Abstract
Nowadays, the use of algae is prevalent for both industrial and agricultural purposes. The determination of chlorophyll (Chl) content is a commonly used method for estimating the phytoplankton abundance in different water bodies or biomass density of algal cultures. The aim of the present work is to optimise the efficiency of the Chl extraction from the green alga Tetradesmus obliquus using methanol as extracting solvent. The extraction efficiency was estimated by measuring the Chl a concentration of the extracts using fluorescence spectroscopy. To increase the extraction yield, glass fibre filters with algal cells on top were treated with 10% (v/v) formalin prior to the extraction. We found that this pretreatment significantly enhanced the extraction yield of Chl without its chemical decomposition. We also found that the optimal cell concentration for Chl determination ranged from 1.44 × 104 to 3.60 × 105 cells/mL and the extraction efficiency was lower when the cell density of the culture was out of this range. These results highlight the importance of the optimization of the pigment extraction for the studied algal species.
Similar content being viewed by others
Introduction
Algal biomass has been used for various agricultural purposes, such as food source, fodder, soil fertiliser, and biostimulant (Kotrbáček et al. 2015; Piwowar and Harasym 2020; Trentacoste et al. 2015; Trifan and Bularda 2015). Besides, algae have also great potential in future biofuel production (Brennan and Owerde 2010; Khan et al. 2018; Nagy et al. 2018; Selvarajan et al. 2015) as well as in diverse pharmaceutical and cosmetic industry applications (e.g. Gateau et al. 2017; Heydarizadeh et al. 2013; Mimouni et al. 2012). Remarkably, algae can provide pigments, vitamins, fatty acids, and proteins for human use (Michalak and Chojnacka 2015; Priyadarshani and Rath 2012). Green algae, for example, members of the genus Scenedesmus, are also widely studied for potential use in water management (Li and Chi 2021, Mulbry et al. 2005, Sánchez-Zurano et al. 2021, Tam and Wong 1989, Yuan et al. 2023).
For estimating the phytoplankton abundance in different water bodies or for determining the biomass density of algal monocultures, chlorophyll (Chl) concentration is a commonly used indicator. Very often, instead of determining total Chl content (sum of Chl a, Chl b and/or Chl c), only the concentration of Chl a is quantified (Gregor and Maršálek 2004). The rationale behind, that Chl a is the primary photosynthetic pigment in all oxygenic photoautotrophs, whilst the accessory pigments Chl b and c are present only in certain taxonomic groups (see e.g. Wetzel 2001). Hence, Chl a concentration is useful to characterise phytoplankton biomass, especially with unknown taxonomic composition.
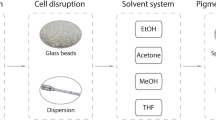
There are various methods to extract Chl (and other extractable pigments, such as carotenoids) from algae and other photoautotrophic organisms. In case of algae, differences in the architecture, composition, and permeability of the cell wall (Pepe et al. 2001) as well as the applied solvent largely determine the efficiency of the applied protocol (Henriques et al. 2007). In order to enhance the yield of pigment extraction, various types of cell disruption methods are commonly used (Jeffrey et al. 1997; Schagerl and Künzl 2007; Wiltshire et al. 2000). The determination of the pigment content of some green algae, such as Scenedesmus armatus (currently known as Desmodesmus armatus), Ankistrodesmus spiralis, or Coelastrum microporum culture, for example, clearly showed the importance of cell disruption and proper selection of the extraction solvent. In these strains, homogenisation provided a twofold higher Chl extraction yield compared to the non-homogenised samples. Amongst the applied methods, the dispersion was more efficient compared to sonication and 90% acetone was proven to be the most effective solvent (Schagerl and Künzl 2007). The combination of sonication and dispersion did not improve the extraction efficiency any further. However, in the case of Scenedesmus quadricauda, Selenastrum capricornutum and the cyanobacterium Microcystis aeruginosa, both methanol and ethanol extraction were proven to be more efficient than acetone alone or in combination with dimethyl sulfoxide (DMSO) (Sartory and Grobbelaar 1984). Amongst the two alcoholic solvents, in the majority of water management laboratories, 90% ethanol is used as the accredited solvent (ISO 10260, 1992). Yet, comparative studies revealed that in case of Nannochloropsis gaditana (= Microchloropsis gaditana)) (Lichtenthaler and Wellburn 1983), Synechococcus elongatus, and Scenedesmus acutus (= Tetradesmus obliquus) (Pápista et al. 2002) methanol provides a significantly more complete extraction compared to ethanol. Nonetheless, Hagerthey and coworkers (Hagerthey et al. 2006) found cold methanol to be a poor solvent for Chl extraction from green algae, cyanobacteria, and diatoms, even after grinding. They obtained the highest extraction yield using freeze-dried samples extracted with the methanol/acetone/N,N-dimethylformamide (DMF) solvent (Hagerthey et al. 2006).
In the study of Schumann and coworkers (Schumann et al. 2005), different preservation methods, storage times and solvents were compared in terms of Chl extraction efficiency. They found that Chl a extraction efficiency is strongly species-specific and also depends on growth conditions. To achieve maximum Chl a extraction yield from algal samples of unknown composition, they suggest implementing 2–3 subsequent 24-h extraction runs using DMF as extraction solvent. Their results also showed that mechanical cell disruption techniques could lead to pigment degradation, in accordance with the results of Henriques and coworkers (Henriques et al. 2007). As long-term storage of frozen or lyophilised samples may also cause pigment loss, some protocols recommend immediate Chl extraction from fresh algae (Jeffrey et al. 1997).
Due to its high oil content and relatively simple cultivation, Tetradesmus obliquus is identified as a promising microorganism with various potential biotechnological applications, including biofuel production (Kim et al. 2019). However, it has only been poorly studied so far. In this study, we systematically investigated i) the effect of formalin pretreatment, ii) the number of extraction steps, and iii) cell density on the Chl extraction efficiency from the green alga T. obliquus. The main objective of these trials was to optimise the yield of chlorophyll extraction from this microorganism. We assumed that both formalin pretreatment and repeated extractions should enhance chlorophyll yield, and also that the cell density (i.e. accessibility of the extraction solvent) should also influence that. Our results emphasise the importance of such characterization for choosing a suitable method for pigment extraction and Chl content determination.
Materials and methods
Alga strain identification and culture conditions
The studied Scenedesmus sp. BEA D01_12 strain was isolated in May 1995 from Lake Balaton (Hungary, 46°45′39.5"N 17°15′29.8"E), the largest lake in Central Europe (Kutasi et al. 2013). The strain was purified and cultured on agar plates.
In order to determine the species level, we performed a DNA-based identification. Total genomic DNA was extracted using a DNeasy Plant Mini kit (Qiagen, Hilden, Germany). PCR amplification and sequencing of the 18S ribosomal RNA (rRNA) gene and the ribosomal ITS region (containing ITS–1, the 5.8S rRNA gene, ITS–2 and a short region from the 28S rRNA gene) were conducted as described in detail by Somogyi et al. (Somogyi et al. 2013), with the exception that the amplification of the ITS region was performed according to Liu et al. (Liu et al. 2014). The obtained sequences were deposited in the GenBank database under the accession codes MW065553 (18S rRNA gene) and MW065554 (ITS). Taxonomic identification was performed using the NCBI BLAST/n tool (Altschul et al. 1990), considering only taxonomically accepted species names in the AlgaeBase (Guiry and Guiry 2020). Comparison of the nucleotide sequences of the rRNA gene and the ribosomal ITS region showed 100% pairwise similarities between BEA D01_12 and various strains of Tetradesmus obliquus (Turpin) M. J. Wynne (formerly also known as Scenedesmus obliquus and Acutodesmus obliquus; (Wynne and Hallan 2016)), including the authentic strain of this species, SAG 276–1. Thus, we identified the studied strain as Tetradesmus obliquus.
Liquid cultures were grown in a modified PHM-1 medium (Table 1) at 24 °C under 16:8 h light–dark cycles. The applied photosynthetic photon flux density (PPFD) was 180 µmol photons m−2 s−1, provided by Tungsram FT8/36W/840/GE/LL/SL1/25 fluorescent tubes. The cultivation flasks were shaken at 150 rpm. The sampling for each experiment was started at 9 am from 7-day-old cultures.
Cell counting
Algal cell counting was performed using a Sceptre™ 2.0 cell counter (Merck) equipped with a 40 µm aperture sensor. Prior to counting, 100 µL algal culture was diluted with 1400 µL of 10 mM phosphate buffer (pH 7.4) and was sonicated in an ultrasound bath (Bandelin Sonorex Super 10P, Berlin, Germany) at 35 kHz for 10 min.
Pigment extraction
From an initial culture with a cell concentration of about 9.0 × 106 cells/mL, we prepared four pairs of a fivefold dilution series, with final cell concentrations of 2.88 × 103, 1.44 × 104, 7.20 × 104, and 3.60 × 105 cells/mL, respectively. Then 10 mL of the samples were filtered through glass fibre filters (GF/C, d = 47 mm, Whatman) using a water jet pump. Notably, no cell disruption was applied; instead, we used intact cells. The filtration was done using a glass filter funnel (11P02). One sample of each pair with different cell concentrations (count/mL) and algal biomass on the fibre filters was soaked in 10% formalin (v/v) for 2 min. After drying to constant mass, all alga-containing filters were immersed in 10 mL of boiling methanol for 5 min. The pigment extracts were cooled to room temperature and decanted into new test tubes, and their fluorescence emission spectra were recorded. Since the fibre filters remained green after a single extraction (Fig. 1), the pigment extraction with boiling methanol was repeated three more times.
Fluorescence emission spectroscopy
The Chl concentration of phytoplankton samples is conventionally determined spectrophotometrically. However, in this work, all but the first extraction steps result in methanolic solutions with considerably low Chl content, which cannot be precisely determined using that approach. Instead, to determine Chl a concentration in these highly diluted pigment extracts, we applied the more sensitive fluorescence spectroscopy method to obtain a good signal–to–noise ratio even at very low concentrations. We recorded fluorescence emission spectra from 580 to 780 nm with 0.5 nm increments using a Jobin–Yvon Horiba FluoroMax-3 spectrofluorometer (Paris, France). The excitation and emission slits were set to 2 nm and 5 nm, respectively. For Chl a, Chl b, and pheophytin excitation, we used 430 nm, 470 nm, and 410 nm excitation wavelengths, respectively. Three consecutive spectra were recorded and averaged in each measurement. The spectra were then corrected for the wavelength-dependent sensitivity change of the fluorometer detector. Five-point linear smoothing and baseline corrections were applied. The spectra were exported and analysed using the SPSERV V3.41 software (Copyright: Csaba Bagyinka, Szeged Biological Research Centre, Szeged, Hungary).
Concentration determination
For the determination of Chl a concentration in methanolic extracts with different pigment concentrations, the intensity of the 673 nm fluorescence emission peak, the fluorescence emission maximum of Chl a in methanol (Materová et al. 2017), was compared to a calibration curve, which was generated using a dilution series of samples with known Chl a concentrations. The concentration of the initial, the most concentrated sample was determined spectrophotometrically using a Perkin Elmer Lambda 25 UV/VIS spectrophotometer (Norwalk, CT, USA) according to Porra et al. (Porra et al. 1989). The Chl a contents obtained after the four repeated extractions were summed in order to determine the total Chl a concentrations.
Statistical analysis
The Chl a concentrations obtained either via the first extraction or via four consecutive extractions (see Section “Concentration determination”), were compared between pretreated and untreated samples at four different levels of sequential fivefold dilutions (cell concentrations). For optimal scaling, the power-law relationship between Chl a concentrations and cell concentrations were first transformed into a linear by logarithmic transformation of the variables. Natural and base 5 logarithm of the Chl a concentrations and cell concentrations were taken, respectively. Linear mixed models were fitted to these log-transformed values with Chl a concentration (obtained either by a single or four repeated extractions) as a response variable, and cell concentration and treatment as well as their interaction as fixed effects. By including the interaction term, we investigated whether the predicted effect of formalin pretreatment is influenced by the cell concentration of the sample. To account for correlation between values obtained using samples originating from the same (batch) culture, the culture number was added as a random effect, i.e. adding a random term to the intercept.
An appropriate variance structure (weighted variance according to the magnitude of cell concentration) was applied. Goodness-of-fit and homoscedasticity were checked with diagnostic plots. Model selection was performed by removing the interaction term if it was not significant. For interpretation, values were transformed back to the original scale. Transformed model coefficients inform about the fold-change in the outcome variable in association with the studied parameter. The model was thus used to estimate the fold change in the outcome variable (Chl a concentration obtained either by a single or four repeated extractions) in association with treatment (control vs. formalin) across different cell concentrations. The results are given as point and interval estimates [confidence interval (CI)]. The level of significance was set at p < 0.05. All statistical analysis and visualisation were performed in the R statistical environment (version 3.6.3.) using base, ggplot2, patchwork, Rcmdrmisc, nlme, and emmeans packages (R Core Team, 2019).
Results
Investigation of formalin treatment
The effect of formalin pretreatment was tested using fluorescence spectroscopy to see if it causes any Chl degradation. Fluorescence emission spectra upon preferential excitation of Chl a, pheophytin, and Chl b at 430, 410 and 470 nm, respectively, were recorded using control and formalin-treated samples. The corresponding spectra were essentially the same in both cases; however, formalin treatment resulted in higher fluorescence intensity (Fig. 2). The spectra recorded upon 430 nm (Chl a) excitation showed a maximum at 673 nm and a broad shoulder at around 730 nm in accordance with literature data (Fig. 2, spectra 1 and 2; Balny et al. 1969). The spectra recorded upon 410 nm (pheophytin) excitation were similar to those recorded upon 430 nm excitation (Fig. 2, spectra 3 and 4), suggesting the absence or very low amount of pheophytin in the samples. At 470 nm (Chl b) excitation, in agreement with the shorter wavelength of the corresponding Qy band, the emission maximum also appeared at a shorter wavelength (at 668 nm) with a broad shoulder around 720 nm. Here, the fluorescence intensities were much weaker (Fig. 2, spectra 5 and 6), in agreement with the low Chl b level in the samples and the low fluorescence yield of Chl b in methanol (Forster and Livingston 1952).
The high similarity in pigment composition of the control and formalin-treated samples was well supported by comparing the 4th derivatives (Fig. 3) of the corresponding fluorescence emission spectra obtained either upon 410 or 430 nm excitation (spectra 1, 2 3, and 4 in Fig. 2a). In these derivative spectra (Fig. 3), not only the shapes and positions of the central derivation bands but also those of the four derivation sidebands were almost identical in the control and formalin-treated samples (Fig. 3), which, in turn, indicates very high similarity between the pigment contents of these two types of samples.
4th derivatives of the fluorescence emission spectra measured upon 430 and 410 nm excitation. Solid lines: spectra of non-treated (control) samples, dashed lines formalin treated samples. For clarity, the spectra of the treated samples are shifted from those of control ones with 0.2 units upwards, and the spectra measured with 410 nm are shifted with 6.0 units upwards along the y-axis
Chl a concentrations obtained by the first extraction
Although the pretreatment of the fibre filters with formalin did not result in any detectable Chl a degradation (see above), it remarkably enhanced the yield of its extraction, regardless of the applied cell concentration over a broad range, i.e. from 2.88 × 103 to 3.60 × 105 cells/mL (Table 2, Fig. 4A). The concentration of extracted Chl a increased proportionally in both cases with increasing cell densities. As expected, one unit increase in the logarithmic (log5) cell concentration entailed a mean fivefold increase in Chl a concentrations (95% CI: 3.9–6.0; p < 0.0001). Extracts of formalin-pretreated samples contained significantly, about 2.2-fold higher Chl a concentrations at all applied culture densities, compared to the control extracted only with hot methanol (95% CI: 1.35–3.60, p = 0.0007) (Table 2, Fig. 4A). Model fitting showed the interaction between treatment and cell concentration not to be significant (p = 0.8053), which means the effect of formalin pretreatment did not depend on the cell density of the applied culture.
Chl a concentrations obtained by repeated extractions
Repeated extractions with hot methanol increased the yield of Chl a extraction from both the control and formalin pretreated samples, yet, with different efficiency (compare Figs. 3A and B, see also Table 2). Four consecutive methanolic extractions without formalin pretreatment resulted in a 70–100% increase in the level of extracted Chl a, as compared to a single extraction. However, repeated extractions of formalin pretreated samples resulted in a much smaller, only around 10–40% relative increase in the overall extraction yield, suggesting a more complete pigment extraction after a single extraction, which cannot be considerably improved with consecutive extractions (Table 2, Fig. 3B). This was also supported by statistical analysis which revealed a non-significant effect of formalin pretreatment on Chl a yield (p = 0.095).
Discussion
In research articles, dealing with pigment extraction from algal cultures, the efficiency of the extraction is usually not reported, which limits the reliability of the results. In the present study, we have investigated the efficiency of Chl extractions by considering the impact of three factors: (1) repeated vs. single methanolic extractions of Chl from T. obliquus cells collected on fibre filter, (2) pretreatment of the cells collected on fibre filter with formalin prior to extraction, and (3) dependence of the extraction yield on the cell density of the cultures.
Using a spectrofluorometric approach, we were able to quantitatively determine even very low Chl concentrations of T. obliquus extracts. Our results clearly showed that methanol is an efficient solvent for extracting Chl a from T. obliquus. Conversely, methanol is less suitable for the determination of Chl b concentration, since the fluorescence yield of this pigment is much lower due to the formation of an H-bond between the methanol and the formyl group on pyrrole ring B which distorts the electron cloud of Chl b (Forster and Livingston 1952).
Nonetheless, the determination of Chl b concentration was not relevant in this study. Essentially, for determining algal biomass in aquatic ecosystems or algae (mono) cultures, the determination of the Chl a concentration as an indicator is of much higher importance than that of Chl b.
In this study, prior to methanolic extraction, a formalin pretreatment proved to significantly increase the yield of Chl a release. Such an approach, i.e. formalin pretreatment is a novel approach to enhancing Chl extraction yield from algal cultures. Mechanical cell disruption methods are more commonly applied (Safi et al. 2015). However, in the case of T. obliquus, we could not further improve the Chl extraction yields with mechanical cell disruption (data not shown). Formaldehyde penetrates membranes rapidly and cross-links proteins, including Chl-containing photosystems, which protein structure destabilisation (Thavarajah et al. 2012) may explain the observed increase in the extraction yield of Chl a. However, importantly, formalin pretreatment did not cause any measurable damage of Chl molecules (Fig. 2). Moreover, although the algal cells on the surface of fibre filters distributed unevenly (Fig. 1), this did not cause any major experimental error (i.e. after formalin pretreatment and methanolic extraction the filters were evenly white (Fig. 1)).
In conclusion, our results highlighted that formalin pretreatment largely facilitates the extraction of Chl a from T. obliquus. Using this approach, even a single, 5-min extraction time was sufficient, in contrast to the commonly applied 24-h duration (Liu et al. 2014). In the case of T. obliquus, the optimal cell density for methanolic extraction ranged from 1.44 × 104 to 3.60 × 105 cells/mL. However, these numbers may be different in other algal species due to strain-specific (e.g. structural) reasons. Hence, our results also show that pigment extraction must be optimised for the studied algae species.
Conclusions for future biology
Since many valuable bio-products including pigments, vitamins, fatty acids, and proteins can be produced using microalgae, the economic implementation of their mass production has a great importance. The accurate determination of biomass density, e.g. by measuring chlorophyll concentration, is essential in industrial scale algal biotechnology, where even a small inaccuracy can have significant economic consequences.
The presented method allows rapid and efficient chlorophyll extraction from a biotechnologically relevant green algae. Our work revealed that formalin pretreatment improves chlorophyll extraction from microalgae. Without such pretreatment, a significant amount of chlorophyll may remain in the cells after the first extraction. We suggest the formalin pretreatment for laboratory tests, safer substances with similar effects are to be studied in the future. The developed method can be used both in algal biotechnology and in research.
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Balny C, Brody SS, Hui Bon Hoa G (1969) Absorption and fluorescence spectra of chlorophyll-a in polar solvents as a function of temperature. Photochem Photobiol 9:445–454
Borowitzka A, Borowitzka LJ (1988) Micro-algal biotechnology. Cambridge university press, Cambridge
Brennan L, Owende P (2010) Biofuels from microalgae—a review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sustain Energy Rev 14:557–577
Forster LS, Livingston R (1952) The Absolute quantum yields of the fluorescence of chlorophyll solutions. J Chem Phys 20:1315–1320. https://doi.org/10.1063/1.1700727
Gateau H, Solymosi K, Marchand J, Schoefs B (2017) Carotenoids of microalgae used in food industry and medicine. Mini-Rev Med Chem 17:1140–1172
Gregor J, Maršálek B (2004) Freshwater phytoplankton quantification by chlorophyll a: a comparative study of in vitro, in vivo and in situ methods. Water Res 38(3):517–522. https://doi.org/10.1016/j.watres.2003.10.033
Guiry MD, Guiry GM (2020) AlgaeBase. World-wide electronic publication, National university of Ireland, Galway
Hagerthey SE, Louda JW, Mongkronsri P (2006) Evaluation of pigment extraction methods and recommended protocol for periphyton chlorophyll a determination and chemotaxonomic assesment. J Phycol 42:1125–1136. https://doi.org/10.1111/j.1529-8817.2006.00257.x
Henriques M, Silva A, Rocha J (2007) Extraction and quantification of pigments from a marine microalga : a simple and reproducible method. Commun Curr Res Educ Topics Trends Appl Microbiol 2:586–593
Heydarizadeh P, Poirier I, Loizeau D, Ulmann L, Mimouni V, Schoefs B, Bertrand M (2013) Plastids of marine phytoplankton produce bioactive pigments and lipids. Mar Drugs 11(9):3425–3471. https://doi.org/10.3390/md11093425
ISO 10260, 1992.Water quality, measurement of biochem. parameters; spectrometric determination of the chlorophyll-a concentration. Beuth verlag GmbH Berlin Vien—Zürich
Jeffrey SW, Mantoura RFC, Wright SW (1997) Phytoplankton pigments in oceanography: Guidelines to modern methods. UNESCO, Paris
Khan MI, Shin JH, Kim JD (2018) The promising future of microalgae: current status, challenges, and optimisation of a sustainable and renewable industry for biofuels, feed, and other products. Microb Cell Fact 17(1):1–21. https://doi.org/10.1186/s12934-018-0879-x
Kim YS, Yi J, Do JM, Chang J, Yoon HS (2019) Characterisation of fatty acid components from tetradesmus obliquus knua019 (chlorophyta, scenedesmaceae) for a resource of biofuel production. Revista Brasileira De Botanica 42(3):431–439. https://doi.org/10.1007/s40415-019-00556-9
Kotrbáček V, Doubek J, Doucha J (2015) The chlorococcalean alga chlorella in animal nutrition: a review. J Appl Phycol 27:2173–2180. https://doi.org/10.1007/s10811-014-0516-y
Kutasi J, Bencsik A, Gráf I (2013) Algae leaf-dung. Espace net patent search, https://worldwide.espacenet.com/patent/search?q=HU1300274A2&queryLang=en%3Ade%3Afr Accessed: 23 February 2021
Li L, Chi K (2021) A comparative study of scenedesmus dimorphus cultured with synthetic and actual wastewater. Water 13(21):3060. https://doi.org/10.3390/w13213060
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 11(5):591–592
Liu J, Gerken H, Li Y (2014) Single-tube colony PCR for DNA amplification and transformant screening of oleaginous microalgae. J Appl Phycol 26:1719–1726. https://doi.org/10.1007/s10811-013-0220-3
Materová Z, Sobotka R, Zdvihalová B, Oravec M, Nezval J, Karlický V, Vrábl D, Štroch M, Špunda V (2017) Monochromatic green light induces an aberrant accumulation of geranylgeranyled chlorophylls in plants. Plant Physiol Biochem 116:48–56. https://doi.org/10.1016/j.plaphy.2017.05.002
Michalak I, Chojnacka K (2015) Algae as production systems of bioactive compounds Eng. Life Sci 15:160–176. https://doi.org/10.1002/elsc.201400191
Mimouni V, Ulmann L, Pasquet V, Mathieu M, Picot L, Bougaran G, Cadoret J-P, Morant-Manceau A, Schoefs B (2012) The potential of microalgae for the production of bioactive molecules of pharmaceutical interest. Curr Pharm Biotechnol 13(15):2733–2750
Mulbry WW, Westhead EK, Pizarro C, Sikora L (2005) Recycling of manure nutrients: use of algal biomass from dairy manure treatment as a slow release fertiliser. Biores Technol 96:451–458
Nagy V, Podmaniczki A, Vidal-Meireles A, Tengölics R, Kovács L, Rákhely G, Scoma A, SzZ T (2018) Water-splitting-based, sustainable and efficient h2 production in green algae as achieved by substrate limitation of the calvin-benson-bassham cycle. Biotechnol Biofuels 11(1):1–16. https://doi.org/10.1186/s13068-018-1069-0
Pápista É, Ács É, Böddi B (2002) Chlorophyll-a Determination with ethanol—a critical test. Hydrobiologia 485:191–198
Pepe M, Giardino C, Borsanib G, Cardoso AC, Chiaudani G, Premazzic G, Rodaric E, Ziliolia E (2001) Relationship between apparent optical properties and photosynthetic pigments in the sub-alpine lake Iseo. Sci Total Environ 268(1–3):31–45
Piwowar A, Harasym J (2020) The Importance and prospects of the use of algae in agribusiness. Sustainability 12(14):5669. https://doi.org/10.3390/su12145669
Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. BBA—Bioenergetics 975(3):384–394
Priyadarshani I, Rath B (2012) Commercial and industrial applications of micro algae—a review. J Algal Biomass Utln 3(4):89–100
Safi C, Frances C, Ursu AV, Laroche C, Pouzet C, Vaca-Garcia C, Pontalier P-Y (2015) Understanding the effect of cell disruption methods on the diffusion of chlorella vulgaris proteins and pigments in the aqueous phase. Algal Res 8:61–68. https://doi.org/10.1016/j.algal.2015.01.002
Sánchez-Zurano A, Lafarga T, Morales-Amaral M, Gómez-Serrano C, Fernandez-Sevilla JM, Acién-Fernández F, Molina-Grima E (2021) Wastewater treatment using Scenedesmus almeriensis: effect of operational conditions on the composition of the microalgae-bacteria consortia. J Appl Phycol 33(6):3885–3897. https://doi.org/10.1007/s10811-021-02600-2
Sartory DP, Grobbelaar JU (1984) Extraction of chlorophyll a from freshwater phytoplankton for spectrophotometric analysis. Hydrobiologia 114(3):177–187
Schagerl M, Künzl G (2007) Chlorophyll a extraction from freshwater algae—a reevaluation. Biologia 62(3):270–275. https://doi.org/10.2478/s11756-007-0048-x
Schumann R, Häubner N, Klausch S, Karsten U (2005) Chlorophyll extraction methods for the quantification of green microalgae colonising building facades. Int Biodeteriorat Biodegrad 55:213–222. https://doi.org/10.1016/j.ibiod.2004.12.002
Selvarajan R, Felföldi T, Tauber T, Sanniyasi E, Sibanda T, Tekere M (2015) Screening and evaluation of some green algal strains (Chlorophyceae) isolated from freshwater and soda lakes for biofuel production. Energies 8:7502–7521. https://doi.org/10.3390/en8077502
Somogyi B, Felföld T, Solymosi K, Flieger K, Márialigeti K, Böddi B, Vörös L (2013) One step closer to eliminating nomenclatural problems of minute coccoid green algae: Pseudochloris wilhelmii gen. et sp. nov. (Trebouxiophyceae, Chlorophyta). Eur J Phycol 48:427–436. https://doi.org/10.1080/09670262.2013.854411
Tam NFY, Wong YF (1989) Wastewater nutrient removal by chlorella pyrenoidosa and scenedesmus sp. Environ Pollut 58(1):19–34. https://doi.org/10.1016/0269-7491(89)90234-0
Thavarajah R, Mudimbaimannar VK, Elizabeth J, Rao UK, Ranganathan K (2012) Chemical and physical basics of routine formaldehyde fixation. J Oral Maxillofac Pathol 16(3):400–405. https://doi.org/10.4103/0973-029X.102496
Trentacoste EM, Martinez AM, Zenk T (2015) The place of algae in agriculture: policies for algal biomass production. Photosynth Res 123:305–315. https://doi.org/10.1007/s11120-014-9985-8
Trifan D, Bularda M (2015) Studies regarding efficiency of biological fertilisation with algafix on winter rape and spring barley. Sci Pap Ser Agron 58:340–343
Wetzel RG (2001) Limnology. Academic press, San Diego, USA
Wiltshire KH, Boersma M, Möller A, Buhtz H (2000) Extraction of pigments and fatty acids from the green alga Scenedesmus obliquus (Chlorophyceae). Aquat Ecol 34:119–126
Wynne MJ, Hallan JK (2016) Reinstatement of Tetradesmus G. M. Smith (Sphaeropleales, Chlorophyta). Feddes Repert 126:83–86
Yuan D, Wang L, Wang H, Miao R, Wang Y, Jin H, Tan L, Wei C, Hu Q, Gong Y (2023) Application of microalgae Scenedesmus acuminatus enhances water quality in rice-crayfish culture. Front Bioeng. Biotechnol Sec Ind Biotechnol. 11:1143622
Acknowledgements
The authors are grateful to Károly Bóka (ELTE Eötvös Loránd University, Department of Plant Anatomy) for his kind help in several experiments and valuable advice, to Hajnalka Nagy (ELTE Eötvös Loránd University, Department of Microbiology) and Péter Balázs (Albitech Ltd.) for their technical assistance.
Funding
Open access funding provided by Eötvös Loránd University. The research was supported by the grants No. 2018–1.1.1-MKI-2018–00034 (to Albitech Ltd.), K 140351 and RRF-2.3.1–21-2022–00014 (to G.B.) by the National Research, Development and Innovation Office, Budapest, Hungary, by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences (Grant No. BO/00837/20/8, to T.F.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Greipel, E., Kósa, A., Böddi, B. et al. Extraction of chlorophyll a from Tetradesmus obliquus—a method upgrade. BIOLOGIA FUTURA (2024). https://doi.org/10.1007/s42977-024-00209-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42977-024-00209-3