Abstract
Despite broad interest and recent experimentation, there is no single ecological model accounting for the adaptive significance of the diversity of avian eggshell colouration. The often blue-green eggs of Turdus thrushes are a charismatic example of this, having long captured cultural and scientific attention. Although the biology and evolutionary history of “true” thrushes is well understood, little is known about correlated evolution between shifts in habitat and eggshell pigmentation, and how these shifts map with Turdus biogeography. We applied phylogenetic comparative methods to assess the evolutionary timing of divergence and variation of life history traits and eggshell colouration and maculation presence in the genus. We found that eggshell colour diversified independently on several occasions in the past 11 million years, with much of the variation occurring within the last 4 million years. The majority of Turdus species lay blue-green eggs and also tend to be sedentary and forest-dwelling. Diet generalist species and species which have transitioned to a forest habitat are more likely to lay white eggs (10% of studied species). In turn, lineages in any habitat were more likely to transition to blue-green eggs. We found that variation in egg colour is increased in some clades, of which two lineages radiated in South America and the East Palearctic, in the past 2–4 million years. These findings provide support for the hypothesis that white eggs are more conspicuous to predators in open environments and that multiple, non-mutually exclusive constraints operate on the adaptive function of avian eggshell colour.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The appearance of bird eggs is highly variable, and most species exhibit some degree of eggshell colouration in their clutches. Avian egg colouration is the product of variable levels and relative concentrations of two main eggshell pigments: biliverdin (responsible for green–blue hues) and protoporphyrin IX (responsible for brown–red hues, e.g. Cassey et al. 2012). These pigments allow shells to appear on a spectrum of blue-green to rusty-brown, or as beige-white when unpigmented (Hanley et al. 2015). The eggs of at least some non-avian dinosaurs within the lineage ancestral to birds, the eumaniraptorans, already contained these pigments and expressed these same coloured phenotypes (Wiemann et al. 2018, but see also Kilner 2006, Shawkey and D’Alba 2019). Avian egg colouration expanded to its present diversity as a function of the interplay between anti-predator constraints, thermoregulation, and intra- or interspecific signalling functions (Moreno and Osorno 2003; Cassey et al. 2012; Lahti and Ardia 2016). For example, parasitic eggshell mimicry is a significant and dramatic driver of egg colouration among obligate avian brood parasites (Aidala and Hauber 2010), and both the antipredator crypsis (Westmoreland 2008) and intraspecific signalling (e.g. Moreno et al. 2005; 2006; but see Riehl 2011) functions for egg pigment have been supported (Cassey et al. 2008). Egg phenotype, including colouration, shows correlated evolution with nest structure (Nagy et al. 2019b). These two elements of the (female) parent birds’ extended phenotypes are thus likely to be also functionally interrelated.
Where to build a nest is one of the most important decisions a bird faces, with food availability and risk of predation being fitness-critical factors in nest-site selection (Martin 1987; Fontaine and Martin 2006; Bellamy et al. 2018). Avian eggs are typically deposited in nests which have been, in turn, constructed in diverse microhabitats. Thus, the structure and visibility of the nest and the eggs within are dependent on that environmental milieu (Hansell 2000). It follows that as egg traits should (and do) exhibit correlated evolution with nest traits (e.g. Cassey et al. 2012; Nagy et al. 2019b) and nest traits in turn are dependent on habitat type, the evolutionary history of habitat use, and egg traits will show interplay. Yet correlated evolution between egg colouration and nesting habitat use remains poorly explored.
The true thrushes (genus Turdus) are a moderately speciose clade presently native to five continents and introduced to a sixth, with constituent species occurring in a wide range of habitat types, including Neotropical jungle, Nearctic and Palearctic woodlands and grasslands, African savannahs, and East Palearctic forests (Nagy et al. 2019a). A parsimonious revision of the clade supporting a dispersal-extinction-cladogenesis plus founder event model has been provided by Nagy et al. (2019a). Their results suggest that the clade originated in East Asia (but see Batista et al. 2020), with two early paths of dispersal, the ancestral species probably being a within-zone or East to West (along-latitudinally) migratory insectivorous species.
However, thrush species have adapted to a diverse range of diets, with some generalists and some specialists within the genus. Dietary generalism is associated with other dimensions of plasticity in avian species, such as technical innovation (Ducaez et al. 2015) and adaptation to anthropogenic environments (Callaghan et al. 2019). Burin et al. (2016) found that omnivorous avian lineages speciate less often and become extinct at higher rates than specialists. In turn, a greater diversity of avian dietary specialists is found in forested areas (Benedetti et al. 2022). Whereas within a passerine species maculation has been shown to inversely correlate with body condition (Badás et al. 2017), the evidence linking dietary breadth and eggshell colouration is ambiguous (e.g. O’Hanlon et al. 2020).
Furthermore, both the plumage diversity (e.g. the repeatedly evolved sexual dichromatism: Luro and Hauber 2022) and the conspicuous egg colours (particularly those on the blue-green spectrum) and maculation of Turdus eggshells have been a consistent subject of interest for researchers (Cassey et al. 2008; Lahti and Ardia 2016). Coexistence attributes such as ecological differences in breeding aspects and nest site selection have been also investigated in some Turdus species (e.g. Götmark et al 1995; Lomáscolo et al. 2010; Mikula et al. 2014). However, correlated evolutionary patterns between egg colouration and habitat use in these phylogenetically and ecologically similar species remain poorly understood.
Here, we aimed to examine (1) the biogeographic history of Turdus in the context of a potential interplay between habitat use and egg colouration and (2) the relationship between dietary breadth and eggshell colouration by applying phylogenetically controlled comparative methods.
Materials and methods
Data collection
We collected egg size (length and width in millimetres) and colouration data for 72 Turdus species from the Birds of the World series (Cornell Lab of Ornithology, Ithaca, USA) as our main source. A rudimentary egg shape (or the inverse of elongation) metric was then calculated by dividing egg width by egg length (sensu Escalona et al. 2018). The colour of the eggs was determined by human-visual investigation of available egg photos and descriptions; this is appropriate since we are not analysing spectral information inclusive of the ultraviolet range in this study: Both biliverdin- and protoporphyrin IX-based eggshell pigments cause dramatically different human-visible coloration of avian eggshells (Cassey et al. 2012). Each species’ egg colouration was determined to fall into one or more of the following categories: white, cream, beige, brown, blue, and green. There is vast variation in thrushes’ eggshell hue, however, and so we further grouped these categories into a binary of white/beige or blue-green coloured eggs for further analyses (there were no exclusively brown eggs in our sample). We also collected data on egg maculation (human-visible: yes/no), however, none of the predictor variables showed any statistical association with this or the egg shape variable (Table 1), and so we excluded them both from further comparisons.
Information on the life history and ecology of species, including average body mass, average clutch size, the duration of the breeding season, diet, and habitat selection, was gathered by revisiting multiple sources described in Nagy et al. (2019a). Duration of avian breeding season if often extended in urban settings (e.g. Møller et al. 2015), and multibrooding species increase breeding season duration in warmer habitats, suggesting greater ecological plasticity as a covariate of breeding season length (Halupka and Halupka 2017). Thus, it is plausible that, as discussed above with dietary breadth, these species do not allocate as much pigment to each clutch. Where it could be clearly identified in the literature, we recorded seven categories of diet (invertebrates, amphibians, reptiles, birds, mammals, fruits, seeds). Diet breadth was the total number of consumed categories by a species. We used this variable as a measure of diet specialisation, as we considered a species being more specialist if it forages on fewer food categories compared to other species (Nagy et al. 2019a). The habitat of each species was recorded as one of the four categories (open, open-woody, forest-open, forest) based on the details available in the literature (e.g. del Hoyo et al. 2016; Rodewald 2016, for more detail see Nagy et al. 2019a). The description of most species clearly stated that a species more likely occupy ‘open’ or ‘forest’ habitats. However, in the case of some species, the descriptions suggested distinguishing between habitat types on a finer scale (Nagy et al. 2019a). If a species’ habitat description included information suggesting the dominance of open habitats over forested ones, we categorized it as open-woody: 'Lighter open woodland, agricultural areas with scattered trees' (e.g. T. falcklandii), 'open woodland, thickets, savannah woodland' (T. pelios), 'mosaic of wooded and open country, open grassland with scrub' (T. viscivorus). Similarly, we categorized species’ habitats as forest-open if they included information on forest preference over open habitats, such as the following: 'Forest-open country mosaic, clearings in primary forest, regenerating managed forest' (T. iliacus), 'woodland, edges, scrub, and adjacent clearings' (T. reevei), “broadleaf deciduous and mixed evergreen forest, also forest edge, light deciduous forest, secondary growth” (T. cardis).
Statistical analyses
We applied a phylogenetic generalized linear mixed model (PGLMM) approach for binomial data available in the ‘phyr’ package (Ives et al. 2020) to investigate the effect of life history predictors on egg colouration (white vs. coloured) in Turdus thrushes. All predictors were included in a full model, from which least significant predictors (α > 0.05) were dropped by stepwise selection until the minimal model was obtained. All predictors were centred and scaled before entering them in the models.
We were also interested in estimating correlated evolutionary patterns between egg colouration and habitat use in thrushes. Therefore, we used the Discrete module of BayesTraits v3.0 (Pagel et al. 2004; Pagel and Meade 2006) to test if transitions occurred dependently or independently during the evolution of egg colouration and habitat selection, i.e. changes in egg colour caused changes in habitat use (or vice versa). We provided 1,000 randomly selected phylogenetic trees for the maximum-likelihood estimations and performed likelihood-ratio tests to evaluate significant differences between the dependent and independent models.
To investigate the correlated changes in habitat use, and egg colouration connected to the biogeographic history of thrushes, we mapped the changes in both traits on the phylogeny of the species by applying stochastic character map simulations 1,000 times using an ‘all-rates-different’ model and the default settings of the function. This method is available in the ‘phytools’ package (Revell 2012).
In case of BayesTraits and stochastic character mapping, we grouped ‘open’ and ‘open-woody’ species together as ‘open’ and the rest as ‘forest’. The phylogenetic trees used in the analyses were originally created by Nagy et al. (2019a) and Nagy (2019). Data preparation, PGLMM, stochastic character mapping, and the processing of the results were performed in R v4.0.3 (R Core Team 2020).
Results
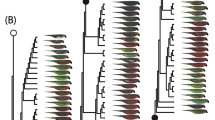
The vast majority of the studied Turdus species lay colourful eggs (n = 60) and only ~ 10% have white eggs (Fig. 1). We excluded 6 species from PGLMMs due to the lack of egg colour information; however, they were used in the correlated evolutionary analysis that can handle missing data. After removing the non-significant predictors from the full model (Table 2), we found that diet generalist species are more likely to lay white eggs, similarly to species living in ‘open-woody’ habitats. Larger species, however, are less likely to do so.
We found that the dependent and independent evolutionary models, investigating the evolutionary transitions in egg colour and habitat use on all trees, are statistically similar based on the likelihood-ratio tests. Despite the non-significant differences, dependent models had higher log-likelihood values, indicating better fit; therefore, we only show the results for them (Fig. 2). Evolutionary transitions to forest habitats occurred with a higher rate in lineages laying white eggs compared to lineages laying colourful eggs. However, lineages laying colourful eggs were less likely to have switched to open habitats during evolution. When species of either colourful- or white-egg lineages transitioned to open or forest habitat, eggs tended to remain or become colourful.
The stochastic character mapping of habitat changes and the diversification of egg colouration allowed us to identify and assign a time scale to the most important diversification events during the genus’ evolution (Fig. 3). By comparing the branches to the results of previous ancestral biogeographic estimations, we found that the geographic and climatic events during the last 2–4 million years could have induced these changes in at least two different lineages in the Eastern Palearctic and in the Neotropical regions.
Discussion
Most Turdus species are mainly sedentary, tropical, and blue-green egg-laying. Having evolved in the East Palearctic (Nagy et al. 2019a; Nagy 2020), true thrushes likely spread to the West Palearctic and Africa (Nagy et al. 2019a). Furthermore, Nagy et al. (2019a) proposed that along-latitude migratory behaviour of early thrush species may have led to the re-centring of habitat ranges for some populations, and consequently been a driving force in the speciation and geographic distribution of thrush species. One possible consequence here could be the evolution of species native to the West Palearctic and Africa. Alternatively, Batista et al. (2020) recovered monophyletic African and South American clades from a lineage dispersing to the Antilles in the early Pliocene, with a Central American radiation separately colonized North America in this phylogeny.
Despite the available information on the biology and evolutionary history of the Turdus species (see references above), the existence of correlated changes in habitat uses and eggshell colouration, as well as its connection to their biogeography, have remained poorly understood. Here, we report that the diversification of Turdus egg colour appears to have happened multiple times in independent lineages. Most species laying eggs with colourful shells are sedentary, forest-dwelling, and invertebrate-and-fruit eaters. Our findings that white egg-laying lineages are more likely to also feature transitions to forest habitat (Fig. 2) and to live in open-woody habitat are consistent with the hypothesis that white eggs are visually conspicuous to predators in the absence of dense vegetation (Nagy et al. 2019b). In birds, life history traits are known to covary with nest predation risk, which may differ substantially among nest sites (Martin 1995). In particular, predation is an important selective force in the evolution of eggshell colouration, but selective pressures on colour evolution may vary between nest sites within open-nesting species (Weidinger 2001). The white egg layers are also large-bodied diet generalists (Table 1), with the notable exception of T. ignobilis and T. unicolor, which lay exclusively white eggs and are not phylogenetically close (Fig. 3) yet are open-habitat dwelling species.
Colourful eggs appear in the basal lineages Psophocichla litsitsirupa and T. mupinensis, as well as the early-diverging West Palearctic T. philomelos. and T. viscivorus, recovered here as the sister taxon to T. philomelos, lays blue-green eggs—the earliest evidence of egg colour diversification in the genus. The divergence between T. philomelos and T. viscivorus appears to have occurred about 10 million years ago (Fig. 3b) and would likely have occurred in the West Palearctic. Although Turdus species are typically described as forest-dwelling, some species may have a generalist character and occupy open or mosaic habitats. While T. philomelos generally occupies forest habitat, T. viscivorus more recently, colonises open-woody habitats, mainly mosaic of wooded and open country, or open grassland with scrub (Fig. 3a).
The common ancestor of the remaining 68 species discussed here appears to have an Afrotropical/Neotropical origin (Nagy et al. 2019a). Thrushes are robust over-water dispersers (Batista et al. 2020) and a likely route between these two tropical zones would be by way of the Antilles (Nagy et al. 2019a; Batista et al. 2020). The presence of the blue egg-laying T. lherminieri in tropical forest habitat in the Lesser Antilles, and its status as the sister taxon to the remaining 67 species, is consistent with this biogeographic history. Multiple trans-Atlantic migrations followed this migration event (Nagy et al. 2019a).
The phylogenetic and biogeographic distribution of eggshell colour characterized in this study suggests that egg colour diversity is likely a response to a multiple interacting constraints, rather than being predicted by a single or few consistent ecological factors (consistent with: Kilner 2006; Cassey et al. 2008, 2012). However, diversity appears to be somewhat clustered phylogenetically, with some clades more disposed to diversify than others. The presence of white eggs in lineages transitioning to forest habitat indirectly supports a crypsis explanation for the relatively colourful eggs of species nesting in more open environments (English and Montgomerie 2011, but see Lahti and Ardia 2016). A non-mutually exclusive alternative is that blue and green eggshells are acting as thermoregulatory and photoprotective 'parasols” (Hanley et al. 2015; Lahti and Ardia 2016; Wysocki 2015). Typically, Turdus nests are open-cup structures with relatively high exposure to solar radiation (e.g. Wysocki et al. 2015; Ganai et al. 2018; Tomiałojć and Neubauer 2018; Jara et al. 2019; Yang et al. 2019; Yi et al. 2020; Batisteli et al. 2021; Turner and Hauber 2021), and heating or cooling beyond thermal limits, as well as prenatal exposure to UV-B radiation, might have detrimental effects on embryonic development (Maurer et al. 2011). Thus, open-nest forest-dwelling bird eggs exhibit a trend to matching the ambient light colour, which can vary from blue to green depending on the density of surrounding vegetation and the height of the nest (Lahti and Ardia 2016).
As in other studies assessing specific ecological traits in relation to the diversity of avian egg colour, our results suggest that multiple, non-mutually exclusive constraints operate on this trait (e.g. Kilner 2006; Cassey et al. 2008, 2012; Cherry and Gosler 2010; Lahti and Ardia 2016).
Conclusions for future biology
We find evidence for phylogenetic grouping of more colourful eggshells in the Turdus thrushes. At least two of these groups radiated in the last 2–4 million years in South America (the Nesocichla eremita, T. ignobilis, T. amaurochalinus clade) and in a lineage that returned to the East Palearctic (T. torquatus, T. naumanni, T. ruficollis, T. pilaris clade). Given the stochastic distribution of other-than-blue egg colour in this phylogeny, we encourage further work to focus on comparing specific ecological (in particular nesting biology) habits of these taxa; for example, the typical ambient lighting of these nests and the umwelt of specific predators likely to constrain eggshell-coloration based crypsis in these habitats. Finally, future studies on the functional basis of eggshell colouration, and the associated evolutionary and behavioural traits, will benefit from incorporating advances in avian visual modelling, which may yield different conclusions than data using human visual assessment (Stevens 2011).
Data availability
No new data have been generated during the preparation of this article. All sources have been listed in the text.
References
Aidala Z, Hauber ME (2010) Avian egg coloration and visual ecology. Nat Edu Knowl 3:53
Badás EP, Martínez J, Rivero-de Aguilar J, Stevens M, Van Der Velde M, Komdeur J, Merino S (2017) Eggshell pigmentation in the blue tit: male quality matters. Behav Ecol Sociobiol 71(57):1–12
Batista R, Olsson U, Andermann T, Aleixo A, Ribas CC, Antonelli A (2020) Phylogenomics and biogeography of the world’s thrushes (Aves, Turdus): new evidence for a more parsimonious evolutionary history. Proc R Soc B 287:20192400. https://doi.org/10.1098/rspb.2019.2400
Batisteli AF, Sarmento H, Aurélio Pizo M (2021) Nest reuse by Pale-breasted Thrushes reduces the chance of cowbird parasitism and allows earlier initiation of breeding. J Field Ornithol 92:105–114. https://doi.org/10.1111/jofo.12363
Bellamy PE, Burgess MD, Mallord JW, Cristinacce A, Orsman CJ, Davis T, Grice PV, Charman EC (2018) Nest predation and the influence of habitat structure on nest predation of Wood Warbler Phylloscopus sibilatrix, a ground-nesting forest passerine. J Ornithol 159:493–506. https://doi.org/10.1007/s10336-017-1527-7
Benedetti Y, Morelli F, Callaghan CT, Fuller R (2022) Distribution and protection of avian specialization in Europe. Glob Ecol Biogeogr 31(1):10–24. https://doi.org/10.1111/geb.13405
Burin G, Kissling WD, Guimarães PR Jr, Şekercioğlu ÇH, Quental TB (2016) Omnivory in birds is a macroevolutionary sink. Nat Commun 7(1):11250. https://doi.org/10.1038/ncomms11250
Callaghan CT, Major RE, Wilshire JH, Martin JM, Kingsford RT, Cornwell WK (2019) Generalists are the most urban-tolerant of birds: a phylogenetically controlled analysis of ecological and life history traits using a novel continuous measure of bird responses to urbanization. Oikos 128(6):845–858. https://doi.org/10.1111/oik.06158
Cassey P, Honza M, Grim T, Hauber ME (2008) The modelling of avian visual perception predicts behavioural rejection responses to foreign egg colours. Biol Lett 4:515–517. https://doi.org/10.1098/rsbl.2008.0279
Cassey P, Thomas GH, Portugal SJ, Maurer G, Hauber ME, Grim T, George Lovell P, Mikšík I (2012) Why are birds’ eggs colourful? Eggshell pigments co-vary with life-history and nesting ecology among British breeding non-passerine birds. Biol J Linn Soc 106:657–672. https://doi.org/10.1111/j.1095-8312.2012.01877.x
Cherry MI, Gosler AG (2010) Avian eggshell coloration: new perspectives on adaptive explanations. Biol J Linn Soc 100:753–762. https://doi.org/10.1111/j.1095-8312.2010.01457.x
del Hoyo J, Elliott A, Sargatal J, Christie DA, de Juana E (2016) Handbook of the Birds of the World Alive. Lynx, Barcelona
Ducatez S, Clavel J, Lefebvre L (2015) Ecological generalism and behavioural innovation in birds: technical intelligence or the simple incorporation of new foods? J Anim Ecol 84(1):79–89. https://doi.org/10.1111/1365-2656.12255
English PA, Montgomerie R (2011) Robin’s egg blue: does egg color influence male parental care? Behav Ecol Sociobiol 65:1029–1036. https://doi.org/10.1007/s00265-010-1107-9
Escalona T, Adams DC, Valenzuela N (2018) A lengthy solution to the optimal propagule size problem in the large-bodied South American freshwater turtle, Podocnemis unifilis. Evol Ecol 32:29–41. https://doi.org/10.1007/s10682-017-9922-3
Fontaine JJ, Martin TE (2006) Habitat selection responses of parents to offspring predation risk: an experimental test. Am Nat 168:811–818. https://doi.org/10.1086/508297
Ganai GR, Fazili MF, Bhat BA, Ahanger FA, Bashir M, Khanday AL (2018) Aspects of breeding of Tickell’s Thrush (Turdus unicolor Tickell, 1833) in Kupwara, Jammu and Kashmir, India. J Himalayan Ecol Sustain Dev 13:134–140
Götmark F, Blomqvist D, Johansson OC, Bergkvist J (1995) Nest site selection: a trade-off between concealment and view of the surroundings? J Avian Biol 26:305–312. https://doi.org/10.2307/3677045
Halupka L, Halupka K (2017) The effect of climate change on the duration of avian breeding seasons: a meta-analysis. Proc R Soc Lond B Biol Sci 284(1867):20171710
Hanley D, Grim T, Cassey P, Hauber ME (2015) Not so colourful after all: eggshell pigments constrain avian eggshell colour space. Biol Lett 11:20150087. https://doi.org/10.1098/rsbl.2015.0087
Hansell M (2000) Bird nests and construction behaviour. Cambridge University Press, Cambridge
Ives A, Dinnage R, Nell LA, Helmus M, Li D (2020) phyr: Model Based Phylogenetic Analysis. R package version 1.1.0. https://cran.r-project.org/package=phyr. Accessed 9 July 2022
Jara RF, Crego RD, Arellano FJ, Altamirano TA, Ibarra JT, Rozzi R, Jiménez JE (2019) Breeding strategies of open-cup-nesting birds in sub-Antarctic forests of Navarino Island, Chile. Rev Chil De Hist Nat 92:1–10. https://doi.org/10.1186/s40693-019-0082-4
Kilner RM (2006) The evolution of egg colour and patterning in birds. Biol Rev 81:383–406. https://doi.org/10.1017/S1464793106007044
Lahti DC, Ardia DR (2016) Shedding light on bird egg color: pigment as parasol and the dark car effect. Am Nat 187:547–563. https://doi.org/10.1086/685780
Lomáscolo SB, Monmany AC, Malizia A, Martin TE (2010) Flexibility in nest-site choice and nesting success of Turdus rufiventris (Turdidae) in a montane forest in northwestern Argentina. Wilson J Ornithol 122:674–680. https://doi.org/10.1676/09-167.1
Luro AB, Hauber ME (2022) Pressure for rapid and accurate mate recognition promotes avian-perceived plumage sexual dichromatism in true thrushes (genus: Turdus). J Evol Biol (in press)
Martin TE (1987) Food as a limit on breeding birds: a life-history perspective. Annu Rev Ecol Evol Syst 18:453–487
Martin TE (1995) Avian life history evolution in relation to nestsites, nest predation, and food. Ecol Monogr 65:101–127
Maurer G, Portugal SJ, Cassey P (2011) An embryo’s eye view of avian eggshell pigmentation. J Avian Biol 42:494–504. https://doi.org/10.1111/j.1600-048X.2011.05368.x
Mikula P, Hromada M, Albrecht T, Tryjanowski P (2014) Nest site selection and breeding success in three Turdus thrush species coexisting in an urban environment. Acta Ornithol 49:83–92. https://doi.org/10.3161/000164514X682913
Møller AP, Díaz M, Grim T, Dvorská A, Flensted-Jensen E, Ibáñez-Álamo JD, Jokimäki J, Mänd R, Markó G, Szymański P, Tryjanowski P (2015) Effects of urbanization on bird phenology: a continental study of paired urban and rural populations. Climate Res 66(3):185–199
Moreno J, Osorno JL (2003) Avian egg colour and sexual selection: does eggshell pigmentation reflect female condition and genetic quality? Ecol Lett 6:803–806. https://doi.org/10.1046/j.1461-0248.2003.00505.x
Moreno J, Morales J, Lobato E, Merino S, Tomás G, Martínez-de la Puente J (2005) Evidence for the signaling function of egg color in the pied flycatcher Ficedula hypoleuca. Behav Ecol 16:931–937. https://doi.org/10.1093/beheco/ari072
Moreno J, Lobato E, Morales J, Merino S, Tomás G, Martínez-de la Puente J, Sanz JJ, Mateo R, Soler JJ (2006) Experimental evidence that egg color indicates female condition at laying in a songbird. Behav Ecol 17:651–655. https://doi.org/10.1093/beheco/ark014
Nagy J (2020) Biologia Futura: rapid diversification and behavioural adaptation of birds in response to Oligocene-Miocene climatic conditions. Biologia Futura 71:109–121. https://doi.org/10.1007/s42977-020-00013-9
Nagy J, Végvári Z, Varga Z (2019a) Phylogeny, migration and life history: filling the gaps in the origin and biogeography of the Turdus thrushes. J Ornithol 160:529–543. https://doi.org/10.1007/s10336-019-01632-3
Nagy J, Hauber ME, Hartley IR, Mainwaring MC (2019b) Correlated evolution of nest and egg characteristics in birds. Anim Behav 158:211–225. https://doi.org/10.1016/j.anbehav.2019.10.015
Nagy J (2019) Interactions of climate, migration and life history traits in the evolutionary history of birds. PhD thesis, University of Debrecen
O’Hanlon NJ, Alonso S, Miller JA, McGill RA, Nager RG (2020) Landscape-mediated variation in diet is associated with egg size and maculation in a generalist forager. Ibis 162(3):687–700. https://doi.org/10.1111/ibi.12739
Pagel M, Meade A (2006) Bayesian analysis of correlated evolution of discrete characters by reversible-jump Markov chain Monte Carlo. Am Nat 167:808–825. https://doi.org/10.1086/503444
Pagel M, Meade A, Barker D (2004) Bayesian estimation of ancestral character states on phylogenies. Syst Biol 53:673–684. https://doi.org/10.1080/10635150490522232
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2022) nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1–155. https://cran.r-project.org/package=nlme. Accessed 9 July 2022
R Core Team. (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.r-project.org/. Accessed 9 July 2022
Revell LJ (2012) phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol Evol 3:217–223. https://doi.org/10.1111/j.2041-210X.2011.00169.x
Riehl C (2011) Paternal investment and the “sexually selected hypothesis” for the evolution of eggshell coloration: revisiting the assumptions. Auk 128(1):175–179
Rodewald P (2016) The Birds of North America online. Cornell Laboratory of Ornithology, Ithaca.
Shawkey MD, D’Alba L (2019) Egg pigmentation probably has an early Archosaurian origin. Nature 570:E43–E45. https://doi.org/10.1038/s41586-019-1282-4
Stevens M (2011) Avian vision and egg colouration: concepts and measurements. Avian Biol Res 4:168–184. https://doi.org/10.3184/175815511X13207790177958
Tomiałojć L, Neubauer G (2018) Song Thrush Turdus philomelos and Hawfinch Coccothraustes coccothraustes exhibit non-random nest orientation in dense temperate forest. Acta Ornithol 52:209–220. https://doi.org/10.3161/00016454AO2017.52.2.008
Turner AM, Hauber ME (2021) The American robin (Turdus migratorius): a focal species for anti-parasitic egg rejection studies among hosts of the brown-headed cowbird (Molothrus ater). Ethology 127:490–503. https://doi.org/10.1111/eth.13158
Weidinger K (2001) Does egg colour affect predation rate on open passerine nests? Behav Ecol Sociobiol 49:456–464. https://doi.org/10.1007/s002650100324
Westmoreland D (2008) Evidence of selection for egg crypsis in conspicuous nests. J Field Ornithol 79:263–268. https://doi.org/10.1111/j.1557-9263.2008.00172.x
Wiemann J, Yang TR, Norell MA (2018) Dinosaur egg colour had a single evolutionary origin. Nature 563:555–558. https://doi.org/10.1038/s41586-018-0646-5
Wysocki D, Jankowiak Ł, Greño JL, Cichocka A, Sondej I, Michalska B (2015) Factors affecting nest size in a population of Blackbirds Turdus merula. Bird Study 62:208–216. https://doi.org/10.1080/00063657.2015.1030722
Yang CC, Wang LW, Liang W, Møller AP (2019) High egg rejection rate in a Chinese population of grey-backed thrush (Turdus hortulorum). Zool Res 40:226–230
Yi T, Sun YH, Liang W (2020) Egg rejection and egg recognition mechanism of chestnut thrushes (Turdus rubrocanus). Behav Proc 178:104158. https://doi.org/10.1016/j.beproc.2020.104158
Acknowledgements
JN thanks for the invitation by Gábor Sramkó, Zoltán Barta and Tamás Székely to contribute to this honorary issue to Prof Zoltán Varga, who was the supervisor of JN during his doctoral studies. It is a great honour for JN and all co-authors to accept this invitation. We are grateful to David Ocampo and all anonymous referees for their comments and suggestions that helped us improving our work. MEH was supported by a Humboldt Award at the University of Bielefeld during the preparation of this manuscript.
Funding
Open access funding provided by University of Debrecen. MEH was supported by a Humboldt Award at the University of Bielefeld during the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nagy, J., Fulmer, A.G., Löki, V. et al. Biogeographic history, egg colouration, and habitat selection in Turdus thrushes (Aves: Turdidae). BIOLOGIA FUTURA 74, 467–474 (2023). https://doi.org/10.1007/s42977-023-00191-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42977-023-00191-2