Abstract
An increasing number of studies have investigated spatial and temporal patterns in species richness and assemblage composition in mountain ecosystems along altitudinal gradients. Small mammals have been successfully used as indicators of environmental health and as proxies of biodiversity. However, information about the composition and distribution of species assemblages in the mosaic of habitat and rocky landform types at a high altitude is still lacking for most of the mountain regions. Through the use of live traps and camera trapping, we described the small mammal community living above the treeline of the Western Dolomites (Italian Alps), investigating the species richness, abundance of individuals and community composition in relation to topographic, micrometeorological, mesohabitat, and biological correlates. A total of five species and 50 individuals were sampled, analysed, and released. At the extremes of the analysed altitudinal range (i.e. 1900 vs 2900 m a.s.l.), community composition was completely different and species richness was related to elevation, steepness, and vegetation cover. At the same time, the taxonomic distinctness of ground-dwelling arthropods (namely carabid beetles and spiders), a proxy of habitat complexity, showed higher values in areas with a greater small mammal species richness. We found a positive effect of steepness and rocky landform type “carsism” on the number of captured individuals, showing the importance of the availability of shelters and underground burrows for the sampled species. As a confirmation of the altitudinal shift for these species in relation to the ongoing climate change, we detected a negative impact of sub-surface ground temperature on small mammal abundance during the monitoring period. In conclusion, small mammals represent an excellent model for understanding the evolutionary processes of ecosystems, population dynamics under changing environmental conditions, and habitat vulnerabilities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Evidence about the biotic responses to ongoing climate change is continuously growing (Chen et al., 2011; Feeley et al., 2020; Lenoir & Svenning, 2015; Parmesan, 2006; Parmesan & Yohe, 2003; Root et al., 2003; Walther et al., 2002; Zhu et al., 2022), and we also realized species vulnerability to future change world-wide (Moritz & Agudo, 2013; Rowe et al., 2015). There is a general trend towards upward and poleward shifts of elevational and latitudinal boundaries of species’ ranges (Chen et al., 2011; Lenoir et al., 2008; Parmesan, 2006; Parmesan & Yohe, 2003; Root et al., 2003; Thomas & Lennon, 1999; Walther et al., 2002), with “leading edge” expansions, where populations shift at northern or upper range margins following climate changes, detected more often than “lagging edge” contractions, where populations reduce their ranges (Angert et al., 2011; Hill et al., 2011; Morelli et al., 2012).
A number of recent studies have investigated spatial and temporal patterns in species richness and life history traits along altitudinal gradients (e.g. Billman et al., 2021; Gobbi et al., 2021; Ross et al., 2021; Shoo et al., 2006; Viterbi et al., 2013). Mountain ecosystems are sensitive and reliable indicators of climate change, encompassing steep environmental gradients in small geographic areas and thus being outstanding natural laboratories for biodiversity studies (Sun et al., 2020). In these areas, many environmental factors correlate with elevation, including temperature (e.g. Wu et al., 2013) and productivity (e.g. Ramirez-Bautista & Williams, 2019).
However, information about the composition and distribution of species assemblages in the mosaic of habitat and rocky landform types available at a high altitude is still lacking for most of the mountain regions and most taxonomic groups. This is probably due to the difficulties in reaching high-altitude areas and collecting quantitative data under harsh environmental conditions, specifically on alpine fellfields.
Small mammals, Rodentia and Eulipotyphla, are the most diverse group of mammalian species, showing a wide range of sizes, behaviours, and niche uses (Nowak & Walker, 1999), also in the Italian peninsula (Loy et al., 2019). They play key roles in food webs, including important ecological roles in mountain ecosystems such as ground-dwelling arthropod predators and seed predators and dispersers (Martin-Regalado et al., 2019; Mortelliti et al., 2019), as ecosystem engineers in soil aeration and nutrient mixing through their burrowing habit (Zhang et al., 2003), and as a prey for mammalian and avian predators (Love et al., 2000; Moore et al., 2003). These taxa are particularly suitable for examining elevation patterns because they are commonly found along mountain slopes, have higher speciation rates and species turnover among habitats than larger mammals (Lopez et al., 2016; Sun et al., 2020).
In this context, small mammals are useful indicators of environmental health and biodiversity (Flowerdew et al., 2004; McCain & Colwell, 2011). Due to the ongoing climate change, low-elevation species expanded their ranges and high-elevation species contracted theirs, leading to changed community composition at mid and high elevations, also with possible species competition and replacement (Moritz et al., 2008). Indeed, during elevational migrations, abiotic factors can affect species composition by limiting the species to those who have specific functional traits necessary to live under these abiotic conditions (Graham et al., 2009; Webb, 2000). These abiotic conditions may act as a filter that prevents the establishment of species that have disfavored traits (Weiher et al., 1998).
The aim of this study was (1) to describe the small mammal community and the species distribution above the treeline of the Western Dolomites (Italian Alps) from 1900 to 2900 m a.s.l., (2) to investigate the species richness and the small mammal abundance in relation to topographic (elevation, steepness), micrometeorological (temperature and relative humidity), biological (abundance of ground-dwelling arthropod acting as potential prey and/or proxy of habitat complexity), and mesohabitat (habitat and landform types) correlates and iii) to evaluate the activity rhythms during day-night time slots (and related temperature and humidity conditions). We expected lower species richness and abundance at higher elevation and in extreme temperature and relative humidity conditions.
Materials and methods
Study area
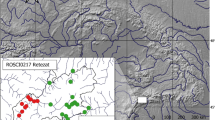
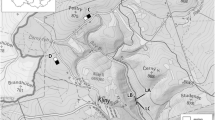
The fieldwork was carried out on the Brenta Dolomites (46°12′N, 10°53′E), in the Adamello Brenta Nature Park (Trento Province, Central-Eastern Alps, Northern Italy; Fig. 1). Round sample areas (n = 6; radius = 100 m) were set up every 200 m from 1900 to 2900 m a.s.l. with a general western aspect (Fig. 1). Sample areas spatial arrangement provided the independence and representativeness of the data collected for each type of environment (see ESM 1 for major details on environmental and micrometeorological description of each sample area). The environments include the uppermost larch forest (Larix decidua) at lower altitudes, alpine prairie with outcropping rock alternating with limestone floors and Grostè peak characterized by alpine calcareous grassland (PAT Geoportal: http://www.territorio.provincia.tn.it, accessed on 12 February 2022; Geomorphologic database of BioMiti Project, Zanoner et al., 2019).
Potential predators are represented by diurnal birds of prey (e.g. Aquila chrysaetos, Falco tinnunculus), opportunistic birds in finding sources of nutrition (e.g. Corvus corax) and night-active raptors (Strix aluco, Asio otus, Aegolius funereus, Glaucidium passerinum, Bubo bubo). Terrestrial predators were mammals (Vulpes vulpes, Mustela nivalis, Mustela erminea, Martes foina, Martes martes) and reptiles (Hierophis viridiflavus; Zamenis longissimus, Vipera aspis, Vipera berus) (Adamello Brenta Nature Park – official monitoring data).
Data collection
Small mammals were monitored by setting up in each sampling area a catch grid of 16 Sherman LFA live traps (size = 7.6 × 8.9 × 22.9 cm; mass = 227 g; 4 × 4 grid; step = 5 m) with 10 g of food bait (i.e. a mixture of sunflower seed, corn, dried fruit, apple, pork cubes and water) and dry grass, to attract animals and allow them to build a temporary shelter (Gagliardi & Tosi, 2012; Gurnell & Flowerdew, 1982; Hoffmann et al., 2010). To ensure safe monitoring, 1, 2 or 3 replicas were made, depending on the thickness of the snow cover and the accessibility to the high-altitude monitoring areas. Trapping was conducted between June and August 2020. After a prebaiting night, traps were set out for 3 consecutive 24 h periods with collection occurring at 8.00–9.00 AM and PM of each day. For each sampled individual, species identity, sex, reproductive status (Hoffmann et al., 2010), body mass and body measurements (Gagliardi & Tosi, 2012; Hoffmann et al., 2010) were recorded. Biometrical measures were used as a confirmation of species identification (i.e. length of head-body, tail, hind foot, and ear) and, together with reproductive status, to assess the age class (young, sub-adult, adult) according to available literature and technical reports in Trento Province (Northern Italy, e.g. Caldonazzi et al., 2018a, 2018b; Ferrari, 2016; Locatelli & Paolucci, 1996, 1998). Before release, animals were marked by shaving their hair in specific areas of the skin, a method that makes the animals temporarily recognizable in accordance with the timing of the monitoring sessions (Gagliardi & Tosi, 2012; Hoffmann et al., 2010).
In four sample areas (ID = 2, 3, 4 and 6; see Fig. 1), 8 camera-traps (SG2060-Xe) were placed alternatively in each catch grid 1-m in front of the live traps during prebaiting and capture periods to analyse the rhythms of daily activity (e.g. Viviano et al., 2022). Cameras were placed at a height of ~ 10–30 cm from the ground and were kept active 24 h, to take one video (10 s) at each animal passage (trigger time = 1 s; see ESM2 for major details).
Elevation (m a.s.l.), steepness (degree), vegetation cover (%), large rock slide deposit (%) and carsism (%) were derived from the Digital Elevation Model (DEM) raster at a 1 m spatial resolution (PAT Geoportal: http://www.territorio.provincia.tn.it, accessed on 12 February 2022), the Habitat Nature 2000 code vector map (PAT Geoportal: http://www.territorio.provincia.tn.it, accessed on 12 February 2022) and the landform type vector map (Geomorphologic database of BioMiti Project; Zanoner et al., 2019) in QGIS 3.16 (QGIS Development Team, 2021) (see Table 1 for a complete list of variables describing the ecological context).
In addition, data logger (Tinytag Plus 2 TGP-4500) was installed in each sampling area to record micrometeorological parameters (i.e. the hourly temperature and hourly percentage of relative humidity) at 5–10 cm depth in the ground and at the height of 150 cm from the ground on a wooden support covered by a white coating to prevent direct sunlight that can lead to the overheating.
We associated with each monitoring session and area data on species richness (i.e. the total number of species collected), abundance (i.e. the total number of individuals sampled), taxonomic distinctness (i.e. a measure that emphasizes the average taxonomic relatedness between species within a community; Clarke & Warwick, 1998; Paschetta et al., 2013) and average body length (mm; i.e. a proxy of available biomass, Moretti et al. 2017) of carabid beetles (Coleoptera: Carabidae) and spiders (Arachnida: Araneae). As reported by Clarke and Warwick (1998), we calculated the taxonomic distinctness as the average path length between any two randomly chosen individuals, conditional on them being from different species (i.e. the ratio between taxonomic diversity and the value it would take were there to be no taxonomic hierarchy). We selected these two taxonomic groups because (1) they are potential ground-dwelling prey for some of the captured small mammals (i.e. Soricidae; Klenovšek et al., 2013), (2) they are among the most common and most studied ground-dwelling and top-predator arthropods living at high altitudes, and (3) previous data were already available for the investigated sampling areas (see Gobbi et al., 2021; Petri et al., 2021 for major details about data collection, species identification and measurements).
Data analysis
After evaluating the trapping success and mortality rate, for each sampling area we reported the list of sampled species, the species richness (i.e. the number of detected species), the number of individuals trapped for each species, and the dominance index (i.e. the probability that two individuals randomly selected from the sample belong to the same species: Simpson, 1949). Data were considered according to day-night trapping sessions.
We fitted linear models testing the effect of the topographic (elevation, steepness), micrometeorological (temperature and relative humidity), biological (abundance of ground-dwelling arthropod acting as potential prey) and mesohabitat variables on species richness and abundance of small mammals in the study areas (see Table 1). We tested for collinearity among independent variables using a correlation matrix (Pearson correlation coefficient, rp) and did not include correlated variables in the same model if |rp|≥ 0.7 (Sokal & Rohlf, 1995).
We z-transformed continuous independent variables to compare the relative effects of predictors on species richness and aspecific abundance (i.e. the total number of captured individuals, without considering the species) of small mammals. We used Akaike's Information Criterion for small sample size (AICc; Burnham & Anderson, 2002) to select the best‐fitting sets of models. We selected all models within 2 AICc units of the best fitting model (i.e. ΔAICc ≤ 2, indicating substantial evidence to support the candidate model; Burnham & Anderson, 2002). We checked assumptions of normality, homoscedasticity, and independence by inspecting standardized residual plots (Zuur et al., 2010). We obtained the effect of each variable (i.e. parameter estimation) included in the confidence set of models via model averaging (model.avg function in MuMin package for R; Burnham & Anderson, 2002; Symonds & Mousalli, 2011). We assessed model goodness of fit using the adjusted R2.
Daily activity rhythms were evaluated considering “activity” as the cumulated period animals spend outside their shelter sites, regardless of their behaviour (Lashley et al., 2018). For all videos, we recorded the date and the time of capture and number of individuals. In order to limit bias and lack of reliability in our analyses, we classified all the captured animals into two group: Sorex sp. and Arvicolinae.
The close morphological resemblance makes the discrimination inside the Sorex genus and the Arvicolinae subfamily not always possible from camera-trap records. Number of small mammal video-captures (N/h) in each 1 h time slot inside the sample area during 24 h/day period was evaluated and considered in relation to mean temperature (°C) at 5–10 cm depth in the ground.
Statistical analyses were performed in R version 4.1.2 (www.r-project.org, R Core Team, 2021).
Results
Live trapping results and small mammal community structure
Trapping success for small mammals was 8.0% representing 97 captures from 1216 trap-sessions (day trapping success: 4.4% representing 21 captures from 480 trap-days; night trapping success: 10.3% representing 76 captures from 736 trap-nights). During the study, the 97 capture-events led to the identification of 50 individuals of small mammals of 5 species, including one insectivore and four rodents (Fig. 2 and Table 2). We identified the snow vole (Chionomys nivalis; n = 21), the bank vole (Myodes glareolus; n = 13), the common vole (Microtus arvalis; n = 3), the genus Microtus subgenus Terricola (Microtus (Terricola) spp.; n = 3 represented by Microtus multiplex and/or Microtus subterraneus), and the Valais shrew (Sorex antinorii; n = 10). At the extremes of the altitudinal range (i.e. ~ 1900 vs 2900 m a.s.l.), community compositions were completely different: we trapped only Chionomys nivalis (n = 4) at 2892 m a.s.l., while we detected Myodes glareolus (n = 12), Microtus (Terricola) spp. (n = 3) and Sorex antinorii (n = 9) at 1867 m a.s.l.. However, it should be considered that only one session was performed in the highest sample area, instead of three sampling sessions, due to the persistence of snow cover. Mortality rate was 13.4% (69.2% Sorex antinorii, 23.1% Chionomys nivalis and 7.7% Myodes glareolus).
Environmental correlates
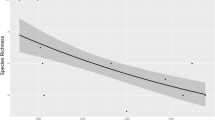
Among potential predictors of small mammal species richness, we revealed the importance of three variables: elevation, steepness, and vegetation cover. While an increase in altitudinal range had a negative effect on species richness, greater steepness and a higher percentage of vegetation cover allowed an increase in the number of species (Table 3a). The taxonomic distinctness of Carabidae and Araneae showed higher values in areas with a greater small mammal species richness (Table 3a).
Throughout the Brenta Dolomites, steepness and rocky landform type “carsism” had a positive effect on the number of captured individuals. A negative impact on small mammal abundance was related to higher value of sub-surface ground temperature during the monitoring period, detecting fewer individuals in areas with the highest temperature values (Table 3b). Also, in this case the taxonomic distinctness of Carabidae and Araneae showed higher values in areas with a greater density of small mammals (Table 3b).
Camera trapping results
In total, 898 h of video-trapping (51% during daytime and 49% during nighttime) collected 295 videos of small mammals, of these 251 were considered as independent events (i.e. recognized a different individual or registration made after at least 15 min). Forty-three events (17.13%) were recorded during daylight, while 208 events (82.87%) during nighttime. Other 63 videos were discarded as it was not possible to identify the species with enough confidence (21.4% of total, in accordance with Viviano et al., 2022). The daily activity of small mammals showed an important peak after the sunset, while a further increase was recorded before the sub-superficial ground temperatures reached the highest daily values (2–4 PM; Fig. 3). This peak was mostly due to the activity of the Sorex sp. recorded in the lowest altitude sample area (see supplementary material ESM3 for more details). The relative humidity was always ≥ 95% for the whole video-trapping period in each sample area, so the variance of this factor was negligible.
Discussion
The data collected gave the possibility to highlight the variables affecting the species richness and abundance of small mammal community above the treeline.
In Italy, the snow vole is distributed in the continental area (Alps and Northern-Central Apennines; Toschi, 1965; Amori et al., 1986; Nappi & Aloise, 2015; Loy et al. 2019).
It is very common in high mountain land, and it is the only European mammal found at 4700 m on the French slope of the Monte Bianco massif (Saint Girons, 1973). Nappi (2002), analysing 157 bibliographical, museum and unpublished data described the altitudinal range in Italy from 170 to 3700 m a.s.l.. In the Alps, the upper altitudinal limit is determined by the height of the peaks (e.g. 1820–2959 m in the Western Italian Alps; Patriarca & Debernardi, 1997); however, in Italy the snow vole predominantly occurs between 1500 and 2000 m of altitude, as it is mainly influenced by the ecological niche rather than by temperatures (Nappi, 2002). In the Dolomites, it had previously been reported at 2700 m a.s.l. (Locatelli & Paolucci, 1996) and this study updates the presence of this species up to 2829 m a.s.l. at Grostè peak. The optimal habitat is represented by open areas with a rocky substrate consisting of large boulders that occupy 90% of the surface, with a maximum of 10% of vegetation cover (Janeau, 1980). In our study, these characteristics were recorded over the 2700 m a.s.l., where the greatest abundance of individuals was found (see Fig. 1).
At the lowest investigated altitude (1900 m a.s.l.), we revealed the presence of the bank vole, the Valais shrew, and the genus Microtus subgenus Terricola. Among the fossorian rodents, we confirmed the presence of M. multiplex and M. subterraneus, distinguishable only with genetic investigation or analysing the dental and cranial morphology. All these species are linked to wooded areas from the hills to the mountains (up to over 2000 m of altitude; Patriarca & Debernardi, 1997). In some cases, it is also present in open or rocky areas, as long as they are covered with shrubs.
The areas located at higher altitudes and with a lower degree of vegetation cover showed, as expected, a lower small mammal species richness. However, it should be considered that the climatic and environmental changes underway, and which occur more rapidly in extreme environments, are causing an upward shift of species (Rowe et al., 2010). It is interesting to note that the steepness contributes positively to describe the species richness, highlighting the choice of safer and more protected areas (i.e. areas with greater abundance of shelters to prevent predation). Indeed, the presence of shelters plays a fundamental role for these species, in all the different environments but above all in areas with limiting climatic-environmental characteristics (Dickman et al., 2011; Ecke et al., 2001; Sabino-Marques & Mira, 2011; Torre et al., 2022).
At the same time, the taxonomic distinctness of carabid beetles and spiders, as well as a habitat complexity index (Clarke & Warwick, 1999; Warwick & Clarke, 1998), showed higher values in areas with more small mammal species. Since taxonomic diversity is a proxy of environmental maturity/stability (Paschetta et al., 2013), high values should be related to a high availability of trophic niches (i.e. areas with lower competition, greater availability of trophic resources, shelter areas, and suitable environmental conditions).
Daily activity rhythms of small mammals. Number of small mammal video-captures (N/h; grey bars; upper panel = Arvicolinae, lower panel Sorex sp.) in each 1 h time slot in relation to mean temperature (°C; red line) at 5–10 cm depth in the ground. Blue background indicates time slot between sunset and sunrise. Data were reported for the whole study area (see supplementary materials ESM3 for the evaluation of activity in the 4 sample areas along the elevation range) located in the Brenta Dolomites (46°12′N, 10°53′E), within the Adamello Brenta Nature Park (Trento Province, Central-Eastern Alps, Northern Italy)
Considering small mammal abundance, our results confirmed the importance of shelter areas and habitat complexity. Indeed, a greater abundance of small mammals was revealed in areas with more underground burrows.
We detected a negative impact of sub-surface ground temperature on small mammal abundance during the monitoring period, detecting fewer individuals in areas with the highest temperature values. This relationship might suggest a possible altitudinal shift for these species in relation to the ongoing climate changes. Also the daily activity showed the lowest values when the sub-surface ground temperatures reached the highest daily values. These high temperatures are also avoided by the genus Sorex which shows a bimodal activity, with the need to feed even during the daytime hours. Despite the presence of a high vegetation cover (in fact the genus Sorex was detected only in the sample area at a lower altitude), the hottest hours are in any case avoided. It is important to note that the activity of Arvicolinae in this high-altitude environment differs greatly from the activity found in wooded areas (Viviano et al., 2022). However, this can be due not only to the environmental and climatic characteristics but also to an anti-predatory strategy (e.g. O’Brien et al., 2018).
As small mammals play a crucial ecological role in food chains, as well as for bioindication, there is an urgent need to continuously monitor their status, population trends, and altitudinal shift. They represent excellent models for understanding the evolutionary processes in ecosystems, population dynamics under changing environmental conditions, and habitat vulnerabilities.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Amori, G., Cristaldi, M., & Contoli, L. (1986). Sui Roditori (Gliridae, Arvicolidae, Muridae) dell’Italia peninsulare ed insulare in rapporto all’ambiente bioclimatico mediterraneo. Animalia, 11(13), 217–269. (in Italian).
Angert, A. L., Crozier, L. G., Rissler, L. J., Gilman, S. E., Tewksbury, J. J., & Chunco, A. J. (2011). Do species’ traits predict recent shifts at expanding range edges? Ecology Letters, 14, 677–689.
Billman, P. D., Beever, E. A., McWethy, D. B., Thurman, L. L., & Wilson, K. C. (2021). Factors influencing distributional shifts and abundance at the range core of a climate-sensitive mammal. Global Change Biology, 27(19), 4498–4515.
Burnham, K. P., & Anderson, D. R. (2002). Model selection and multimodel inference: A practical information-theoretic approach (2nd ed.). Springer.
Caldonazzi, M., Paolucci, P., & Zanghellini, S. (2018a). Roditori. In Deflorian, M. C., Caldonazzi, M., & Zanghellini, S., Pedrini, P. (Eds.), Atlante dei Mammiferi della provincia di Trento. Monografie del Museo delle Scienze, 6, pp 37–66. (in Italian)
Caldonazzi, M., Paolucci, P., & Zanghellini, S. (2018b). Roditori. In Deflorian, M. C., Caldonazzi, M., Zanghellini, S., & Pedrini, P. (Eds.) Atlante dei Mammiferi della provincia di Trento. Monografie del Museo delle Scienze, 6, 155–214. (in Italian)
Chen, I., Hill, J. K., Ohlemuller, R., Roy, D. B., & Thomas, C. D. (2011). Rapid range shifts of species associated with high levels of climate warming. Science, 333, 1024–1026.
Clarke, K. R., & Warwick, R. M. (1998). A taxonomic distinctness index and its statistical properties. Journal of Applied Ecology, 35, 523–531.
Clarke, K. R., & Warwick, R. M. (1999). The taxonomic distinctness measure of biodiversity: Weighting of step lengths between hierarchical levels. Marine Ecology Progress Series, 184, 21–29.
Dickman, C. R., Greenville, A. C., Tamayo, B., & Wardle, G. M. (2011). Spatial dynamics of small mammals in central Australian desert habitats: The role of drought refugia. Journal of Mammalogy, 92(6), 1193–1209.
Ecke, F., Löfgren, O., Hörnfeldt, B., Eklund, U., Ericsson, P., & Sörlin, D. (2001). Abundance and diversity of small mammals in relation to structural habitat factors. Ecological Bulletins, 1, 165–171.
Feeley, K. J., Bravo-Avila, C., Fadrique, B., Perez, T. M., & Zuleta, D. (2020). Climate-driven changes in the composition of New World plant communities. Nature Clinical Practice Endocrinology and Metabolism, 10, 965–970.
Ferrari, G. (2016). La comunità di micromammiferi in un ambiente che cambia: un caso studio nelle Alpi Centrali italiane. Master's degree in Ecology and Nature Conservation, University of Parma, Italy. (in Italian)
Flowerdew, J. R., Shore, R. F., Poulton, S. M. C., & Sparks, T. H. (2004). Live trapping to monitor small mammals in Britain. Mammal Review, 34, 31–51.
Gagliardi, A., & Tosi, G. (2012). Monitoraggio di Uccelli e Mammiferi in Lombardia. Tecniche e metodi di rilevamento. Ed. Regione Lombardia, Università degli Studi dell’Insubria, Istituto Oikos, Italy. (in Italian)
Gobbi, M., Armanini, M., Boscolo, T., Chirichella, R., Lencioni, V., Ornaghi, S., & Mustoni, A. (2021). Habitat and landform types drive carabid beetles distribution at high-altitude. Diversity, 13, 142.
Graham, C. H., Parra, J. L., Rahbek, C., & McGuire, J. A. (2009). Phylogenetic structure in tropical hummingbird communities. Proceedings of the National Academy of Sciences of the United States of America, 106(2), 19673–19678.
Gurnell, J., & Flowerdew, J. R. (1982). Live trapping small mammals. A practical guide. An occasional publication of the Mammal Society. Haervest House, 62 London Road, Reading, Berkshire.
Hill, J. K., Griffiths, H. M., & Thomas, C. D. (2011). Climate change and evolutionary adaptations at species’ range margins. Annual Review of Entomology, 56, 143–159.
Hoffmann, A., Decher, J., Rovero, F., Schaer, J., Voigt, C., & Wibbelt, G. (2010). Field methods and techniques for monitoring mammals. In J. Eymann, J. Degreef, C. Häuser, J. C. Monje, Y. Samyn, & D. VandenSpiegel (Eds.), Manual on field recording techniques and protocols for All Taxa Biodiversity Inventories and Monitoring (pp. 482–529). Pensoft Publishers.
Janeau, G. (1980). Répartition écologique des micromammifères dans l’étage alpin de la région de Briançon. Mammalia, 44(1), 2–25. (in French).
Klenovšek, T., Janzekovič, F., & Novac, T. (2013). Notes on invertebrates preyed by shrews (mammalia: Insectivora: Soricidae) in Slovenia. Series Historia Naturalis, 23(2), 153–160.
Lashley, M. A., Cove, M. V., Chitwood, M. C., Penido, G., Gardner, B., DePerno, C. S., & Moorman, C. E. (2018). Estimating wildlife activity curves: Comparison of methods and sample size. Science and Reports, 8, 1–11.
Lenoir, J., & Svenning, J. C. (2015). Climate-related range shifts—A global multidimensional synthesis and new research directions. Ecography, 38, 15–28.
Lenoir, J., Gégout, J. C., Marquet, P. A., de Ruffray, P., & Brisse, H. (2008). A significant upward shift in plant species optimum elevation during the 20th century. Science, 320, 1768–1771.
Locatelli, R., & Paolucci, P. (1996). L‘arvicola delle nevi (Microtus nivalis Martins, 1842) nell’Italia Nord Orientale: Biometrie, morfologia dentale e scelte dell’habitat. Boll Mus Civ St Nat Venezia, 45, 195–209. (in Italian).
Locatelli, R., & Paolucci, P. (1998). Insettivori e piccoli roditori del Trentino. Ed. Giunta della Provincia autonoma di Trento, Servizio Parchi e foreste demaniali (TN), Italy. (in Italian)
Lopez, L. C. S., Figueiredo, M. S. L., Fracasso, M. P. D. A., Mesquita, D. O., Anjos, U. U., & Grelle, C. E. V. (2016). The role of local versus biogeographical processes in influencing diversity and body-size variation in mammal assemblages. Ecology and Evolution, 6(5), 1447–1456.
Love, R. A., Webbon, C., Glue, D. E., & Harris, S. (2000). Changes in the food of British Barn Owls (Tyto alba) between 1974 and 1997. Mammal Review, 30, 107–129.
Loy, A., Aloise, G., Ancillotto, L., Angelici, F.M., Bertolino, S., Capizzi, D., Castiglia, R., Colangelo, P., Contoli, L., Cozzi, B., Fontaneto, D., Lapini, L., Maio, N., Monaco, A., Mori, E., Nappi, A., Podestà, M., Russo, D., Sarà, M., Scandura, M., & Amori, G. (2019) Mammals of Italy: an annotated checklist. Hystrix, 30(2), 87–106.
Martin-Regalado, C. N., Briones-Salas, M., Lavariega, M. C., & Moreno, C. E. (2019). Spatial incongruence in the species richness and functional diversity of cricetid rodents. PLoS ONE, 14(6), e0217154.
McCain, C. M., & Colwell, R. K. (2011). Assessing the threat to montane biodiversity from discordant shifts in temperature and precipitation in a changing climate. Ecology Letters, 14(12), 1236–1245.
Moore, N. P., Askew, N., & Bishop, J. D. (2003). Small mammals in new farm woodlands. Mammal Review, 33, 101–104.
Morelli, T., Smith, A. B., Kastley, C. R., Mastroserio, I., Moritz, C., & Beissinger, S. R. (2012). Anthropogenic refugia ameliorate the severe climate-related decline of a montane mammal along its trailing edge. Proceedings of the Royal Society B, 279, 4279–4286.
Moretti, M., Dias, A. T., De Bello, F., Altermatt, F., Chown, S. L., Azcárate, F. M., Bell, J. R., Fournier, B., Hedde, M., Hortal, J., & Ibanez, S. (2017). Handbook of protocols for standardized measurement of terrestrial invertebrate functional traits. Functional Ecology, 31(3), 558–567.
Moritz, C. M., & Agudo, R. (2013). The future of species under climate change: Resilience or decline? Science, 341, 504–508.
Moritz, C., Patton, J. L., Conroy, C. J., Parra, J. L., White, G. C., & Beissinger, S. R. (2008). Impact of a century of climate change on small mammal communities in Yosemite National Park, USA. Science, 322, 261–264.
Mortelliti, A., Grentzmann, I. P., Fraver, S., Brehm, M. A., Calkins, S., & Fisichelli, N. (2019). Small mammal controls on the climate-driven range shift of woody plant species. Oikos, 128, 1726–1738.
Nappi, A. (2002). Vertical distribution of the snow vole Chionomys nivalis (Martins, 1842) (Rodentia, Arvicolidae) in Italy. Hystrix, the Italian Journal of Mammalogy, 13(1–2), 45–52.
Nappi, A., & Aloise, G. (2015). About the presence of the snow vole, Chionomys nivalis (Martins, 1842), in Calabria: Data review and critical considerations (Mammalia, Rodentia, Cricetidae). Biodiversity Journal, 6(1), 7–10.
Nowak, R. M., & Walker, E. P. (1999). Walker’s mammals of the world (Vol. 1). JHU Press.
O’Brien, S. L., Cook, J. A., & Newsome, S. D. (2018). Niche differentiation among small mammals of the Alexander Archipelago in southeastern Alaska. Journal of Mammalogy, 99(1), 108–116.
Parmesan, C. (2006). Ecological and evolutionary responses to recent climate change. Annual Review of Ecology Evolution and Systematics, 37, 637–669.
Parmesan, C., & Yohe, G. (2003). A globally coherent fingerprint of climate change impacts across natural systems. Nature, 421, 37–42.
Paschetta, M., Giachino, P., & Isaia, M. (2013). Taxonomic relatedness of spider and carabid assemblages in a wetland ecosystem. Zool Stud, 51, 1175–1187.
Patriarca, E., & Debernardi, P. (1997). Insectivora, Chiroptera, Lagomorpha, Rodentia and Carnivora of the Gran Paradiso National Park: Checklist and preliminar ecological characterisation. Ibex, 4, 17–32.
Petri, I., Bernasconi, M., Ballarin, F., Pantini, P., Armanini, M., Caccianiga, M., Chirichella, R., LencioniV, M. A., & Gobbi, M. (2021). Ragni (Arachnida: Araneae) d’alta quota delle Dolomiti di Brenta. Studi Trentini Di Scienze Naturali, 101, 73–81. (in Italian).
QGIS Development Team. (2021). QGIS Geographic Information System. http://qgis.osgeo.org. Accessed on 20th June, 2021
R Core Team. (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Ramirez-Bautista, A., & Williams, J. N. (2019). The importance of productivity and seasonality for structuring small rodent diversity across a tropical elevation gradient. Oecologia, 190(2), 275–286.
Root, T., Price, J. T., Hall, K. R., Schneider, S. H., & Pounds, J. A. (2003). Fingerprints of global warming on wild animals and plants. Nature, 421, 57–60.
Ross, J. G. B., Peters, W., Ossi, F., Moorcroft, P. R., Cordano, E., Eccel, E., Bianchini, F., Ramanzin, M., & Cagnacci, F. (2021). Climate change and anthropogenic food manipulation interact in shifting the distribution of a large herbivore at its altitudinal range limit. Science and Reports, 11(1), 1–12.
Rowe, K. C., Rowe, K. M., Tingley, M. W., Koo, M. S., Patton, J. L., Conroy, C. J., Perrine, J. D., Beissinger, S. R., & Moritz, C. (2015). Spatially heterogeneous impact of climate change on small mammals of montane California. Proceedings of the Royal Society B, 282(1799), 20141857.
Rowe, R. J., Finarelli, J. A., & Rickart, E. A. (2010). Range dynamics of small mammals along an elevational gradient over an 80-year interval. Global Change Biology, 16(11), 2930–2943.
Sabino-Marques, H., & Mira, A. (2011). Living on the verge: Are roads a more suitable refuge for small mammals than streams in Mediterranean pastureland? Ecological Research, 26(2), 277–287.
Saint Girons, M. C. (1973). Les mammifères de France et du Bénélux. (faune marine exceptée). Doin, Paris. (in French)
Shoo, L. P., Williams, S. E., & Hero, J. M. (2006). Detecting climate change induced range shifts: Where and how should we be looking? Austral Ecology, 31(1), 22–29.
Simpson, E. H. (1949). Measurement of diversity. Nature, 163, 688–688.
Sokal, R. R., & Rohlf, F. J. (1995). Biometry: The principles and practice of ptatistics in biological research (3rd ed.). Freedman.
Sun, J., Wen, Z., Feijó, A., Cheng, J., Wang, Y., Li, S., Ge, D., Xia, L., & Yang, Q. (2020). Elevation patterns and critical environmental drivers of the taxonomic, functional, and phylogenetic diversity of small mammals in a karst mountain area. Ecology and Evolution, 10(19), 10899–10911.
Symonds, M. R. E., & Mousalli, A. (2011). A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behavioral Ecology and Sociobiology, 65, 13–21.
Thomas, C. D., & Lennon, J. J. (1999). Birds extend their ranges northwards. Nature, 399, 213.
Torre, I., Jaime-González, C., & Díaz, M. (2022). Habitat suitability for small mammals in Mediterranean landscapes: How and why shrubs matter. Sustainability, 14(3), 1562.
Toschi, A. (1965). Mammalia. Lagomorpha, Rodentia, Carnivora, Ungulata, Cetacea. Fauna d’Italia (Vol. 7). Edizioni Calderini, Bologna. (in Italian)
Viterbi, R., Cerrato, C., Bassano, B., Bionda, R., Von Hardenberg, A., Provenzale, A., & Bogliani, G. (2013). Patterns of biodiversity in the northwestern Italian Alps: A multi-taxa approach. Community Ecology, 14, 18–30.
Viviano, A., Scarfò, M., & Mori, E. (2022). Temporal partitioning between forest-dwelling small rodents in a Mediterranean Deciduous Woodland. Animals, 12(3), 279.
Walther, G., Post, E., Convey, P., Menzel, A., Parmesan, C., Beebee, T. J. C., Fromentin, J., Hoegh-Guldberg, O., & Bairlein, F. (2002). Ecological responses to recent climate change. Nature, 416, 389–395.
Warwick, R. M., & Clarke, K. R. (1998). Taxonomic distinctness and environmental assessment. Journal of Applied Ecology, 35(4), 532–543.
Webb, C. O. (2000). Exploring the phylogenetic structure of ecological communities: An example for rain forest trees. The American Naturalist, 156(2), 145–155.
Weiher, E., Clarke, G. P., & Keddy, P. A. (1998). Community assembly rules, morphological dispersion, and the coexistence of plant species. Oikos, 81(2), 309–322.
Wu, Y., Yang, Q., Wen, Z., Xia, L., Zhang, Q., & Zhou, H. (2013). What drives the species richness patterns of non-volant small mammals along a subtropical elevational gradient? Ecography, 36(2), 185–196.
Zanoner, T., Seppi, R., & Carton, A. (2019). Database Geomorfologico del Progetto BioMiti; Tecnical Report and Vector Data; Adamello Brenta Natural Park: Strembo, Italy. (in Italian)
Zhang, Y., Zhang, Z., & Liu, J. (2003). Burrowing rodents as ecosystem engineers: The ecology and management of plateau zokors Myospalax fontanierii in alpine meadow ecosystems on the Tibetan Plateau. Mammal Review, 33(3–4), 284–294.
Zhu, G., Papeş, M., Armsworth, P. R., & Giam, X. (2022). Climate change vulnerability of terrestrial vertebrates in a major refuge and dispersal corridor in North America. Diversity and Distributions, 28, 1227–1241.
Zuur, A. F., Ieno, E. N., & Elphick, C. S. (2010). A protocol for data exploration to avoid common statistical problems. Methods in Ecology and Evolution, 1, 3–14.
Acknowledgements
We are grateful to all the students and the Adamello Brenta Nature Park staff for their support in collecting data. G. Lövei, E. Mori and an anonymous reviewer provided helpful comments on earlier drafts of the manuscript.
Funding
Open access funding provided by Università degli Studi di Sassari within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no competing interests exist.
Ethical approval
The study complies with all relevant national, regional and provincial Italian laws and with the ethical standards of scholarly research.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chirichella, R., Ricci, E., Armanini, M. et al. Small mammals in a mountain ecosystem: the effect of topographic, micrometeorological, and biological correlates on their community structure. COMMUNITY ECOLOGY 23, 289–299 (2022). https://doi.org/10.1007/s42974-022-00104-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42974-022-00104-8