Abstract
Ethyl carbamate (EC), a genotoxic and carcinogenic compound, is an undesirable trace component in spirits and whisky. The precursor of EC produced from barley (Hordeum vulgare L.) malt is primarily a cyanogenic glycosidic nitrile (GN) known as epiheterodendrin (EPH). EPH production is genetically controlled in barley, low or non-GN varieties exist and EPH production can be controlled by using low or non-GN barley varieties for malt production. Here, we report on a rapid and inexpensive colorimetric assay modified from Cook and Oliver (in: European Brewery Convention (eds) Proceedings of the 23rd Congress, Lisbon. European Brewery Convention, Zoeterwoude, Netherlands, 1991). The new low-volume, high-throughput, semi-quantitative test method can be used for the selection of low or non-GN breeding lines through samples of acrospires. This method is based on the detection of cyanide by the reaction with chloramine-T followed by the addition of the reagent (pyridine-barbituric acid) to form a soluble violet-blue product measured at 590 nm. Absorbance measurements and the visual color are used to estimate the presence of EPH production in acrospires. In this report, the level of EPH production was compared among some commercial European non-GN lines, Canadian malt varieties and advanced barley breeding lines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glycosidic nitrile (GN), also known as cyanogenic glycoside, is a major precursor of cyanides produced in fermented malt barley wort (Cook et al. 1990). Catalyzed by the copper ion CuII, cyanides turn into cyanate which reacts with ethanol to give rise to ethyl carbamate (EC) (Beattie and Polyblank 1995; Aresta et al. 2001). Epiheterodendrin (EPH) is the only cyanogenic glycoside among the five hydroxynitrile glucosides derived from the amino acid L-leucine in barley leaf tissue (Nielsen et al. 2002).
In fermented beverages and foods, EC is naturally produced and Canada was the first country to introduce maximum levels for EC in a variety of alcoholic beverages, from 30 μg/L for wine to 400 μg/L for fruit brandies (Health Canada 2018). In whisky, the EC content can be significantly higher, with up to 1000–1719 µg kg-1 reported in some products (Ryu et al. 2015). With increasing market diversification of the North American malt/beverage industry, EC has become an important issue, especially for malts intended for the production of single malt whiskey.
Significant differences in GN levels have been observed between barley varieties, which is the result of one or more cytochromes in the biosynthetic pathway being downregulated (Knoch et al. 2016; Nielson et al. 2002). The varieties, Emir and Aramir registered in the late 1960s and early 1970s, do not produce EPH (Swantson et al. 1999a). It was found that a DNA deletion on the short arm of the 1H barley chromosome within the hydroxynitrile glucoside biosynthetic gene cluster prevents the production of EPH (Ehlert et al. 2019). In the UK, EC in single malt Scotch whisky has been effectively controlled by utilizing non-GN producing (Bringhurst 2015) varieties such as LG’s Concerto or Syngenta’s Laureate. These varieties were developed by introducing the EPH disruptive 1H deletion into elite European malting barley varieties. At the writing of this paper, there are no non-GN barley varieties registered for production in Canada.
Breeders can integrate the 1H deletion using marker-assisted selection (MAS) which is commercially available for screening at the James Hutton Institute (Swanston et al. 1999a; www.huttonltd.com/barley). However, screening large numbers of lines and shipping samples to Europe for testing may be time consuming and too costly for breeders. Additionally, the genotype test will only screen for the 1H deletion and not detect other genetic variants that could result in the low and non-GN trait. An alternative approach is to test and phenotype the actual EPH levels in germinating seeds. Swantson et al. (1999b) found that testing acrospires of germinated seed was equally effective at determining EPH production as directly testing malt. From a colorimetric assay reported by Cook and Oliver (1991), a rapid, high-throughput method was developed to detect the production of EPH in the acrospires from germinated barley seed. Cook and Oliver (1991) based their work on methods developed by Lambert et al. (1975) and the method published by Brinker and Seigler (1989). GN detection methods were recently reviewed by Gleadow et al. (2011). The levels of GN in foods are always a concern and increasingly becoming important to the barley industry in North America, as there is increasing diversification of craft brewing and single malt distilling. It is important that breeding programs develop high-producing and competitive non- or low-GN varieties for production in Canada. We present a practical, low-cost, simplified and updated semi-quantitative method to allow cereal breeding programs to quickly identify non-, low or high GN producing barley genotypes. Our phenotyping compared known GN-producing varieties, non-GN producing varieties and breeding lines.
Materials and methods
Seed and germplasm
Seeds were collected from 24 varieties or breeder lines (Table 1) from the malting barley and germplasm breeding programs at Field Crop Development Centre (FCDC), Lacombe AB Canada. Seeds for GN testing were collected from mature plants propagated using a standard protocol (light, temperature, water and nutrients) in a growth chamber in 2019 except for CDC Churchill, FB485, TR03662, TR05671, and TR14617 which were obtained from a seed storage cold room (5–8 °C). Non-GN varieties, Odyssey and Overture, were kindly provided by Limagrain Cereals Research.
Seed germination and acrospire collection
For each genotype, about 5 g of seed was placed in a metal sieve and rinsed with running tap water. Approximately, 50 seeds were germinated in a petri dish on Whatman filter paper dampened using deionized H2O (diH2O). Seeds were incubated at room temperature (RT) in darkness and rinsed with sterile diH2O daily over the first 3 days of incubation. On day five, 3–4 acrospires (1.5–2.0 cm in length) were dissected from the germinated seeds. These acrospires were transferred to a sterile 2-mL microcentrifuge tube preloaded with 8–10 steel beads (2–5 mm). The weights of the tubes with beads only and with acrospires added were measured and recorded. Approximately, 140–250 mg of sample per tube were flash-frozen with liquid nitrogen. Frozen samples were ground for 60 s with a Retsch (MM301) mixer mill. Ground samples were either stored at − 80 °C or used immediately for testing.
Colorimetric assay
The assay reagents were prepared similar to Cook and Oliver (1991) but with the following modified testing procedures. To each ground acrospires sample, 0.8 ml of 50 mM phosphate extraction buffer (pH 6.0) was added. The extraction buffer was made fresh by a four times dilution of 200 mM phosphate stock solution (pH 6.0) with sterile diH20. Then, samples (with the tube lid open) were incubated for 3 min at 100 °C on a heat block and cooled in ice thereafter. Samples were then centrifuged for 5 min in an Eppendorf 5417C centrifuge at 16,400 rpm, and then, 0.2 mL of supernatant was transferred to designated wells of a 2 mL 96 well plate (Biobasic, CAT # BR581-96 N). Eight wells on the plate were reserved for cyanide standards (prepared from 10% potassium cyanide, Ricca Chemical): 0.2 mL for each well ranging from 100 to 1 ppm cyanide (details are supplied in the supplemental materials). To each well (standards, blanks and samples), 0.1 ml of 4 mg mL−1 β-glucosidase solution was added and allowed to stand for 30 min at room temperature. The reaction was stopped by adding 0.1 mL of 0.1 M NaOH using a multi-channel pipet. After two minutes of incubation, 0.5 mL of 200 mM stock phosphate buffer (pH 6) was added to stabilize the produced cyanide. The final step was the addition of 25 µL reagent A (0.5% aqueous chloramine-T) followed by 25 µL reagent B, (6 g 1,3 dimethylbarbituric acid, 30 mL pyridine, and diH2O added to 100 mL) to each well. The barbituric acid-pyridine solution, reagent B, must be stored in the dark (Cook and Oliver 1991). Well contents were mixed with a multichannel pipette, 200 µL from each well was transferred to corresponding wells of a 96 well microplate. After a 5 min incubation, absorbance was measured using a spectrophotometer (Bio-Rad iMark microplate reader) at 590 nm. Color production indicated the presence of soluble alkali-metal cyanide resulting from EPH in the acrospires, and it could be quickly visualized. EPH production in acrospires can be semi-quantitatively estimated by comparing the absorbance value of samples with a standard curve produced from cyanide standards. For checking the effect of sample treatment, we compared acrospire samples with and without grinding (as described above).
Results
Previously reported non-GN varieties and our breeding lines identified as non-GN producers based on the 1H eph deletion (Ehlert et al. 2019; Holtz et al. 2020) showed no visible color formation (Fig. 1), and their absorbance reads were below 0.5 (Table 1). GN-producer varieties were visually distinguishable from non-GN producing varieties or lines such as Odyssey, Overture, Leo, FB485, TR03662, TR05671, and TR14617. EPH producing varieties included AAC Synergy, Bentley, CDC Bow, CDC Churchill, CDC Connect, CDC Fraser, CDC Kendall, CDC Meredith, CDC Polarstar, Celebration, Cervesa, Legacy, Lowe, Major, Merit 57, Newdale, and Tradition. Abs values for GN-producing samples ranged between 1.137 and 2.248 (data not shown), while the Abs value from the 30 ppm cyanide standard was 2.312. From this test, all Abs values from GN-producing varieties are higher than 1.049 but lower than 2.3.
Colorimetric assay on various barley varieties and lines. Standard curve (column 1 on the left plate) was prepared from cyanide standards (1–100 ppm). After 5 min reaction time, non-GN lines remained colorless and could be quickly identified on plates. Results of non-GN varieties are as follows (with plate locations in brackets): Odyssey (A2-D2), Overture (E2-H2), Leo (A5-D5), TR14617 (A12, B12), TR03662 (C12, D12), TR05671 (E12, F12), FB485 (G12, H12) on the left assay plate and Grit (A5-H5) right assay plate. Well from GN producing varieties were visibly blue. Averaged OD values are reported in Table 1. Blank controls are wells: G1, H1 of the left plate, and C2-H2 of the right plate
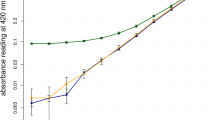
We were able to develop a semi-quantitative method for measuring potential EPH productivity from acrospires using cyanide standards. The minimum detectable level appears to be 5 ppm in the testing solution with a corresponding absorbance (Abs) value of 0.582 (data not shown). The Abs from blank controls and non-GN producer varieties showed no obvious difference (Table 1). From the cyanide standards curve, the relation between absorbance and cyanide concentration was linear within the range of 10 ppm to 30 ppm (Fig. 2). Therefore, the following equation is developed for calculating cyanide levels and correlating EPH productivity (ppm) from acrospires:
where 10 = 10 ppm cyanide standard, Abs = mean absorbance value from a line, 1.049 is the Abs of 10 ppm cyanide standard, Abs30 is the Abs of 30 ppm cyanide standard, 0.8 is the 0.8 mL 1:4 phosphate buffer dilution added to a sample tube.
From the calculation using the above equation, some GN-producer varieties have lower EPH productivity, including AAC Synergy, CDC Kendall, and Merit 57 (Table 1). Results of the ground samples are shown in Fig. 3. Non-ground samples yielded absorbance values 2.1 to 2.9 lower than ground samples (Fig. 3) with little visual color formation (picture not taken).
Discussion
The colorimetric method for the detection of cyanogenic glycoside in barley acrospires was developed by Cook and Oliver (1991). In the method, Cook and Oliver used 8 mL glass vials and boiling water to extract cyanogenic glycoside. With our modified protocol, the assay volumes were reduced by 75%, and the reduced volume allowed the use of 2 mL microcentrifuge tubes in a heating block. Therefore, the benefits of our revised method for plant breeders include lower material costs, less labour, and improved safety, as it requires less plant material and test reagents.
Quantification of cyanogenic glycoside production from barley acrospires was reported by Swanston (1999) based on the colorimetric method developed by Cook and Oliver (1991). Swanston (1999) found that 400 mg of acrospires was adequate to compare genotypes for EPH production and there was no significant genotype × weight interaction. As cyanide standards were not used in their tests, the results were presented as absorbance values ranging from about 0.7 to 1.9. From our test, 1.9 Abs would be equivalent to 23 ppm in the testing solution and over 80 ppm in acrospires, and the cyanogenic glycoside (EPH) from GN-producer varieties ranged from 46.97 ppm to 113.77 ppm in acrospires (Table 1). There are other methods reported for cyanogenic glycosides or cyanide detection as well. Testing paper methods were reported by Brinker and Seigler (1989) and Gleadow et al. (2012). Quantification of cyanogenic glycosides using a spectrophotometer was reported by Woodrow et al. (2002), and their sample incubation time was 15 h.
It is understandable that EPH produced during barley malting would be lower than acrospires which undergo longer germination, but the use of acrospores can increase sensitivity for GN detection. Alternate tests are available for testing GN in malted barley, however, breeding programs may not always have access to a micro-malting facility, while this quick colorimetric assay provides an easy preliminary test for early line selection.
Overall, the cyanogenic glycoside levels in acrospires of major GN-producing malt varieties are high, suggesting the need to develop non-GN malting barley varieties for Canadian malt whisky producers. Due to its higher boiling point of 185 °C, EC can be removed during distillation (Cook et al. 1990) and most EC in the distillate is formed 24–48 h after distillation (Riffikin et al. 1989; Aylott et al. 1990). Therefore, double distillation should dramatically reduce EC contents in whisky, but it will increase production costs. Thus, using non-GN varieties is the best approach for malt whisky distilleries.
The use of a non-GN variety that can exceed the performance of current major malting varieties will not only benefit barley malt whisky producers in Canada, but also brewing industries because malt made from a non-GN variety can mitigate the presence of cyanide in the fermenting wort. The concentration of EC in regular beer should not exceed 15 µg L−1 as regulated by the Liquor Control Board of Ontario (LCBO). The improved protocol can be used for confirming non-GN genotypes in malting barley breeding programs. The breeding lines FB485, TR03662, TR05671, and TR14617 were all confirmed as non-GN lines from our assay. They have either the variety Leo or Grit in their pedigree and were confirmed to carry the deletion on the 1H chromosome (Holtz et al. 2020).
Among the GN-producer varieties, a few malting barley varieties showed slightly lower EPH levels, including AAC Synergy, CDC Kendall, and Merit 57, demonstrating how absorbance readings could be used to semi-quantitatively estimate GN production. Overall, the EPH level among commercial GN-producing varieties was high. Our results demonstrate that this improved protocol can be used for identifying low-GN producers as well as confirming non-GN genotypes and offers a quick, low volume alternative that is scalable for high-throughput screening of breeding materials.
References
Aylott RI, Cochane GC, Leonard MJ, McDonald LS, McKenzie WM, McNeish AS, Walker DA (1990) Ethyl carbamate in grain spirits. Part I: post-distillation ethyl carbamate formation in maturing grain whisky. J Inst Brew 96:213–221. https://doi.org/10.1002/j.2050-0416.1990.tb01030.x
Aresta M, Boscolo M, Franco DW (2001) Copper (II) catalysis in cyanate conversion into ethyl carbamate in spirits, and relevant reactions. J Agric Food Chem 49:2819–2824. https://doi.org/10.1021/jf001346w
Beattie JK, Polyblank GA (1995) Copper-catalysed oxidation of cyanide by peroxide in alkaline aqueos solution. Aust J Chem 48:861–868. https://doi.org/10.1071/CH9950861
Battaglia R, Conacher BS, Page BD (1990) Ethyl Carbamate in alcoholic beverages and foods: a review. Food Addit Contam 7:477–496. https://doi.org/10.1080/02652039009373910
Bringhurst TA (2015) Barley research in relation to scotch whisky production: a journey to new frontiers. J Inst Brew 121:1–18. https://doi.org/10.1002/jib.192
Brinker AM, Seigler DS (1989) Methods for the detection and quantitative determination of cyanide in plant materials. Phytochem Bull 21:24–31
Cook R, MacCaig N, Macmillian JMB, Lumsden WB (1990) Ethyl carbamate formation in grain-based spirits. Part III The Primary Source. J Inst Brew 96:233–244. https://doi.org/10.1002/j.2050-0416.1990.tb01032.x
Cook R, Oliver WB (1991) Rapid detection of cyanogenic glycoside in malted barley. In: European Brewery Convention (eds) Proceedings of the 23rd Congress, Lisbon, pp. 513–519. European Brewery Convention, Zoeterwoude, Netherlands
Ehlert M, Jagd LM, Braumann I, Dockter C, Crocoll C, Motawia MS, Møller BL, Lyngkjær MF (2019) Deletion of biosynthetic genes, specific SNP patterns and differences in transcript accumulation cause variation in hydroxynitrile glucoside content in barley cultivars. Sci Rep 9:5730. https://doi.org/10.1038/s41598-019-41884-w
Gleadow RM, Bjarnholt N, Jørgensen K, Fox, J. Miller RE (2012) Detection, identification and quantitative measurement of cyanogenic glycosides. Chapter 12. pp 283–310 in Research Methods in Plant Science: Vol. 1. Soil Allelochemicals (Eds S S Narwal, L. Szajdak, D. A. Sampietro). Published by International Allelopathy Foundation. Studium Press, USA. (Series ISBN: 1-933699-64-7; Vol. ISBN: 1-933699-65-5)
Health Canada (2018) Health Canada's Maximum Levels for Chemical Contaminants in Foods. https://www.canada.ca/en/health-canada/services/food-nutrition/food-safety/chemical-contaminants/maximum-levels-chemical-contaminants-foods.html Accessed 2 July 2020
Holtz MD, Xue S, Juskiw P, Capettini F, Zantinge J (2020) Look and you will find: New non-GN malt barley varieties are on the way. In: Poster, The 9th Canadian Barley Symposium and 24th BMBRI Triennial Meeting. Winnipeg, Feb 24–25 2020
Knoch E, Motawie MS, Olsen CE, Møller BL, Lyngkjær MF (2016) Biosynthesis of the leucine derived α-, β- and γ-hydroxynitrile glucosides in barley (Hordeum vulgare L.). Plant J 88:247–256. https://doi.org/10.1111/tpj.13247
LCBO. 2013. QUALITY ASSURANCE LABORATORY: Guidelines for Chemical Analysis. http://www.doingbusinesswithlcbo.com/tro/Packaging-Quality/Downloads/PPS_EN.pdf Accessed Sept 8 2020
Nielsen KA, Olsen CE, Pontoppidan K, Møller BL (2002) Leucine-derived cyano glucosides in barley. Plant Physiol 129:1066–1075. https://doi.org/10.1104/pp.001263
Ough CS (1976) Ethyl carbamate in fermented beverages and foods. I. Naturally occurring ethyl carbamate. J Agric Food Chem 24:323–328. https://doi.org/10.1021/jf60204a033
Riffikin HL, Wilson R, Bringhurst TA (1989) The possible involvement of Cu2+ peptide/protein complexes in the formation of ethyl carbamate. J Inst Brew 95:121–122. https://doi.org/10.1002/j.2050-0416.1989.tb04619.x
Ryu D, Choi B, Kim E, Park S, Paeng H, Kim C, Lee JY, Yoon HJ, Koh E (2015) Determination of ethyl carbamate in alcoholic beverages and fermented foods Sold in Korea. Toxicol Res 31:289–297. https://doi.org/10.5487/TR.2015.31.3.289
Seigler DS (1975) Isolation and characterization of naturally occurring cyanogenic compounds. Phytochemistry 14:9–29
Swanston JS, Thomas WTB, Powel W, Young GR, Lawrence PE, Ramsey L, Waugh R (1999) Using molecular markers to determine barleys most suitable for malt distilling. Mol Breeding 5:103–109. https://doi.org/10.1023/A:1009606705925
Swanston JS (1999) Quantifying cyanogenic glycoside production in the acrospires of germinating barley grains. J Sci Food Agric 79:745–749. https://doi.org/10.1002/(SICI)1097-0010(199904)79:5%3c745::AID-JSFA245%3e3.0.CO;2-E
Woodrow IE, Slocum D, Gleadow RM (2002) Influence of water stress on cyanogenic capacity in Eucalyptus cladocalyx. Funct Plant Biol 29:103–110
Acknowledgements
We would like to thank the Brewing and Malting Barley Research Institute (BMBRI) and the Canadian Agricultural Partnership (CAP) project # 2019AO21AF for their financial support toward our project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. Özkan.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xue, S., Zantinge, J.L., Holtz, M.D. et al. A simplified low volume colorimetric assay for rapid comparison of cyanogenic glycoside production between barley genotypes. CEREAL RESEARCH COMMUNICATIONS 51, 457–462 (2023). https://doi.org/10.1007/s42976-022-00297-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42976-022-00297-8