Abstract
During the last years, much of the diversity studies of myxomycetes (plasmodial slime molds) have been concentrated mostly in the Southern region of Vietnam. Moreover, information on leaf litter inhabiting myxomycetes for the country is still in scarcity. Hence, this study aims to assess the occurrence and distribution of leaf litter inhabiting myxomycetes in different forest types in the subtropical northern and coastal tropical monsoon central part of the country. Samples of aerial and ground leaf litter that were used to prepare moist chamber cultures in the laboratory were collected in (1) Ba Vi National Park, Ha Noi, (2) Ho Nui Coc, Thai Nguyen, and (3) coastal forest patches in Da Nang. A total of 24 species belonging to 10 genera, wherein the majority of these myxomycete species appeared abundantly (11 species) is reported for this study. Based on species richness, Ha Noi harbored the highest number of myxomycete species. Leaf litter inhabiting myxomycete communities between aerial and ground substrates shared a high level of similarity based on their species composition and relative abundance. Highest level of similarity of leaf litter inhabiting myxomycete asssemblages is also reported between Ha Noi and Da Nang (CC = 0.78, PS = 0.56). This research study is the first step in understanding the complex myxomycete ecology of leaf inhabiting myxomycetes and would help filling now the large gap in one of the unexplored tropical areas of the world.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With its unique and diverse topographic, climatic and hydrographic conditions, Vietnam is recognized as one of the mega hotspot for biodiversity around the world. This is evident from the high degree of landscape-level variation among ecoregions in the country ranging from tropical rain forests and mangrove habitats to marine ecosystems. Furthermore, this is also the home of many endemic species inhabiting the diverse terrestrial and aquatic habitats. Currently, a hot topic for the country is the exploration of its rich biodiversity since it is quite clear that biodiversity indeed plays a very important role in contributing to the direct benefits for people and national economy of the country, especially in the field of agriculture, forestry and aquatics. This is also the basis for ensuring food security and maintaining the genes that builds new species of plants and animals. Comparing the large number of records of plants and animals in Vietnam, a great gap about the country’s microbial diversity is very evident. Microorganisms like saprophytic organisms have an important influence on the decaying process in nature (Lonsdale et al. 2008). Thus, assessing the biological diversity of these microorganisms is very essential, since they play a vital role in maintaining the balance in soil biota (Stephenson et al. 2011; Dagamac et al. 2017a). One of the microorganism groups that play the aforementioned roles are the unknown group of terrestrial fungus-like protists called the myxomycetes.
Myxomycetes or plasmodial slime molds, are small group of amoeboid protists which is abundant in many terrestrial ecosystems (Massingill and Stephenson 2013; Schnittler et al. 2017a) with ca. 1000 morphological species described worldwide. The life cycle of myxomycetes can be divided into two trophic stages: microscopic and macroscopic stage. Everhart and Keller (2008) described the first stage of myxomycetes having a uninucleated amoebae and the second stage called the plasmodium with a multinucleated structure. Interestingly, under unfavorable environmental conditions, the plasmodium can develop into delicate and colorful fruiting bodies containing haploid spores (Schnittler and Mitchell 2000). Studies about myxomycetes in the tropical Southeast Asia are steadfastly increasing during the last decades. Some previous countries in the region that have information for myxomycete studies are the Philippines with 159 species (Dagamac and dela Cruz 2015; Macabago et al. 2017; Bernardo et al. 2018), Thailand with 145 (Ko Ko et al. 2010; Dagamac et al. 2017b), Indonesia with 119 (Farr 1990; Rosing et al. 2011), Republic of Singapore with 76 (Rosing et al. 2011), Myanmar with 67 (Ko Ko et al. 2013a), and Laos with 44 (Ko Ko et al. 2013b). In Vietnam, most of the myxomycete studies have been investigated in the southern part of the country (Novozhilov et al. 2018, 2020) while the number of myxomycete studies on the other parts of the country is relatively scarce. Furthermore, the first myxomycete report for Vietnam started only in 2009 that initially annotated 23 species by Van Hooff (2009), wherein the rare species Cribraria tecta was reported as a new species for science. Then, two new additional species named Diderma cattiense and Diderma pseudotestaceum were reported by Novozhilov et al. (2014). This is then followed by an ecological study in three lowland tropical forests in Vietnam by Tran et al. (2014). A comprehensive biodiversity assessment of myxomycetes conducted in southern Vietnam was then reported by Novozhilov et al. (2017) which accounted for an additional 69 new records for Vietnam. Two new papers that reported about myxomycetes association with agricultural litter (Redeña-Santos et al. 2017) and comparisons of myxomycete assemblages in protected and unprotected plantation forest (Redeña-Santos et al. 2018) in Thai Nguyen, Northern Vietnam resulted to additional five new records for Vietnam. Stephenson et al. (2019) have reported the presence of Barbeyella minutissima, the rare temperate myxomycetes that are commonly associated with Picea trees, on higher elevation areas of Southern Vietnam. But perhaps, the recent publication of Novozhilov et al. (2020) about the long term systematic survey in Bidoup Nui Ba and Chu Yang Sin national parks is the most extensive work conducted for the country so far with 105 taxa being reported, forty-two of which are new records for Vietnam including a Badhamia species suspected to be new to science. All these efforts now amounted to 174 myxomycetes species for Vietnam.
In spite of these rapid increase of myxomycete records for Vietnam, no profiling of myxomycete species in terms of comprehensive comparison on leaf litters on different types of forest in northern and coastal central Vietnam, the regions with rich and diversified vegetation, and contrasting seasonality, have been so far reported. In addition, biodiversity of Vietnam is facing many potential threats due to the increasing habitat loss, population growth and climate change. A large area of forest is now destroyed to make space for economic development. Illegal logging, hunting and mining lead to a dramatic decrease in the biodiversity of the country. This also causes many changes in the diversity not only with the country’s flora and fauna but also with its many unexplored microbial communities, particularly the leaf litter inhabiting myxomycetes. A baseline study focusing on underexplored forest areas in the country is deemed necessary especially on the threats of biodiversity in the country. Many undiscovered records of myxomycetes need to be annotated for further addition of biogeographic information about the global distribution of this unfathomable organism. Therefore, this study wants to answer the following questions: (a) What are the myxomycetes species found on forest leaf litter in the North and the central part of Vietnam? and (b) What are the (i) occurrences and (ii) species diversity of these myxomycetes species in terms of substrates and collecting localities?
Materials and methods
Study area
Vietnam is situated in the tropical Southeast Asian region, but the whole country is divided into three regions along its length: the North with humid subtropical climate, the coastal Centre and the South with monsoon tropical climate (Tran et al. 2014). In this study, two localities in the North including Ha Noi and Thai Nguyen and one locality in the coastal Centre were selected (Fig. 1). Five collecting points were randomly chosen for each area summing up a total of 15 collecting points used for this research study.
Area 1 (Ha Noi): samples were collected in Ba Vi National Park which is 50 km to the West of central Ha Noi, at 21° 55′ N and 105° 18′ E. Ba Vi is a low mountain with the average elevation of 400 masl and has the total natural area of 10,814 ha. It has typically humid subtropical climate with two clear seasons: cold and dry winter from November to March; hot, humid and rainy summer from April to October and is influenced by the monsoon mechanism. The vegetation is diversed and typical for the subtropical evergreen forest (https://vuonquocgiabavi.com.vn). In general, this is a quite steep mountain with the average of 25 °C. The diverse vegetation appears in different forest layers. The covering canopy only appears in moist areas. These woody trees are very rare, mostly are remnants of the former primary forest, such as Castanopsis sp., Dracontomelon duppreanum, Quercus sp. The dominant woody tree layer includes sparse woody trees with the average height between 14–25 m, but they do not exist continuously. There are some common species like Alangium kurzii, Aphananthe aspera, Castanopsis sp., Cinnamomum sp., Claoxylon indicum, Cryptocarya sp., Dracontomelon duperreanum, Ficus tristylis, Macaranga denticulata, Magnolia balansae, Mallotus paniculatus, Schefflera sp., Syzygium sp.,Vernicia montana, Wrightia sp. The shrub layer is also sparse with uneven growth. On the forest floor, there are different kinds of grasses and mosses that cover the forest floor.
Area 2 (Thai Nguyen): Samples were collected within the forested vicinities of Coc Lake- plantation forest with the main purpose of wood production through logging. However, illegal logging activities of local people are still taken place. This area is 16 km far from the Centre of Thai Nguyen city to the East and 96 km from Ha Noi to the North, at 21° 34′ N and 105° 41′ E with the total natural area of 11,283 ha, at the average elevation of 150–200 masl (https://vietnamtourism.gov.vn). Similar to Ba Vi National park, this area also has the subtropical climate with two distinct seasons: cold and dry winter from November to March, hot and rainy summer from April to October. There are a large proportion of protected plantation forests with two main species Acacia mangium and Acacia auriculiformis (https://honuicoctourism.gov.vn).
Area 3 (Da Nang): is a coastal tourism city in the Centre of Vietnam, which is located in 15° 55′ N and 107° 18′ E, one side heads to the Pacific Ocean and the other sides are mountainous. Da Nang is 765 km far from Ha Noi to the North, and 964 km to Ho Chi Minh City in the South. It is in tropical monsoon climate with high annual temperature. There are two distinct seasons: the dry season from January to September, the rainy season from October to December. This area is well-known for the natural forest near the coast named Son Tra, which is 10 km from Da Nang Centre to the North, three sides heading to the sea and one side heading to the urbanized Da Nang (https://danang.gov.vn). The samples here were collected in the lowland natural tourism areas along the coastal city (one sites), and four sites in a small island called Cu Lao Cham, which is about 35 km from central Da Nang.
Substrate collection and preparation of moist chamber cultures
Samples were randomly collected at five points for each locality between February and April 2017. On each site, 10 ground leaf litters which are the leaf substrates being in a direct contact with the soil environment; and 10 aerial leaf litters which are leaf substrates not being in a direct contact with the soil were carefully picked up and put in dry papers. The geographical coordinates were identified by a GPS device. In some cases when GPS reading was difficult to obtain, recording points were referenced using Google Earth. In total, 300 substrate samples were used for moist chamber cultures in the laboratory. Moist chamber cultures have been used already in many studies of myxomycetes in the tropics (Macabago et al. 2010; Alfaro et al. 2015; Dagamac et al. 2015a) especially for practicality reason wherein finding myxomycetes fruiting in the field is such a challenging feat. The moist chamber cultures were set-up following the protocol of Stephenson and Stempen (1994). Air dried leaf substrates were divided into tiny pieces (smaller than 9 cm length) and was placed above 3 thin layers of soft filter papers in Petri dishes of 9 cm diameter. Substrates were then soaked with distilled water and then were left covered overnight. After 24 h, pH of each petri dish was checked at three points after pouring off excess water. Then the samples were incubated in an ambient light condition and in room temperature for almost 12 weeks. Regular checking was implemented after 7, 14, 25, 45, 60, 67 days. Within the incubation, distilled water was regularly added when the samples became dry so that the moist conditions can be maintained.
Collection of fruiting bodies and species determination
The appearance of the fruiting body of myxomycetes was noted. Initial description of the characteristics including type, shape, and presence of lime, color or height was identified by using the stereomicroscope (Zeiss Stemi DV4). Each specimen was determined using the published literature (Poulain et al. 2011) and the web-based identification key (https://slimemold.uark.edu/) current valid names were counter-checked using an online nomenclatural database for the eumycetozoans (https://nomen.eumycetozoa.com). Then, the pre-identified matured fruiting bodies were put in match boxes, cooled up in the freezer for up to 24 h and then transferred to the herbarium of the university.
Microscopic examinations were also conducted to further verify the identity of some specimens. For those specimens which are enough to identify as a species but holding some difficulties to identify with a strong certainty, the abbreviation “cf” was added in the determination. Then a small amount of matured fruiting bodies was taken and transferred to a glass slide with a drop of either the Hoyer’s medium or lactophenol. After that, this piece of fruiting body was carefully crushed to exposed microscopic structures such as the spore morphology and capillitial threads. They were preserved within one day or until all the spores became accurately round. The prepared microscopic slides were then observed using a Leica DFC450 compound light microscope.
Data analysis
According to the calculation of percentage yield of Dagamac et al. (2012), moist chambers having plasmodia and/or fruiting bodies were considered as positive records for myxomycetes. Abundance index calculated for each species was in accordance to the categories first proposed by Stephenson et al. (1993) which is based upon the rate of each species to the total number of records: R-rare (< 0.5%), O-occasional (between 0.5 and = 1.5%), C-common (1.5–3%), A-abundant (> 3%). To estimate the survey completeness, the freely downloadable software Estimate S (Version 9.1, 100 randomizations) was used to construct the species accumulation curves (SAC). The Chao 1 estimators, which targets species richness for individual based data, was utilized in accordance with the protocols of Macabago et al. (2017) and Novozhilov et al. (2017). The percentage exhaustiveness of each study site and each substrate was calculated by the ratio of the actual species numbers recorded for that location or that substrate and the mean value of species expected by Chao 1 estimator.
To calculate the different indices of species diversity the software SPADE (Species Prediction and Diversity Estimation) from Chao and Shen (2003) were utilized. The α diversity expressed as Fisher (FIS) indices for species richness, and two heterogeneity index namely Shannon (SHA) and Simpson (SIM) (both species richness and evenness), were generated. Moreover, the taxonomic diversity index (S/G ratio) was computed by dividing the total number of species with the total number of genera recorded. The lower the S/G ratio, the more diverse a particular biota is considered according to the concept of Magurran (2004). For the β diversity the assemblages of myxomycetes associated with different areas and different substrates were compared by using Sorensen’s Coefficient of Community (CC) and the Percentage Similarity (PS) indices. Coefficient of Community (CC) is an index using only the presence or absence of species in the two comparing communities and is computed by following this equation:
where a = the number of shared species between two communities, b = the number of species that only appears in the first community, c = the number of species that only appears in the second community. The coefficient of community (CC) values range between 0 and 1. The value of CC is 0 when there is no common species between two communities, or the highest value is 1 when both communities share all common species. Meanwhile, the Percentage Similarity (PS) index considers both their relative abundance and the presence or absence of species. The PS index was calculated using the following equation:
Result
General data
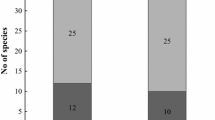
A total of 247 (82%) moist chambers out of 300 were positive for myxomycetes. From these 247 positive moist chambers, 61 (17%) plasmodia and 305 (83%) fruiting body records are herein reported. Comparing the yield of the moist chambers from different collecting localities, Da Nang gave the highest percentage followed by Ha Noi and Thai Nguyen with 86%, 84% and 77%, respectively. For the whole study, a total of 22 morphospecies belonging to 10 genera were determined (Table 1). Additionally two forms (dwarf form, Schnittler 2000; yellow form, Dagamac et al. 2017b) of the genus Arcyria was accounted for this study amounting now a total of 24 myxomycetes species. The calculated relative abundance showed 11 abundant species (Diderma hemisphaericum, Diachea leucopodia, Didymium squamulosum, Arcyria cinerea, Physarum echinosporum, Perichaena vermicularis, Physarum cinereum, Perichaena chrysosperma, Perichaena pedata, Diderma effusum and Physarum melleum). Seven species were common (Physarum tenerum, Arcyria cinerea-dwarf, Didymium nigripes, Perichaena depressa, Arcyria margino-undulata, Physarum globuliferum, Arcyria cinerea-yellow). Four species were recorded to be occasional (Lamproderma scintillans, Craterium rubronodum, Physarum pusillum, Physarum bitectum), and 2 rare species that appeared only in the national park of Ha Noi are Stemonitopsis aequalis var. microspora and Trichia papillata (Fig. 2).
Diversity of myxomycetes between aerial and ground substrates
Using EstimateS, the rarefaction curve (Fig. 3a) constructed for this research study showed a higher number of species for ground substrates (23.0) than aerial substrates (20.5). There were 21 species belonging to 9 genera in aerial litter and 23 species belonging to 9 genera in ground litter. Comparing the taxonomic diversity index between the two different substrates, aerial substrates (S/G = 2.33) was found to be more taxonomically diverse than ground substrates (S/G = 2.55). In terms of α diversity (Table 2), FIS (species richness index only) and SHA (heterogeneity index) are higher in ground litter (7.75 and 2.76) than aerial litter (6.43 and 2.58), respectively. However, the more intuitive SIM index (also a heterogeneity index) showed an opposite trend, by having higher result for aerial litter (0.1) than ground litter (0.08). Nonetheless, all indices have no statistical difference between each other (P > 0.05, diversity t-test). The Venn diagram constructed for two substrate types of aerial and ground shared 20 species. One species found appearing exclusively in only aerial substrate was Stemonitopsis aequalis var. microspora, and three species appearing exclusively in ground substrates were Physarum bitectum, Physarum pusillum and Trichia papillata. A calculation of the similarity index resulted to a high coefficient of community (CC) index and relatively high percentage similarity (PS) index with 0.90 and 0.64 values, respectively (Fig. 4a).
Diversity of myxomycetes among the three localities
Using the rarefied values, the expected numbers of myxomycete species (Chao 1) for Ha Noi, Thai Nguyen and Da Nang were 18.00, 16.86 and 14.85, respectively (Fig. 3b). In terms of taxonomic diversity (Table 2), Ha Noi harbored 18 species belonging to 10 genera, Thai Nguyen hosted 17 species belonging to 7 genera, and Da Nang had 15 species belonging to 7 genera. Therefore, the highest taxonomic diversity index (the S/G ratio) was exhibited in Ha Noi (1.80), followed by Da Nang (2.14) and the lowest taxonomic diversity index was in the plantation forest in Thai Nguyen (2.42). In terms of α diversity (Table 2), the highest value for Fisher (FIS) (species richness index only) was Ha Noi, at 6.77 and the lowest value was Da Nang at 4.73. Two heterogeneity values including Shannon (SHA) and Simpson (SIM) exhibited the opposite trend. For example the SHA index was highest in Ha Noi (2.61), followed by Thai Nguyen (2.47), and lowest in Da Nang (2.42). However, the SIM index was highest in Da Nang (0.12), and lowest in Ha Noi (0.08). Similarly to the myxomycete comparison between 2 substrates, these indices in terms of localities also do not have statistical difference among each other (P > 0.05, diversity t-test). Comparing the similarities of myxomycete communities among those areas (Fig. 4b), Ha Noi and Da Nang (CC: 0.78, PS: 0.56) have higher species shared in comparison to Da Nang and Thai Nguyen (CC = 0.63, PS = 0.57) and Thai Nguyen and Ha Noi (CC = 0.62, PS = 0.42).
Discussion
This rapid assessment of myxomycete diversity focusing on leaf litter inhabiting myxomycetes serves as baseline information for understanding myxomycete ecology and distribution in Vietnam. In spite of limiting challenges on field collections, the result presented herein contributed to the ongoing biodiversity studies about this interesting group of fungus-like protist.
Moist chamber techniques as a method to evaluate myxomycete diversity
A total of 247 (82%) moist chambers that were positive with myxomycetes indicated a relatively high productivity of moist chambers (MC) used in this whole survey. This result then confirms the effectiveness of moist chamber technique for many myxomycete surveys that have been already conducted in the tropical areas where fructifications is quite challenging and a daunting task to find in the field. For instance, there are many case studies conducted in different national parks in the Philippines, wherein most of them showed a comparable number of high percentage yields i.e. Mt. Makulot National Park (84%, Cheng et al. 2013), Quezon National Park (82%, Dagamac et al. 2015b), Puerto Princesa Subterranean River National Park (70%, Pecundo et al. 2017). The recent papers about myxomycetes in heterogenous plantation forests (Redeña-Santos et al. 2018) and monotypic agricultural lands (Redeña-Santos et al. 2017) in Thai Nguyen, northern Vietnam reported high values of 87% and 90%, respectively. Another paper from Schnittler et al. (2015) also reported a high percentage of positive moist chamber (76%) to reveal myxomycetes and myxomycete-like organism (MMLO) in Oman. In contrast, this percentage yield is much higher compared to the one reported in some recent studies in the Philippines in a man-made ecopark (Macabago et al. 2010) and in an anthropogenic disturbed lowland mountainous forest (Dagamac et al. 2011, 2014) with only 51% and 23%, respectively. Thus, in the tropical and subtropical forest, where the fructifications are quickly decayed or can be washed off completely by the heavy rains, the technique of using moist chamber cultures in the laboratory is still considered an effective method (Stephenson et al. 1993; Corpuz et al. 2012; Dagamac et al. 2012; Tran et al. 2014; Carascal et al. 2017). However, an investigation by Novozhilov et al. (2017) implemented in southern Vietnam using both moist chamber technique and fieldwork stated that 29 species found in the field could not be present in the moist chambers, or 47 species found in the moist chamber could not be easily found in the field. In another related studies in Neotropics i.e. Costa Rica (see Rojas et al. 2010, 2011, 2012) and El Salvador (Rojas et al. 2013) the moist chamber and field survey was combined to establish comprehensive diversity assessments of myxomycetes. Therefore, integration of both moist chamber technique and fieldwork component is recommended to have sufficient results about myxomycetes diversity.
Myxomycetes occurrences in leaf litter of northern Vietnam
A total of 24 determinable species belonging to 10 genera (Table 1) of leaf litter inhabiting myxomycetes are reported in this research. Comparing this number of leaf litter inhabiting myxomycetes from other studies, this result is relatively higher than other well-surveyed forest patches in tropical Southeast Asian landscapes like in a karst forest (21 species, Dagamac et al. 2015b) or volcanic soils (19, Rea-Maminta et al. 2015). In contrast, this number is much lesser than leaf litter inhabiting myxomycetes from warm temperate forest patches (48, Takahashi 2013). Furthermore, species categorized as abundantly occurring in this study such as Diderma hemisphaericum (Bull.) Hornem, Arcyria cinerea (Bull.) Pers., Didymium squamulosum (Alb. and Schwein.) Fr., Physarum cinereum (Batsch) Pers. and Diderma effusum (Schwein.) Morgan was previously reported as cosmopolitan in distribution. Nonetheless, we see as well differences in terms of occurrences from different myxomycete species reported in this study since assemblages of myxomycetes are expected to vary considerably from one place to another (Eliasson and Nannenga-Bermekamp1983). Interestingly, this study reported only two rare species namely, Stemonitopsis aequalis var. microspora (Lister) Nann-Bremek. and Trichia papillata Adamonyte. The species Stemonitopsis aequalis var. microspora was first reported in Japan in 1972 and was later found as a new record in Turkey (Ergül and Dulger 2000) but was never found in any studies in tropical Southeast Asia. Recently, this rare species is first reported in Vietnam by Redeña-Santos et al. (2018). Moreover, from this survey, Trichia papillata is reported once again as a record for Vietnam. Trichia papillata was first obtained from the moist chamber cultures of hare (Lapus sp.) and roe deer (Capreolus sp.) dung collected in southern Lithuania (Adamonyte 2003), and some years later, this species was once again reported in plant litter of Taiwan (Liu et al. 2007). This species is initially considered as truly coprophilous, which is known from very few collections in the world. Novozhilov et al. (2020) first reported this species for the county and that is also noteworthy to mention that the species Trichia pappilata is only reported in Vietnam in comparison among other countries in the Southeast Asian region.
Comparing the diversity of myxomycetes between aerial and ground leaf litters
Taking into account the three measurements of species diversity (Table 2) used in this study, our result showed surprisingly no clear trend between the two substrates. Perhaps, this is attributable to factors of sampling and should be treated as a case to case basis. Nevertheless, when comparing the species richness between the two types of substrate, this finding is in contrast with some previous studies in the tropics that reported higher species richness found on ground leaf litter than aerial leaf litter. For example, Dagamac et al. (2015b) examined the distribution and occurrence of myxomycetes in tropical Karst forest showing 19 species inhabiting aerial leaf litter and only 10 species inhabiting ground leaf litter. Other studies of Schnittler and Stephenson (2002) and Rojas et al. (2014) also showed that in tropical rain forest, aerial litters have different levels of moisture and it has a better ability to dry out after rain, which may provide better conditions for myxomycetes (Rojas et al. 2014; Dagamac et al. 2015b). Meanwhile, the ground substrates tend to remain relatively moist, which encourages the development of fungi and limit the growth of myxomycetes. Albeit speculative, it can be stated that in subtropical rain forest, with the close canopy, this can be a challenge for the dispersal ability of myxomycete spore. Instead of dispersing in a long distance, such as the spores disperse up to 1.8 km by a slight breeze or theoretically up to 500 km by a storm with a speed of 100 km/h (Schnittler et al. 2006), perhaps spores can be washed away from above the ground (aerial substrates), causing them to drop on the ground substrate. Since the soil contains high microbial communities (Stämmler et al. 2016; Epelde et al. 2017) with a mantle of dead organic matter in the ground, it becomes an ideal environment for myxomycetes. In terms of the similarities of myxomycete assemblages between aerial and ground substrates both substrates shared 20 species (CC = 0.9, PS = 0.64), showing that many species appearing on aerial leaf litter are also found on the ground leaf litter. This indicates that the species found on both substrates are nearly overlapping (Fig. 4). It is important to note that the only 4 exclusive species (aerial: Stemonitopsis aequalis var. microspora; ground: Physarum bitectum, Physarum pusillum and Trichia pappilata) that are reported in this study were also the rarely or occasionally occurring species which means there will be a difficulty to ascertain whether these species are really exclusive for the abovementioned leaf substrates.
Distribution of myxomycetes among three different collecting localities
Among the three localities investigated in this study, Ba Vi National Park hosted the highest number of species (Fig. 3) compared to the plantation forest (Thai Nguyen) and the tourism forest patches (Da Nang). Similar to some previous studies, Dagamac et al. (2017b) noted higher species in the lowland forest with higher vegetation types, and the forest cover is interrupted by emergent trees and tree fall gaps. Likewise, Takahashi (2013) also suggested higher species in even mixed green forest compared to pure bamboo or homogenous forest. Similarly, Tran et al. (2006) also reported higher species richness in diverse litter than in uniform litter. This also follows the finding of Rojas and Stephenson (2012), that forest structure may form the myxomycete occurrence in tropical forests. Therefore, high level of plant heterogeneity presented the highest number of species like in the case of Ba Vi National Park, Ha Noi. Interestingly, the protected plantation forest of Thai Nguyen hosted a relatively high number of species, wherein four species (Physarum bitectum, Physarum globuliferum, Physarum pusillum and Physarum tenerum) were reported to be exclusive for its area. Comparing this to the findings of Redena-Santos et al. (2017) on selected agricultural leaf litters in the same province, two of these four exclusive species Physarum tenerum and Physarum pusillum was also found here as a common and occasionally occurring species, respectively, while they are noted to be an occasional species in other Southeast Asian countries. The other two Physarum bitectum, P. globuliferum seem to be suitably occurring for this plantation forest in Thai Nguyen. Perhaps, the leaf litters of Acacia mangium and A. auriculiformis can be a suitable microhabitat for some myxomycete specialists. This speculation about association of myxomycetes with plant species of Vietnam is still not fully understood and may warrant further investigation in the future.
Implications and outlook in Vietnam’s biodiversity
Myxomycete biodiversity in Vietnam is such a budding topic to explore. With many threats of habitat loss, population growth, and climate change in Vietnam, it necessitates to annotate many undiscovered myxoymycetes for the country. The results of this study implicates that at a local scale and focused substrate type, we already see a number of morphospecies that can be subjected to rapid assessment of diversity and the discovery of quite rare new records of myxomycetes species. However, with the many modern molecular approach applied nowadays to many biodiversity studies in other organisms such as in birds (Quek et al. 2018) or plants (Xu et al. 2018), applying it to myxomycetes particularly in Vietnam will help disentangle the real hidden diversity of myxomycetes (Shchepin et al. 2019). Similar with other studies that merges barcoding techniques (Feng and Schnittler 2017; Shchepin et al. 2017; Schnittler et al. 2017b) or species distribution modelling (Almadrones-Reyes and Dagamac 2018) with field surveys, it would be such a promising feat for myxomycete diversity in Vietnam to incorporate these modern ecological tools. Nonetheless, this research study is the first step in understanding the complex myxomycete ecology in particular the leaf inhabiting myxomycetes.
References
Adamonyte G (2003) Trichia papillata, a new coprophilous myxomycete species. Mycotaxon 87:379–384
Alfaro JRA, Alcayde DLIM, Agbulos JB, Dagamac NHA, dela Cruz TEE (2015) The occurrence of myxomycetes from a lowland montane forest and agricultural plantations of Negros Occidental, Western Visayas, Philippines. Fine Focus 1:7–20
Almadrones-Reyes KJ, Dagamac NHA (2018) Predicting local habitat suitability in changing climate scenarios: applying species distribution modelling for Diderma hemisphaericum. Curr Res Environ Appl Mycol 8:492–500
Bernardo JLM, Arioder JLQ, Almadrones-Reyes KJ, Dagamac NHA (2018) Myxomycetes communities occurring in fragmented forest patches in two municipalities of Laguna, Philippines. Community Ecol 19:289–299
Carascal MB, Rea MAD, Dagamac NHA, dela Cruz TEE (2017) Myxomycetes associated with grassland litter in the Philippines. Curr Res Environ Appl Mycol 7:56–63
Chao A, Shen T (2003) User’s guide for program SPADE (species prediction and diversity estimation). https://chao.stat.nthu.edu.tw. Accessed 18 Mar 2018
Cheng CBT, Yu KNT, Campos ML, Adora JM, Pascua GCP, Pangilinan MVB, Buaya AT, dela Cruz TEE (2013) Occurrence and Diversity of Myxomycetes (Plasmodial Slime Molds) along the Northern Slope of Mt. Makulot, Cuenca, Batangas, Philippines. Asian J Biodivers 4:65–83
Corpuz IR, Martinez CC, Petilla KN, Baranda JC, Buaya A, dela Cruz TEE (2012) Occurrence and diversity of myxomycetes (plasmodial slime molds) in Mt. Palay-palay National Park, Cavite, Philippines. Acta Manilana 60:57–65
Dagamac NHA, dela Cruz TEE (2015) Myxomycetes research in the Philippines: updates and opportunities. Mycosphere 6:784–795
Dagamac NHA, dela Cruz TEE, Pangilinan MVB, Stephenson SL (2011) List of species collected and interactive database of myxomycetes (plasmodial slime molds) for Mt. Arayat National Park, Pampanga Philippines. Mycosphere 2:449–455
Dagamac NHA, Stephenson SL, dela Cruz TEE (2012) Occurrence, distribution and diversity of myxomycetes (plasmodial slime moulds) along two transects in Mt. Arayat National Park, Pampanga, Philippines. Mycology 3:119–126
Dagamac NHA, Stephenson SL, dela Cruz TEE (2014) The occurrence of litter myxomycetes at different elevations in Mt. Arayat National Park, Pampanga, Philippines. Nova Hedwigia 98:187–196
Dagamac NHA, Rea-Maminta MAD, Batungbacal NS, Jung SH, Bulang CRT, Cayago AGR, dela Cruz TEE (2015a) Diversity of plasmodial slime molds (myxomycetes) in coastal, mountain, and community forests of Puerto Galera, Oriental Mindoro, the Philippines. J Asia-Pacific Biodivers 8:322–329
Dagamac NHA, Rea-Maminta MAD, dela Cruz TEE (2015b) Plasmodial Slime Molds of a Tropical Karst Forest, Quezon National Park, the Philippines. Pac Sci 69:411–422
Dagamac NHA, dela Cruz TEE, Rea-Maminta MAD, Aril-dela Cruz JV, Schnittler M (2017a) Rapid assessment of myxomcete diversity in the Bicol Peninsula, Philippines. Nova Hedwigia 104:31–46
Dagamac NHA, Novozhilov YK, Stephenson SL, Lado C, Rojas CA, dela Cruz TEE, Unterseher M, Schnittler M (2017b) Biogeographical assessment of myxomycete assemblages from Neotropical and Asian Palaeotropical forest. J Biogeogr 44:1524–1536
Eliasson U, Nannenga-Bremekamp NE (1983) Myxomycetes of the Scalesia Forest, Galápagos Islands. Mycol Proc C 86:143–153
Epelde L, Lanzén A, Mijangos I, Sarrionandia E, Anza M, Garbisu C (2017) Short-term effects of non-grazing on plants, soil biota and aboveground-belowground links in Atlantic mountain grasslands. Sci Rep 7:1–11
Ergül C, Dulger B (2000) A New Myxomycetes Genus Record for Turkey (Stemonitopsis (Nann.-Brem.). Turkish J Bot 24:355–357
Everhart SE, Keller HW (2008) Life history strategies of corticolous myxomycetes: the life cycle, plasmodial types, fruiting bodies, and taxonomic orders. Fungal Divers 29:1–16
Farr ML (1990) Contributions toward a Mycobiota of Indonesia: Hypocreales, synnematous hyphomycetes, Aphyllophorales, Phragmobasidiomycetes, and Myxomycetes. Mem NY Bot Gard 59:169–171
Feng Y, Schnittler M (2017) Molecular or morphological species? Myxomycete diversity in a deciduous forest in northeastern Germany. Nova Hedwigia 104:359–380
Ko Ko TW, Hanh TTM, Stephenson SL, Mitchell DW, Rojas C, Hyde KD, Lumyong S (2010) Myxomycetes of Thailand. Sydowia 62:243–260
Ko Ko TW, Rosing WC, Ko Ko ZZW, Stephenson SL (2013a) Myxomycetes of Myanmar. Sydowia 65:267–276
Ko Ko TW, Tran HTM, Clayton ME, Stephenson SL (2013b) First records of myxomycetes from Laos. Nova Hedwigia 96:73–81
Liu CH, Chang JH, Yang FH (2007) Myxomycetous Genera Perichaena and Trichia in Taiwan. Bot Stud 48:91–96
Lonsdale D, Pautasso M, Holdenrieder O (2008) Wood-decaying fungi in the forest: conservation needs and management options. Eur J Forest Res 127:1–22
Macabago SAB, Dagamac NHA, dela Cruz TEE (2010) Diversity and distribution of plasmodial myxomycetes (Slime molds) from La Mesa Ecopark, Quezon City, Philippines. Biotropia 17:51–61
Macabago SAB, Dagamac NHA, dela Cruz TEE, Stephenson SL (2017) Implications of the role of dispersal on the occurrence of litter-inhabiting myxomycetes in different vegetation types after a disturbance: a case study in Bohol Islands, Philippines. Nova Hedwigia 104:221–236
Magurran AE (2004) Measuring biological diversity. Blackwell Science, Oxford
Massingill JM, Stephenson SL (2013) Myxomycetes appearing in moist chamber cultures on samples of bark and wood collected from coarse woody debris. Mycosphere 4:627–633
Novozhilov YK, Mitchell DW, Okun MV, Shchepin ON (2014) New species of Diderma from Vietnam. Mycosphere 5:554–564
Novozhilov YK, Erastova DA, Shchepin ON, Schnittler M, Alexandrova AV, Popov ES, Kuznetsov AN (2017) Myxomycetes associated with monsoon lowland tropical forests in southern Vietnam. Nova Hedwigia 104:143–182
Novozhilov YK, Shchepin ON, Alexandrova AV, Popov ES, Dagamac NHA (2018) Altitudinal patterns of diversity of myxomycetes (Myxogastria) across tropical forests of Southern Vietnam. Protistology 12:73–80
Novozhilov YK, Shchepin ON, Schnittler M, Dagamac NHA, Alexandrova AV, Popov ES, Kuznetsov AN (2020) Myxomycetes associated with mountain tropical forests of Bi Dup-Nui Ba and Chu Yang Sin national reserves (Dalat plateau, southern Vietnam). Nova Hedwigia. https://doi.org/10.1127/nova_hedwigia/2019/0560
Pecundo MH, Dagamac NHA, Stephenson SL, dela Cruz TEE (2017) First myxomycetes survey in the limestone forest of Puerto Princesa Subterranean River National Park, Palawan, Philippines. Nova Hedwigia 104:129–141
Poulain M, Meyer M, Bozonnet J (2011) Les Myxomycètes. Fédération mycologique et botanique Dauphiné-Savoie, Sevrier
Quek MC, Chin NL, Tan SW, Yusof YA, Law CL (2018) Molecular identification of species and production origins of edible bird’s nest using FINS and SYBR green I based real-time PCR. Food Control 84:118–127
Rea-Maminta MAD, Dagamac NHA, Huyop FZ, Wahab RA, dela Cruz TEE (2015) Comparative diversity and heavy metal biosorption of myxomycetes from forest patches on ultramafic and volcanic soils. Chem Ecol 31:741–753
Redeña-Santos JC, Dunca JAU, Thao DV, Dagamac NHA (2017) Myxomycetes occurring on selected agricultural leaf litters. Stud Fungi 2:171–177
Redeña-Santos JC, Thao DV, Schnittler M, Dagamac NHA (2018) The first report of composition and occurrence of myxomycetes assemblages in protected and unprotected plantation forest: a comparative study in Thai Nguyen City, northern Vietnam. Plant Ecol Evol 151:231–240
Rojas C, Stephenson SL (2012) Rapid assessment of the distribution of myxomycetes in a Southwestern Amazon forest. Fungal Ecol 5:726–733
Rojas C, Valverde R, Stephenson SL, Vargas MJ (2010) Ecological patterns of Costa Rican myxomycetes. Fungal Ecol 3:139–147
Rojas C, Stephenson SL, Huxel GR (2011) Macroecology of high-elevation myxomycete assemblages in the northern Neotropics. Mycol Prog 10:423–437
Rojas C, Stephenson SL, Valverde R, Estrada-Torres A (2012) A biogeographical evaluation of high-elevation myxomycete assemblages in the northern Neotropics. Fungal Ecol 5:99–113
Rojas C, Morales RE, Calderon I, Clerc P (2013) First records of myxomycetes in El Salvador. Mycosphere 4:1042–1051
Rojas C, Rollins A, Stephenson SL (2014) Distribution of myxomycetes among the microhabitats available for these organisms in tropical forests. In: Misra JK, Tewari JP, Deshmukh SK, Vagvölgyi C (eds) Fungi from different substrates. Taylor & Francis Group, USA, pp 126–143
Rosing WC, Mitchell DW, Moreno G, Stephenson SL (2011) Additions to the myxomycetes of Singapore. Pac Sci 65:391–400
Schnittler M (2000) Foliicolous liverworts as a microhabitat for Neotropical myxomycetes. Nova Hedwigia 72:259–270
Schnittler M, Mitchell DW (2000) Species diversity in Myxomycetes based on the morphological species concept—a critical examination. Stapfia 73:55–61
Schnittler M, Stephenson SL (2002) Inflorescences of Neotropical herbs as a newly discovered microhabitat for myxomycetes. Mycologia 94:6–20
Schnittler M, Unterseher M, Tesmer J (2006) Species richness and ecological characterization of myxomycetes and myxomycete-like organisms in the canopy of temperate deciduous forest. Mycologia 98:223–232
Schnittler M, Novozhilov YK, Shadwick JDL, Spiegel FW, García-Carvajal E, König P (2015) What substrate cultures can reveal: Myxomycetes and myxomycete-like organisms from the Sultanate of Oman. Mycosphere 6:356–384
Schnittler M, Dagamac NHA, Novozhilov YK (2017) Biogeographical patterns in myxomycetes. In: Stephenson SL, Rojas C (eds) Myxomycetes: biology, systematics, biogeography ecology. Academic Press, UK, pp 299–331
Schnittler M, Shchepin ON, Dagamac NHA, Dahl MB, Novozhilov YK (2017) Barcoding myxomycetes with molecular markers: challenges and opportunities. Nova Hedwigia 104:323–341
Shchepin ON, Dagamac NHA, Sanchez OM, Novozhilov YK, Schnittler M, Zemlyanskaya IV (2017) DNA barcoding as a tool for identification of plasmodia and sclerotia of myxomycetes (Myxogastria) appearing in moist chambers. Mycosphere 8:1904–1913
Shchepin ON, Schnittler M, Dagamac NHA, Leontyev DV, Novozhilov YK (2019) Unexplored diversity of the microscopic myxomycetes: evidences from environmental DNA. Plant Ecol Evol 152:499–506
Stämmler F, Gläsner J, Hiergeist A, Holler E, Weber D, Oefner PJ, Gessner A, Spang R (2016) Adjusting microbiome profiles for differences in microbial load by spike-in bacteria. Microbiome 4:28
Stephenson SL, Stempen H (1994) Myxomycetes: a handbook of slime molds. Timber Press Inc., Portland
Stephenson SL, Kalyanasundaram I, Lakhanpal T (1993) A comparative biogeographical study of myxomycetes in the mid-appalachians of eastern North America and two regions of India. J Biogeogr 20:645–657
Stephenson SL, Fiore-Donno AM, Schnittler M (2011) Myxomycetes in soil. Soil Biol Biochem 43:2237–2242
Stephenson SL, Novozhilov YK, Almadrones-Reyes KJ, Dagamac NHA, Schnittler M (2019) New records of Barbeyella minutissima (Myxomycetes, Echinosteliales) with an updated distribution maps. Nova Hedwigia 109:177–186
Takahashi K (2013) Myxomycete distribution varies among leaf litters of different vegetation in a local secondary forest of warm-temperate western Japan. Mycoscience 54:368–377
Tran HTM, Stephenson SL, Hyde KD, Mongkolporn O (2006) Distribution and occurrence of myxomycetes in tropical forests of northern Thailand. Fungal Divers 22:227–242
Tran DQ, Nguyen HTN, Tran HTM, Stephenson SL (2014) Myxomycetes from three lowland tropical forests in Vietnam. Mycosphere 5:662–672
Van Hooff JPM (2009) Cribraria tecta, a new myxomycete from Vietnam. Boletín de la Sociedad Micológica de Madrid 33:129–136
Xu Y, Wang J, Bonos SA, Meyer WA, Huang B (2018) Candidate genes and molecular markers correlated to physiological traits for heat tolerance in fine fescue cultivars. Int J Mol Sci 19:116
Acknowledgements
Open Access funding provided by Projekt DEAL. LTTN would like to extend her gratitude to the DAAD for the scholarship support and funding during the field collection in Vietnam. The authors would like to also acknowledge the SusEnMan (Sustainable Environmental Management) Project (DAAD Project 57218030) headed by Prof. Wilhelm Steingrube and the International Office of the University of Greifswald for the small financial grant given during the course of the work. We would also like to thank Prof. Martin Schnittler (Director, Institute of Botany and Landscape Ecology) for the technical assistance for the identification of the specimens and for providing the equipment and materials for this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nguyen, L.T.T., Sanchez-Mahecha, O., Almadrones-Reyes, K.J. et al. Occurrence of leaf litter inhabiting myxomycetes from lowland forest patches of Northern and Central Vietnam. Trop Ecol 60, 495–506 (2019). https://doi.org/10.1007/s42965-020-00059-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42965-020-00059-9