Abstract
Gunung (Mount) Talang is an active volcano in West Sumatra that has a number of Nepenthes species, including the endemic N. talangensis, but their ecology has been little been studied. This study found five species of Nepenthes growing in the protected forest area of Gunung Talang, namely N. bongso, N. inermis, N. pectinata, N. spathulata and N. talangensis. The population of N. talangensis is very small (23 individuals) and it grows sympatrically with N. bongso and N. inermis forming natural hybrids. Lithocarpus conocarpus, Camellia lanceolata, Syzygium acuminatissimum, Adinandra dumosa and Dehaasia sp. are the five most dominant tree species found in the Nepenthes habitat, while L. conocarpus and Podocarpus neriifolius had strong positive associations with N. talangensis. Growth rates of the five Nepenthes species were not significantly different, neither were foliar nitrogen (mean = 1.14%) or phosphorus concentrations (mean = 0.11%). We suggest that N. talangensis should be considered as Critically Endangered and outline some possible conservation actions.
Similar content being viewed by others
Introduction
Nepenthes is the only genus in the Nepenthaceae family, which are all classified as carnivorous plants (Phillipps and Lamb 1996; Clarke 2001). Indonesia is the center of the global distribution of Nepenthes (Mansur 2013) with 79 species or 43% of the total number in the world (currently 182) (Mansur et al. 2021). Sumatra is one of the largest and most biodiverse of the Indonesian islands (Whitten et al. 1997); the Bukit Barisan Mountains, which stretch from South Sumatra to Aceh, are one of the most important contributors to Sumatra's high biodiversity, including Nepenthes (Lee et al. 2006). On the island of Sumatra, there are 39 species of Nepenthes of which more than three-quarters are endemic (Hernawati et al. 2022). The high number of Nepenthes species recorded in Sumatra makes it the second largest center of diversity after Borneo (Indonesia, Malaysia and Brunei Darussalam), and Nepenthes experts state that Sumatra is a hotspot for Nepenthes evolution (Wistuba et al. 2007). Indeed, the discovery of several new Nepenthes species from the island of Sumatra in recent years supports its importance as a key area for tropical biodiversity.
In the 1980s, half of Sumatra's landmass was tropical rain forest, but now more than half of that forest has been lost, placing Sumatran forests under some of the greatest threat in Indonesia (Margono et al. 2012). According to Global Forest Watch data, during 2001–2019, deforestation in Indonesia reached 26.8 million ha, with most of that occurring in Sumatra and Kalimantan (Lidwina 2021). Changes in the function of forest areas to non-forest areas such as oil palm plantations and gold/coal mining, along with forest fires, have reduced forest land so that it is predicted that the population of Nepenthes in their natural habitat will also decline. Nevertheless, many Nepenthes species are found at higher elevations which provides some protection from forest loss and land conversion. However, overexploitation for commercial purposes as ornamental plants will result in a reduction in the natural population of Nepenthes and there are several species, such as N. clipeata (Lee 2007; Mansur et al. 2021), N. dubia (Cheek et al. 2017) and N. rigidifolia (Hernawati and Akriadi 2005) whose conservation status, based on the IUCN Red List, is Critically Endangered. Indeed, Indonesia ranks second (26 species) after Brazil (28 species) for threatened carnivorous plants species. There are currently, 12 Critically Endangered species, nine Endangered species and five Vulnerable Nepenthes species in Indonesia (Cross et al. 2020), so it is necessary to make efforts to reduce the threat of further loss of Nepenthes species in Indonesia, and in Sumatra in particular.
Studies of Nepenthes have mostly focused on descriptions of new species, the methods they use to attract, capture and digest prey, and on the food webs contained within their pitchers (Moran and Clarke 2010). In contrast, there is a lack of basic autecological research on habitat requirements and associations of Nepenthes species, or on ecophysiological studies such as foliar nutrient concentrations or growth rates (Mansur et al. 2021, 2022a,b, 2023; Mansur and Brearley 2008; Osunkoya et al. 2007, 2008). Whilst Nepenthes show aggregated spatial patterns (Chua 1995; Damit et al. 2017), we are not aware of any studies that document the associations between Nepenthes and other plant species in natural habitats that might shed further light on their habitat requirements and how these might be shaped by interspecfic interactions.
Gunung Talang is an active volcano in West Sumatra that last erupted between 2005 and 2007. It has an area of 6150 ha with a peak height of 2,597 m asl located in Solok Regency with the status of Protected Forest. In addition to providing ecosystem services regulating water systems, preventing flooding, controlling erosion and maintaining soil fertility, the Gunung Talang forest is also predicted to have a high biodiversity, including of Nepenthes. Puspitaningtyas and Wawangningrum (2007) reported that there were six species of Nepenthes found in the Gunung Talang forest, five of which are endemic to the island of Sumatra. After fifteen years had passed, we were interested to determine how the number of Nepenthes on Gunung Talang might have changed, monitor the state of their current population and habitat, and conduct initial studies on their autecology.
Materials and methods
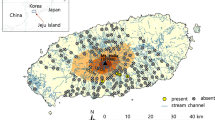
The study was conducted in the Gunung (Mount) Talang protected forest area in November 2021 and October 2022. Administratively, the research location is in Jorong Bukit Gompong Village, Gunung Talang District, Solok Regency (Fig. 1). The mountain is about 30 km east of the city of Padang in West Sumatra.
Plant exploration was carried out by following the summit trail from an elevation of c. 1500 m to c. 2300 m above sea level. Each Nepenthes plant found was recorded; for each individual, their coordinate position, morphological parameters (including stem length and leaf number) and leaf chlorophyll content were also determined. Leaf area was calculated as length × width × 0.66. Leaves from three plant samples from each species (N. bongso, N. inermis, N. pectinata and N. talangensis), namely the second leaf from the tip of the stem (estimated to be six months old), and pitcher fluid were sampled to analyze their nutrient concentrations. We also collected soil samples (c. 100 g) from three elevations where Nepenthes grew (2090, 2150 and 2215 m asl). Each species of Nepenthes was collected for herbarium vouchers that were deposited in Herbarium Bogoriense (BO), Research Center for Ecology and Ethnobiology, National Research and Innovation Agency (BRIN), Indonesia. A number of plants of each species were numbered with small aluminium tags and the top leaf of each individual was punched with a small hole using a paper hole-punch. After 11 months, the plants were re-censused and the stem length and number of leaves of each individual were re-recorded.
Three plots of 0.09 ha each (30 m × 30 m) were established to determine the abundance of Nepenthes and the species of trees growing in their habitat. Each species of Nepenthes in the 27 subplots (10 m × 10 m each) was recorded along with their position (x and y co-ordinates). Tree species (Ø ≥ 5 cm) in each subplot were recorded and their stem diameter, height, and position (x, y) noted. All data collected was processed and analyzed according to the Mueller-Dombois and Ellenberg method (1974) to obtain values for Basal Area (BA), Relative Frequency (RF), Relative Density (RD), Relative Dominance (RDo), and Importance Value Index (IVI). Morisita’s (1959) index of dispersion was calculated for each species in each plot. The calculation of associations between plant species was carried out using a 2 × 2 contingency table (Ludwig and Reynolds 1988; Sutomo et al. 2011; Handayani et al. 2019) on plant species that had an IVI value greater than 10% (Zulkarnaen et al. 2017). The calculated chi-square (χ2) value was then compared with the chi-square (χ 2) table at the 5% test level, which is 3.84. If the value of χ 2 was greater than that in the χ 2 table, then there was an association between the two species, whereas if the value of χ2 was less than that in the χ 2 table, then there was no association between them (Mueller-Dombois and Ellenberg 1974). The strength of the association was then calculated using the Ochiai (1957) index (OI):
\(OI=(a/( \sqrt{a+b})(\sqrt{a+c})\))
a = The number of sampling units (i.e. each 10 m × 10 m subplot) where both species occur.
b = The number of sampling units where species a occurs but not species b.
c = The number of sampling units where species b occurs but not species a.
The Ochiai index value ranges from 0 to 1 with the stronger the association between the two plant species the closer the index value is to 1 (Ludwig and Reynolds 1988).
Leaf and soil samples were dried in an oven at 60 °C for three days, then ground with a pestle and mortar. Two ml of mixed acid (sulfuric acid, nitric acid, and perchloric acid) was added to leaves or soil (0.2 g) and heated on a hotplate at a temperature of 170 °C until the solution was clear with a white precipitate. Samples were then diluted to a final volume of 10 ml before analysis. Determination of P was done by a colorimetric method, where 1 ml of the sample was added to 3 ml of distilled water and then reacted with 1 ml of P dye with the yellow color of the sample measured using a Shimadzu BioSpec-Mini 1240 UV–Vis spectrophotometer at a wavelength of 450 nm. Nitrogen was also determined by a colorimetric method, where 2 ml of the sample was reacted with 4 ml of Na-phenate and 4 ml of 5% NaOCl with the blue color of the sample measured using a spectrophotometer as above at a wavelength of 636 nm. Determination of other elements (Ca, K, Mg and Na) was by atomic absorption spectrophotometry using a Shimadzu AA-6800. The pH of the pitcher fluid was measured using an ATC pH-2011 portable meter.
Data were analysed using Minitab 19.2 using one-way ANOVAs with Tukey’s post hoc tests with a significance level of 0.05.
Results and discussion
Diversity
In a previous study, it was found that there were six species of Nepenthes in the Gunung Talang area, namely N. bongso, N. gracilis, N. inermis, N. pectinata, N. spathulata and N. talangensis (Puspitaningtyas and Wawangningrum 2007), but at the time of this study, only five species were found (Fig. 2), with N. gracilis not observed. Nepenthes gracilis generally grows in open areas in the lowlands and is widespread across Borneo, Sumatra, Sulawesi, the Malay peninsula and Indochina (Phillipps and Lamb 1996; Mansur 2013), so there is little concern regarding its conservation. Nepenthes spathulata has also been recorded from Gunung Singgalang (Mansur et al. 2023) indicating that it is also found in West Sumatra, in addition to provinces further south (Clarke 2001). Nepenthes pectinata and N. spathulata grow at an elevation between 1500 to 2100 m above sea level (asl) in shaded areas. Nepenthes bongso and N. talangensis grow at an elevation of 2150 to 2200 m asl in mossy forest with a shaded canopy, while N. inermis grows at an elevation of 2150 to 2300 m asl in mossy forest with a slightly open canopy (Table 1). As well as Gunung Talang, N. pectinata was also found on Gunung Pangulubao, Gunung Singgalang, Gunung Talakmau (Cheek and Jebb 2001), and Gunung Malintang (Herbarium Bogoriense: BO); N. spathulata was also found on Gunung Tanggamus (Lampung), South Sumatra, Jambi and Bengkulu (Clarke 2001), and Gunung Pesagi (BO); N. bongso was also found on Gunung Kerinci, Gunung Marapi, Gunung Singgalang (BO) and Gunung Gadut (Clarke 2001) and N. inermis was also found in Gunung Belirang (Clarke 2001), whereas N. talangensis is endemic to Gunung Talang, and thus, particularly important to study.
Morphology
The morphology of the five species studied was different, according to their respective characters. The upper pitchers of N. inermis and N. talangensis resemble a funnel (infundibular) and N. inermis is notable by the lack of a peristome. The lower pitchers of N. bongso, N. pectinata and N. spathulata are oval (ovoid) and the upper pitchers are trumpet-shaped. In other locations in Sumatra, both the lower and upper pitchers of N. spathulata are ovoid in the basal portion and cylindrical above as are the upper pitchers of N. pectinata (when produced), while the lower pitchers of both N. bongso and N. pectinata are variable in shape (Clarke 2001). Nepenthes bongso had the greatest stem diameter, stem length, internode length, peristome width and pitcher height. Nepenthes pectinata had the largest leaves and N. inermis had the smallest leaves, while N. talangensis had the greatest chlorophyll content compared to other species (Table 2). The pH of the pitcher fluid of N. bongso, N. inermis, N. spathulata and N. talangensis, was the same, namely between 4.3 and 4.5, whilst N. pectinata had a pH closer to neutral of 6.2. (Table 3).
Population
In the plot area of 0.27 ha, only two individuals of N. bongso and N. spathulata were recorded whereas we found six individuals of N. inermis, 36 individuals of N. pectinata and nine individuals of N. talangensis (Table 1). Outside the plots, we also found one individual of N. bongso and 14 individuals of N. talangensis. Although N. bongso, N. inermis and N. spathulata, have very small populations, these three species can be found in other locations in West Sumatra, therefore, their conservation status, according to the IUCN, is classified as Least Concern. On the other hand, N. pectinata is found quite abundantly on Gunung Talang and is widespread in West Sumatra and North Sumatra (Cheek and Jebb 2001; Mansur et al. 2022a, 2023).
The N. talangensis population is very small and confined to Gunung Talang, therefore, the IUCN classifies it as an Endangered (C2b) species as assessed in 2000 (Clarke et al. 2000). At the time of this study, the population of N. talangensis was very small and threatened, with only 23 individuals found in the study site (nine individuals in the plot, 14 individuals outside the plot), therefore, we suggest to increase the Red List status of the species to ‘Critically Endangered’ (A2a, B2a,b(v), C2b) with the necessity to carry out ex-situ conservation efforts by means of cultivation so that its population is maintained.
Habitat
At the study site, Nepenthes plants grew in primary forest with sloping to very steep topography on Andosol soils and the forest was slightly disturbed by humans. In a plot area of 0.27 ha, 35 tree species (Ø ≥ 5 cm) were recorded, which were dominated by Lithocarpus conocarpus (Fagaceae), Camellia lanceolata (Theaceae) and Syzygium acuminatissimum (Myrtaceae) with IVIs of 47.2, 34.6 and 27.1%, respectively (Table 4).
Distribution and associations
Within the three plots, N. inermis and N. talangensis generally grow in clusters (Median Morisita’s index was 5.5) while N. pectinata has a relatively broader and more randomly distribution (Morisita’s index = 1.4) (there were insufficient individuals of N. bongso and N. spathulata to examine their distribution) (Table 1 and Fig. 3). Nepenthes inermis grows epiphytically on fallen tree trunks in gaps with open canopies on hilltops or small cliffs. Nepenthes talangensis lives sympatrically with N. bongso and N. inermis with a high to very high index of association (Table 5), therefore, hybrid species among these species were found at the study site, namely N. talangensis × N. bongso and N. talangensis × N. inermis, also known as N. × pyriformis (Clarke 2001) (Fig. 4). The Ochiai Index showed that N. talangensis was positively associated with N. bongso and N. inermis as well as with other species such as Lithocarpus conocarpus and Podocarpus neriifolius (Table 5), it was strongly associated with Dehaasia sp. in Plot 1 but not Plot 2. In Plot 3, N. pectinata had positive associations with four species with a very high Ochiai index (Table 5). The clumped distribution of Nepenthes is likely due to similar habitat requirements for e.g. light or sufficiently moist soil. The more random distribution of N. pectinata suggests less precise habitat requirements which may also be a reason for its broader distributional range across Sumatra. Associations between species illustrate that they have a similar ecological niche, which can cause their spatial patterns to be clustered in a habitat (Mueller-Dombois & Ellenberg 1974). Positive associations could be due to similar requirements for (or responses to) a limiting resource, or through species enhancing each other’s fitness when in close association such as through facilitation (Mousaei Sanjerehei and Rundel 2020). In the case of the associations between N. talangensis and L. conocarpus and P. neriifolius this might be because of similar substrate requirements, lack of strong interspecific competition or through the trees enhancing growth of the Nepenthes plants; either way, further research on the possible reasons would be interesting.
Growth rates
Growth rates of the five species (and one hybrid) were not significantly different from one another (Leaves: F = 0.60, p = 0.70; Stem: F = 1.75, p = 0.20; Table 6); stem and leaf growth rates were correlated with each other across the taxa (r = 0.48, p = 0.044). This is in contrast to data from lowland forest of Central Kalimantan where it was found that different Nepenthes species had quite contrasting growth rates and it seemed that species that either produced longer stems with more leaves or produced thicker stems instead (Mansur and Brearley 2008).
Nutrient concentrations
Nitrogen (N) concentrations in the leaves of N. bongso, N. inermis, N. pectinata and N. talangensis, were generally low, with a mean of 1.14% and not significantly different between species; similarly phosphorus (P) and calcium (Ca) concentrations did not differ between species. Potassium (K) was greatest in N. pectinata whereas magnesium (Mg) was greater in N. inermis than N. bongso and sodium (Na) was greater in N. talangensis than N. bongso. The mean N:P ratio was 11.7 ± 1.74 and did not differ among species. These concentrations all fell within the range of those already reported in Mansur et al. (2021, 2022b). The most abundant elements in the pitcher fluid were K (albeit very variable) and Ca as found in previous studies (Buch et al. 2013; Mansur et al. 2021). Concentrations of K and Ca in N. inermis pitcher fluid were greater than in other species—possibly related to its highly viscous fluid (Salmon 1993).
Conclusions
We here describe the ecology of five species of Nepenthes found on Gunung Talang in Sumatra including spatial patterns and associations along with leaf and pitcher nutrient concentrations. Nepenthes talangensis is an endemic species found only on Gunung Talang which needs to be immediately conserved considering its very small population that is threatened by over-harvesting by collectors for commercial purposes. The habitat of Nepenthes on Gunung Talang is still in quite good condition although conservation efforts can be improved.
Data availability
Not applicable.
References
Buch F, Rott M, Rottloff S, Paetz C, Hillke I, Raessler M, Mithöfer A (2013) Secreted pitfall-trap fluid of carnivorous Nepenthes plants is unsuitable for microbial growth. Ann Bot 111(3):375–383
Cheek M, Jebb M (2001) Nepenthaceae. In: Flora Malesiana Vol. 15:1–157. Nationaal Herbarium Nederland, Universiteit Leiden Branch, The Netherlands
Cheek M, Jebb M, Mansur M, Beattie J (2017) Nepenthes dubia. Curtis Bot Mag 34(2):111–122
Chua LSL (1995) Conservation studies with Nepenthes macfarlanei Hemsl. in Peninsular Malaysia. PhD thesis, University of Bath, UK.
Clarke C (2001) Nepenthes of Sumatra and Peninsular Malaysia. Natural History Publications, Kota Kinabalu, Sabah, Malaysia
Clarke C, Cantley R, Nerz J, Rischer H, Wistuba A (2000) Nepenthes talangensis. The IUCN Red List of Threatened Species 2000: e.T39701A10256061. https://doi.org/10.2305/IUCN.UK.2000.RLTS.T39701A10256061.en. Accessed 26 June 2023.
Cross AT, Krueger TA, Gonella PM, Robinson AS, Fleischmann AS (2020) Conservation of carnivorous plants in the age of extinction. Glob Ecol Conserv 24:e01272
Damit A, Nilus R, Suleiman M (2017) Population structure and dispersion pattern of Nepenthes along the Kaingaran Trail of Mount Trus Madi, Sabah, Borneo. Sepilok Bull 25(2)6:1-11
Handayani T, Moro HKEP, Utami LB (2019) Association of herbaceous species on the sand dunes of Parangtritis Yogyakarta as biology learning resource. Adv Soc Sci Educ Humanit Res 422:242–245
Hernawati H, Zuhud EAM, Prasetyo LB, Soekmadi R (2022) Synopsis of Sumatran Nepenthes. Biodiversitas 23(8):4243–4255
Hernawati, Akriadi P (2006) A Field Guide to the Nepenthes of Sumatra. PILI-NGO Movement, Bogor, Indonesia
Lee CC, Hernawati AP (2006) Two new species of Nepenthes (Nepenthaceae) from North Sumatra. Blumea 51(3):561–568
Lee CC (2009) A preliminary conservation assessment of Nepenthes clipeata (Nepenthaceae). In: Lee CC, Clarke C (Eds) Proceedings of the 2007 Sarawak Nepenthes Summit, pp. 96–100. Sarawak Forestry Corporation, Kuching, Malaysia
Lidwina (2021) Deforestasi paling banyak di Sumatra dan Kalimantan. https://databoks.katadata.co.id/datapublish/2021/01/20/deforestasi-paling-banyak-terjadi-di-sumatera-dan-kalimantan. Accessed 13 Mar 2022
Ludwig JA, Reynold JS (1988) Statistical ecology: a primer on methods and computing. John Wiley and Sons, New York, USA
Mansur M (2013) Tinjauan tentang Nepenthes (NEPENTHACEAE) di Indonesia [A review of Nepenthes (NEPENTHACEAE) in Indonesia]. Ber Biol 12(1):1–7
Mansur M, Brearley FQ (2008) Ecological studies on Nepenthes at Barito Ulu, Central Kalimantan, Indonesia. J Teknol Lingkung 9(3):271–276
Mansur M, Brearley FQ, Esseen PJ, Rode-Margono EJ, Tarigan MRM (2021) Ecology of Nepenthes clipeata on Gunung Kelam, Indonesian Borneo. Plant Ecol Divers 14(3–4):195–203
Mansur M, Salamah A, Mirmanto E (2022a) Diversity of Nepenthes species in North Sumatra Province. Ber Biol 21(3):199–209
Mansur M, Salamah A, Mirmanto E, Brearley FQ (2022b) Nutrient concentrations in three Nepenthes species (Nepenthaceae) from North Sumatra. Reinwardtia 21(2):55–62
Mansur M, Salamah A, Mirmanto E, Brearley FQ (2023) Diversity, ecology and conservation status of Nepenthes in West Sumatra province, Indonesia. Biotropia 30(2):220–231
Margono BA, Turubanova S, Zhuravleva I, Potapov P, Tyukavina A, Goetz BA, S, Hansen MC, (2012) Mapping and monitoring deforestation and forest degradation in Sumatra (Indonesia) using Landsat time series data sets from 1990 to 2010. Environ Res Lett 7(3):034010
Moran JA, Clarke CM (2010) The carnivorous syndrome in Nepenthes pitcher plants: current state of knowledge and potential future directions. Plant Signal Behav 5(6):644–648
Morisita M (1959) Measuring of the dispersion and analysis of distribution patterns. Mem Fac Sci Kyushu Uni Ser E (Biol) 2(4):215–235
Mousaei Sanjerehei M, Rundel PW (2020) A comparison of methods for detecting association between plant species. Ecol Inform 55:101034
Mueller-Dombois D, Ellenberg H (1974) Aims and Methods of Vegetation Ecology. John Wiley and Sons, New York, USA
Ochiai A (1957) Zoogeographic studies on the soleoid fishes found in Japan and its neighbouring regions. Bull Jpn Soc Sci Fish 22(9):526–530
Osunkoya OO, Daud SD, Di-Giusto B, Wimmer FL, Holige TM (2007) Construction costs and physico-chemical properties of the assimilatory organs of Nepenthes species in northern Borneo. Ann Bot 99(5):895–906
Osunkoya OO, Daud SD, Wimmer FL (2008) Longevity, lignin content and construction cost of the assimilatory organs of Nepenthes species. Ann Bot 102(5):845–853
Phillipps A, Lamb A (1996) Pitcher-Plants of Borneo. Natural History Publications, Kota Kinabalu, Sabah, Malaysia
Puspitaningtyas DM, Wawangningrum H (2007) Keanekaragaman Nepenthes di Suaka Alam Sulasih Talang—Sumatera Barat [Nepenthes Diversity in Sulasih Talang Nature Reserve—West Sumatra]. Biodiversitas 8(2):152–156
Salmon B (1993) Some observations on the trapping mechanisms of Nepenthes inermis and N. rhombicaulis. Carniv Plant Newsl 22(1–2):11–12.
Shaukat SS, Hussain F, Zafar H, Rao TA, Mahmood K, Raza A (2014) Species composition, spatial heterogeneity, interspecific association and diversity of an early successional plant community: a comparison of some species association indices. Int J Biol Biotechnol 11(4):677–691
Sutomo D, Fardila PLSE (2011) Species composition and interspecific association of plants in primary succession of Mount Merapi. Indonesia Biodiversitas 12(4):212–217
Syaf R (2019) KKI Warsi: Sepanjang 2017–2019 deforestasi sumbar capai 23.352 ha. https://www.antaranews.com/berita/1220708/kki-warsi-sepanjang-2017-2019-deforestasi-sumbar-capai-23352-hektare. Accessed 13 Mar 2022
Whitten T, Damanik SJ, Anwar J, Hisyam N (1997) The Ecology of Sumatra. Periplus Editions (HK) Ltd., Hong Kong.
Wistuba A, Nerz J, Fleischmann A (2007) Nepenthes flava, a new species of Nepenthaceae from the northern part of Sumatra. Blumea 52(1):159–163
Zulkarnaen RN, Peniwidiyanti RRR, Helmanto H, Wanda IF (2017) Struktur dan asosiasi komunitas tumbuhan bawah di Resort Cikaniki, Taman Nasional Gunung Halimun Salak [Structure and associations of understorey plants at Cikaniki resort, Gunung Halimun Salak National Park]. J Ilmu Alam Lingkung 8(2):21–30
Acknowledgements
This research was funded by grants from Beauval Zoo (France) and Chester Zoo (UK). Thanks to Phillip Esseen, Paul Leach, Nicolas Leroux, Johanna Rode-Margono and Stuart Young for help and support of this research. Special thanks to Sunardi, Heru Hartantri, Yusran E. Ritonga, Putra, Rhima and Daniel Ferdinan for assistance during the fieldwork. This paper is a part of the Ph.D. dissertation of the first author.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mansur, M., Salamah, A., Mirmanto, E. et al. Ecology of Nepenthes on Mount Talang, West Sumatra, Indonesia. Trop Ecol (2024). https://doi.org/10.1007/s42965-024-00333-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42965-024-00333-0