Abstract
Barley (Hordeum vulgare L.) is one of the most widely grown cereal crops. Numerous pathogens impair the amount and quality of the grain yield. Blumeria graminis f. sp. hordei (Bgh) is a fungal pathogen causing powdery mildew, a widespread and economically important foliar disease. Since there is a limited number of known resistance genes, efforts of scientists and breeders are focused on searching for new sources of resistance. Barley landraces are a known, but still underexploited source of diversity. A set of 79 barley landraces collected in North Africa and the Middle East was tested against powdery mildew at seedling and adult plant stages. Under a controlled environment, 50% of accessions showed resistance conditioned by major genes. Among them, seven accessions showed broad resistance to Bgh isolates that were virulent to most of the known resistance genes. The field experiments carried out under natural infection over several years indicated all accessions as potential sources for resistance breeding. Twelve landraces were found to be medium resistant or resistant during all six seasons. This report relates to barley landraces as a promising source of potentially novel resistance to powdery mildew.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Barley (Hordeum vulgare L.) is the fourth most important cereal crop according to harvesting area (http://www.fao.org/faostat). It is resistant to harsh environmental conditions, such as soil salinity, high altitude and low rainfall (von Bothmer et al. 2003a), and is used for feeding and malting purposes. The challenge is to control over 250 pathogens infecting the barley crop (Singh et al. 2019). Most of them are a significant economic problem through reduction of the crop quality and yield. Prevention of crop loss is one of the goals of food security (Savary et al. 2012). Powdery mildew caused by Blumeria graminis (D.C.) Golovin ex Speer f. sp. hordei Em. Marchal (Bgh) is one of the most important barley diseases. Bgh is an airborne fungus which causes yield loss of up to 50%, while average losses are about 10–20% (Tratwal and Weber 2006). In contrast to chemical agents, resistance breeding is an economically effective and environmentally friendly method of controlling the spread of the pathogen.
Several race-specific major resistance genes were already mapped on the barley genome: Mla, Mlat, MlGa, Mlk, Mlnn, Mlra on chromosome 1H; MlLa and MlMor on 2H; Mlg and MlBo on 4H; Mlj on 5H; Mlh on 6H; and mlt and Mlf on 7H (Jørgensen 1994; Schönfeld et al. 1996; Chełkowski et al. 2003; Piechota et al. 2019). Some of them, as well as other major resistance genes with unknown location on the barley genome, like Ml(Ab), Ml(Lv); Ml(Lo) and Ml(St), have been introduced to modern European cultivars (Dreiseitl 2017a). Resistance conditioned by major genes is non-durable. Newly appearing fungal pathotypes can overcome resistant genes in a few years. Also the effectiveness of pyramiding two or more major genes is limited in time (Dreiseitl 2003, 2011). The recessive allele mlo carries non-race-specific resistance to Bgh. It is widely used in elite cultivars due to its durability and lack of selection pressure to the pathogen population.
In the face of the limited available gene pool, searching for new resistance sources is in the interests of scientists and breeders. The selection process has narrowed the gene pool of modern crops (Wulff and Dhugga 2018), so exploration should extend beyond cultivars (Pietrusińska et al. 2018). Barley landraces could be promising as they were cultivated according to traditional practices and not subjected to strong selection pressure. They are highly diverse and heterogeneous populations with a variable gene pool (Camacho Villa et al. 2005). Landraces belong to the primary gene pool and can be easily used in breeding programs. The lack of crossing barriers reduces breeding costs and efforts (von Bothmer et al. 2003b; Yun et al. 2006). The aim of this study was to select promising sources of powdery mildew resistance in North African and Middle East barley landraces.
Materials and methods
Plant material
A set of 79 spring barley landraces from the collection of the Plant Breeding and Acclimatization Institute – National Research Institute (Radzików, Poland) was screened for resistance to Bgh. All accessions were obtained from The International Centre for Agricultural Research in the Dry Areas (ICARDA) collection (Table 1). Barley landraces originated from North Africa and the Middle East and included 27 accessions from Algeria, 14 from Jordan, six from Egypt, six from Morocco, six from Libya, one from Tunisia and 19 of unknown origin.
Pathogen isolates
Six barley Bgh isolates, Bgh27, Bgh133, Bgh111, Bgh131, Bgh4714, Bgh314, collected in Poland, were used for artificial inoculation at the seedling stage. The isolates have a known virulence spectra according to the Pallas near-isogenic lines: Pallas (Mla8), P01(Mla1), P02 (Mla3), P03 (Mla6, Mla14), P04B (Mla7, +?), P06 (Mla7, MlLG2), P07 (Mla9, Mlk) P08B (Mla9), P09 (Mla10, MlDu2), P10 (Mla12), P11 (Mla13, Ml(Ru3)), P12 (Mla22), P13 (Mla23), P14 (Mlra), P15 (Ml(Ru2)), P17 (Mlk), P18 (Mlnn), P19 (Mlp), P20 (Mlat), P21 (Mlg, Ml(CP)), P23 (MlLa), P24 (Mlh) (Kølster et al. 1986); and cultivars Borwina (Ml(Bw)), Iron (Ml(1-B-53)), Steffi (Ml(St1), Ml(St2)), Lenka (Mla13, Ml(Ab)), Gunnar (Mla3, Ml(Tu2)) and Triumph (Mla7, Ml(Ab)) (Table 2, Table S1*). All isolates were freshly propagated on the susceptible barley variety Manchuria CIho 2330.
Seedling resistance screening
Seedling tests were performed for resistance against each of six Bgh isolates: Bgh27, Bgh133, Bgh111, Bgh131, Bgh4714, Bgh314. For each test ca. 25 seeds per barley accession were sown. They were grown under controlled chamber conditions with a 16/8 h day/night photoperiod and a 22/16 °C temperature regime. Seedlings with a fully expanded first leaf (DC: 12 (Zadoks et al. 1974)) were inoculated with Bgh by shaking conidia from the susceptible cv. Manchuria. After 8–10 days, infection types were scored according to a 5-level scale (Mains and Dietz 1930), where 0, 1 and 2 represented resistant plants and 3 and 4 represented susceptible plants. Postulation of resistant genes was based on a comparison of reaction spectra designated on landraces and the barley differential set (Table S1). In case of a mixed reaction against Bgh isolate, postulation was performed for the resistance score. Possibility of resistance to Avr genes was concluded on the basis of the gene-for-gene hypothesis (Flor 1956). The infection response spectrum of each landrace was compared with the Bgh virulence spectrum previously found on the set of barley differential varieties.
Adult plant resistance screening
Barley landraces were screened for adult plant resistance under natural infection conditions. Plants were field grown in Radzików, central Poland (52° 13′ 38″ N, 20° 36′ 55″ E) during six seasons: 2010–2013, and 2016–2017. Plants were sown in a 2-m plot with 20 cm row spacing and 7 cm distance between seeds. Susceptible Manchuria was sown every 20 rows as a disease spreader. Disease symptoms were scored on adult plants at the flowering stage (DC: 60–69; Zadoks et al. 1974) according to a 9-level scale, were 1 indicates a very susceptible reaction and extreme infection of the entire plant and 9 indicates a totally resistant plant without visible symptoms of infection (Dreiseitl 2003, 2011) and the lowest infection score per accession and per season was recorded.
Results
Seedling resistance screening
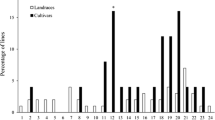
Among 79 barley landraces, 39 (49%) showed resistance to at least one Bgh isolate tested (Table 3). Among them, two accessions (701, 720) were resistant to five isolates, four (695, 719, 737, 740) to four, five to three, and 15 were resistant to two isolates. Postulation of resistance genes was feasible for seven accessions. For all resistant landraces resistance to Avr genes was estimated. Presumption indicated 11 accessions (605, 695, 701, 704, 720, 735, 737, 740, 748, 749, 753) carrying putative resistance to at least nine Avr genes. Two accessions (701, 720) probably carried resistance to 20 Avr genes. For two landraces (739, 742) it was impossible to indicate any known resistance, but they showed an incompatible reaction against one of the Bgh isolates used. Some of the accessions revealed a heterogenic reaction and contained resistant and susceptible plants against at least one Bgh isolate tested. A mixed reaction was observed for 27 landraces, which is 37% of all tested barleys and 67% of accessions resistant to Bgh isolates at the seedling stage.
Adult stage screening
The set of 79 barley landraces was tested under field conditions during six seasons. The lowest infection scores noted during the trial were: 5 (medium resistance) recorded for 21 (26%) landraces and 3 (which is medium susceptible) observed for 58 (74%) accessions (Table 1). Average infection scores calculated for six seasons were: 7 (resistant) for 13 (16%) accessions, 5–6 (medium resistant) for 65 (82%) accessions, and 4 (medium susceptible) for one accession. A set of 12 (612, 613, 621, 631, 647, 648, 651, 694, 727, 730, 748, 753) accessions showed an average score of 7 (resistant) with the lowest score of 5 (medium resistant). These accessions were medium resistant or resistant during all six seasons.
Discussion
Blumeria graminis is a highly adaptive and fast propagating fungal pathogen. New pathotypes, which emerge easily and fast, may manifest virulence to resistance genes currently used in crops. To retard erosion of resistance, gene pyramiding as well as major and minor gene combinations are used. The limited number of known genes carrying resistance to Bgh inspires the effort to discover new potential resources.
Landraces are crucial for resistance breeding and to restore diversity among the resistance gene pool (Akem et al. 2000). Variable genotypes throughout the population and lack of strong selection pressure support genetic differentiation. Previous reports identified barley landraces as potential sources of resistance (Czembor 2000, 2002; Comadran et al. 2009; Newton et al. 2010; Spies et al. 2012). Resistance genes revealed in landraces were successfully introduced to modern cultivars. The best known and used is the mlo recessive allele, which was first identified in an Ethiopian landrace. Other examples are Mlg, originating from the German landrace Weihenstephan; and variants revealed in the multiallelic locus Mla: Mla3 carried by the Uruguayan landrace Ricardo, and Mla12 from Arabische (Jørgensen 1994). Since Bgh isolates collected in Africa revealed higher diversity than European fungi (Dreiseitl and Kosman 2013; Jensen et al. 2013), barley landraces originating from that region are suspected to be variable in Bgh resistance loci. Additionally, landraces originating from regions of barley diversification and domestication, such as North Africa and the Middle East, exhibit a diversity of resistance genes due to long-term coevolution with the pathogen (Camacho Villa et al. 2005; Morrell and Clegg 2007). Screening of Jordanian barley landraces allowed the selection of 165 lines resistant to Bgh (Abdel-Ghani et al. 2008). A collection of 131 barley lines originating from Morocco exhibited seedling resistance (Czembor 2000, 2002).
In this report seedling tests showed that 50% of the investigated accessions harbored major resistance genes (Table 3). The Bgh isolates used for inoculation covered most of the known and used genes (Jørgensen 1994; Dreiseitl 2014), so it can be presumed that these accessions carry new resistance genes. Six accessions (695, 701, 719, 720, 737, 740) have a broad spectrum of resistance and may be of interest to breeders. Some of the accessions revealed mixed infection types after inoculation with Bgh isolates. Since the landraces are non-homogeneous dynamic populations, mixed reaction within plants is expected. Progeny of these accessions should be selected according to the resistance trait before further testing. Previous reports showed the mixed reaction of barley landraces against Bgh (Dreiseitl 2017b). In the present research, postulation of resistance genes was feasible for seven landraces (Table 3), whereas possible resistance to Avr was estimated for 38 seedling resistant landraces. In previous data presented by Czembor (2000, 2002), reaction of 66 (50%) lines selected from barley landraces were unique and distinguished from other known genes. Nevertheless, for 99 (76%) accessions the Mlat gene was postulated or supposed. The latter author used in the experiments a more diverse set of Bgh isolates that were more accurate. The six Bgh isolates used in this study had broad virulence spectra and allowed expression of the maximum number of resistance genes but are less informative than the full differential Bgh set.
Since major genes are usually overcome in a few years, field resistance determined by minor quantitative genes is more promising. It has longer durability and is effective against various pathotypes. That kind of resistance increases during continuous cultivation and quantitative minor genes are still diversifying and changing (Jensen et al. 2012). The multiyear trial examined resistant landraces under different weather conditions and variable pathogen pressure. A set of 12 (612, 613, 621, 631, 647, 648, 651, 694, 727, 730, 748, 753) accessions revealed a good level of resistance during six seasons. These accessions could be valuable material for a more detailed analysis and a good source for resistance breeding. Additionally, selected landraces were collected from the area of barley origin and domestication. Field resistance maintained by long-term coevolution with a pathogen is valuable and economically important for farmers and breeders (Jensen et al. 2012).
This multiyear study did not reveal consistent field resistance to natural pathogen pressure with seedling resistance against artificial inoculation in a climate chamber. Reaction patterns were variable among growth stages. Expression of quantitative resistance becomes more visible at the adult stage and against multipathotype infection. Previous data identified wild barley (H. vulgare ssp. spontaneum) genotypes resistant at seedling stage and highly susceptible at adult stage under field conditions (Dreiseitl and Bockelman 2003). Nevertheless, all landraces resistant to at least three Bgh isolates (701, 720, 695, 719, 737, 740, 698, 694, 729, 794, 730) at the seedling stage were at least medium resistant at the adult stage.
According to Dreiseitl (2017b) searching for and detecting new resistance is desirable for its utility in breeding programs, for easier identification of minor resistance genes frequently masked by major genes and for improving knowledge of resistance. New resistance genes can be useful by combining them with other known genes or partial Mlo resistance in barley cultivars. That approach makes resistance more durable and inhibits Mlo resistance erosion (Dreiseitl 2017b). Six-year field trials and artificial testing against highly virulent powdery mildew isolates, presented in this report, provided the list of barley landraces resistant to B. graminis f. sp. hordei.
References
Abdel-Ghani AH, Al-Ameiri NS, Karajeh MR (2008) Resistance of barley landraces and wild barley populations to powdery mildew in Jordan. Phytopathol Mediterr 47:92–97. https://doi.org/10.14601/Phytopathol_Mediterr-2611
Akem C, Ceccarelli S, Erskine W, Lenne J (2000) Using genetic diversity for disease resistance in agricultural production. Outlook Agric 29:25–30. https://doi.org/10.5367/000000000101293013
Camacho Villa TC, Maxted N, Scholten M, Ford-Lloyd B (2005) Defining and identifying crop land-races. Plant Genet Resour 3:373–384. https://doi.org/10.1079/PGR200591
Chełkowski J, Tyrka M, Sobkiewicz A (2003) Resistance genes in barley (Hordeum vulgare L.) and their identification with molecular markers. J Appl Genet 44:291–309
Comadran J, Thomas WTB, van Eeuwijk FÁ, Ceccarelli S, Grando S, Stanca AM, Pecchioni N, Akar T, Al-Yassin A, Benbelkacem A, Ouabbou H, Bort J, Romagosa I, Hackett CA, Russell JR (2009) Patterns of genetic diversity and linkage-disequilibrium in a high structured Hordeum vulgare associacion-mapping population for the Mediterranean basin. Theor Appl Genet 119:175–187. https://doi.org/10.1007/s00122-009-1027-0
Czembor JH (2000) Resistance to powdery mildew in barley landraces from Morocco. J Plant Pathol 82:187–200. https://doi.org/10.1071/AP00022
Czembor JH (2002) Resistance to powdery mildew in selections from Moroccan barley landraces. Euphytica 125:397–409. https://doi.org/10.1023/A:1016061508160
Dreiseitl A (2003) Adaptation of Blumeria graminis f. sp. hordei to barley resistance genes in the Czech Republic in 1971–2000. Plant Soil Environ 49:241–248
Dreiseitl A (2011) Differences in powdery mildew epidemics in spring and winter barley based on 30-year variety trials. Ann Appl Biol 59:49–57. https://doi.org/10.1111/j.1744-7348.2011.00474.x
Dreiseitl A (2014) Pathogenic divergence of Central European and Australian populations of Blumeria graminis f. sp. hordei. Ann Appl Biol 165:364–372. https://doi.org/10.1111/aab.12141
Dreiseitl A (2017a) Genes for resistance to powdery mildew in European barley cultivars registered in the Czech Republic from 2011 to 2015. Plant Breed 136:351–356. https://doi.org/10.1111/pbr.12471
Dreiseitl A (2017b) Heterogeneity of powdery mildew resistance revealed in accessions of the ICARDA wild barley collection. Front Plant Sci 8:202. https://doi.org/10.3389/fpls.2017.00202
Dreiseitl A, Bockelman HE (2003) Sources of powdery mildew resistance in a wild barley collection. Genet Resour Crop Evol 50:345–350. https://doi.org/10.1023/A:1023953819787
Dreiseitl A, Kosman E (2013) Virulence phenotypes of Blumeria graminis f. sp. hordei in South Africa. Eur J Plant Pathol 136:113–121. https://doi.org/10.1007/s10658-012-0143-x
Flor HH (1956) The complementary genic system in flax and flax rust. Adv Genet 8:29–54. https://doi.org/10.1016/S0065-2660(08)60498-8
Jensen HR, Dreiseitl A, Sadiki M, Schoen DJ (2012) The Red Queen and the seed bank: pathogen resistance of ex situ and in situ conserved barley. Evol Appl 5:353–367. https://doi.org/10.1111/j.1752-4571.2011.00227.x
Jensen HR, Dreiseitl A, Sadiki M, Schoen DJ (2013) High diversity, low spatial structure and rapid pathotype evolution in Moroccan populations of Blumeria graminis f. sp. hordei. Eur J Plant Pathol 136:323–336. https://doi.org/10.1007/s10658-013-0166-y
Jørgensen JH (1994) Genetics of powdery mildew resistance in barley. Crit Rev Plant Sci 13:97–119. https://doi.org/10.1080/07352689409701910
Kølster P, Munk L, Stølen O, Løhde J (1986) Middle-isogenic barley lines with genes for resistance to powdery mildew. Crop Sci 26:903–907
Mains EB, Dietz SM (1930) Physiologic forms of barley mildew, Erysiphe graminis hordei Marchal. Phytopathology 20:229–239
Morrell PL, Clegg MT (2007) Genetic evidence for a second domestication of barley (Hordeum vulgare) east of the Fertile Crescent. Proc Natl Acad Sci USA 104:3289–3294. https://doi.org/10.1073/pnas.0611377104
Newton AC, Akar T, Baresel JP, Bebeli PJ, Bettencourt E, Bladenopoulos KV, Czembor JH, Fasoula DA, Katsiotis A, Koutis K, Koutsika-Sotiriou M, Kovács G, Larsson H, Pinheiro De Carvalho MAA, Rubiales D, Russell J, Dos Santos TMM, Vaz Patto MC (2010) Cereal landraces for sustainable agriculture. Agron Sustain Dev 30:237–269. https://doi.org/10.1051/agro/2009032
Piechota U, Czembor PC, Słowacki P, Czembor JH (2019) Identifying a novel powdery mildew resistance gene in a barley landrace from Morocco. J Appl Genet 60:243–254. https://doi.org/10.1007/s13353-019-00505-y
Pietrusińska A, Żurek M, Piechota U, Słowacki P, Smolińska K (2018) Searching for diseases resistance sources in old cultivars, landraces and wild relatives of cereals. A review. Agron Sci 73:45–60. https://doi.org/10.24326/asx.2018.4.5
Savary S, Ficke A, Aubertot JN, Hollier C (2012) Crop losses due to diseases and their implications for global food production losses and food security. Food Sec 4:519–537. https://doi.org/10.1007/s12571-012-0200-5
Schönfeld M, Ragni A, Fishbeck G, Jahoor A (1996) RFLP mapping of three new loci for resistance genes to powdery mildew (Erysiphe graminis f. sp. hordei) in barley. Theor Appl Genet 93:48–56. https://doi.org/10.1007/BF00225726
Singh B, Mehta S, Aggarwal SK, Tiwari M, Bhuyan SI, Bhatia S, Islam MA (2019) Barley, disease resistance, and molecular breeding approaches. In: Wani SH (ed) Disease resistance in crop plants. Springer Nature, Cham, pp 261–299
Spies A, Korzun V, Bayles R, Rajaraman J, Himmelbach A, Hedley PE, Schweizer P (2012) Allele mining in barley genetic resources reveals genes of race-non-specific powdery mildew resistance. Front Plant Sci 2:113. https://doi.org/10.3389/fpls.2011.00113
Tratwal A, Weber A (2006) Virulence frequency of Blumeria graminis f. sp. hordei and the occurrence of powdery mildew on four winter barley cultivars. J Plant Prot Res 46:221–230
von Bothmer R, Sato K, Knüpffer H, Hintum T (2003a) Barley diversity – an introduction. In: von Bothmer R, Hintum T, Knüpffer H, Sato K (eds) Diversity in barley (Hordeum vulgare). Elsevier Science B.V, Amsterdam, pp 3–8
von Bothmer R, Sato K, Komatsuda T, Yasuda S, Fischbeck G (2003b) The domestication of cultivated barley. In: von Bothmer R, van Hintum T, Knuüpffer H, Sato K (eds) Diversity in barley (Hordeum vulgare). Elsevier Science B.V, Amsterdam, pp 9–27
Wulff BBH, Dhugga KS (2018) Wheat—the cereal abandoned by GM. Science 361:451–452. https://doi.org/10.1126/science.aat5119
Yun SJ, Gyenis L, Bossolini E, Hayes PM, Matus I, Smith KP, Steffenson BJ, Tuberosa R, Muehlbauer GJ (2006) Validation of quantitative trait loci for multiple disease resistance in barley using advanced backcross lines developed with a wild barley. Crop Sci 46:1179–1186. https://doi.org/10.2135/cropsci2005.08-0293
Zadoks JC, Chang TT, Konzak CF (1974) A decimal code for the growth stages of cereals. Weed Res 14:415–421. https://doi.org/10.1111/j.1365-3180.1974.tb01084.x
Acknowledgements
The authors thank to Professor Henryk Czembor for significant and valuable advices provided in their work with barley–powdery mildew pathosystem. The research was conducted within program “Creation of scientific basis for biological improvement and plant genetic resources protection as source of innovation and support of sustainable agriculture and national food security”, Project No. 3-2-00-0-02 (PW task 2.2): “Broadening of barley gene pool” supported by The Ministry of Agriculture and Rural Development Poland.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. Grausgruber.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Piechota, U., Czembor, P.C. & Czembor, J.H. Evaluating barley landraces collected in North Africa and the Middle East for powdery mildew infection at seedling and adult plant stages. CEREAL RESEARCH COMMUNICATIONS 48, 179–185 (2020). https://doi.org/10.1007/s42976-020-00021-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42976-020-00021-4