial cells, interrupts fibroblast responses to TGFβ, and reduces established fibrosis in ex vivo precision-cut lung slices. Thorax. 2017;71(6):565–7. https://doi.org/10.1136/thoraxjnl-2015-208215.
Tauler P, Martínez S, Moreno C, Monjo M, Martínez P, Aguiló A. Effects of caffeine on the inflammatory response induced by a 15-km run competition. Med Sci Sports Exerc. 2013;45(7):1269–76. https://doi.org/10.1249/MSS.0b013e3182857c8a.
Tauler P, Martinez S, Martinez P, Lozano L, Moreno C, Aguiló A. Effects of caffeine supplementation on plasma and blood mononuclear cell interleukin-10 levels after exercise. Int J Sport Nutr Exerc Metab. 2016;26(1):8–16. https://doi.org/10.1123/ijsnem.2015-0052.
Walsh NP, Gleeson M, Shephard RJ, Gleeson M, Woods JA, Bishop NC, Fleshner M, Green C, Pedersen BK, Hoffman-Goetz L, Rogers CJ, Northoff H, Abbasi A, Simon P. Position statement. Part one: immune function and exercise. Exerc Immunol Rev. 2011;17:6–63.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Each author made substantial contributions to: the conception and design of the study, or acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; and final approval of this submission.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
Ethical approval was attained via institution review board.
Informed consent
All participants were aware of the experimental protocol and provided written informed consent for participation and publication.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rahimi, M., Beaven, C.M. Caffeine Modifies the Immune and Anti-inflammatory Responses to Short Incremental Cycling Exercise Until Exhaustion in Humans: A Pilot Study. J. of SCI. IN SPORT AND EXERCISE (2023). https://doi.org/10.1007/s42978-023-00226-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42978-023-00226-z