Abstract
Behavioral epigenetics posits that both nature and nurture must be considered when determining the etiology of behavior or disease. The epigenome displays a remarkable ability to respond to environmental input in early sensitive periods but also throughout the lifespan. These responses are dependent on environmental context and lead to behavioral outcomes. While early adversity has been shown to perpetuate issues of mental health, there are numerous intervention strategies shown efficacious to ameliorate these effects. This includes diet, exercise, childhood intervention programs, pharmacological therapeutics, and talk therapies. Understanding the underlying mechanisms of the ability of the epigenome to adapt in different contexts is essential to advance our understanding of mechanisms of adversity and pathways to resilience. The present review draws on evidence from both humans and animal models to explore the responsivity of the epigenome to adversity and its malleability to intervention. Behavioral epigenetics research is also discussed in the context of public health practice and policy, as it provides a meaningful source of evidence concerning child development and disease intervention and prevention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The debate of nature versus nurture seeks to place a dichotomization on the importance of the genome or environment in determining our propensity for a behavioral phenomenon or disease. Behavioral epigenetics has helped cement the realization that both need to be considered, providing empirical evidence of physical interactions between our genome and environment that can drive changes in behavior or disease etiology. Originally defined by Conrad Waddington in 1942, the term epigenetics has shifted definitions throughout the history of the field; it was originally used to describe how the process of fertilization is able to yield a complex organism through variations in gene expression (Felsenfeld, 2014; Waddington, 1940; Waddington, 1942). Literally translating to “above genetics”, David Moore (2015) more broadly defines epigenetics as the process by which genetic material is activated, or deactivated, in different environmental contexts. Indeed, functioning more like a dimmer switch, epigenetic mechanisms enable our environments to dynamically interact with our genome and alter the degree to which our genes are expressed.
A commonly studied epigenetic phenomenon in terms of behavior or disease is DNA methylation. Briefly, one mechanism that can occur at the molecular level in response to the environment is the addition of methyl groups to cytosine-guanine (CG) dinucleotides, aided by enzymes called DNA methyl-transferases (DNMT) near the promoter region of a DNA sequence (Bestor, 2000; Smith & Meissner, 2013). Typically, albeit not exclusively, the more methylated a promoter region, the less degree of gene expression (Nan et al., 1998; Smith & Meissner, 2013). DNA methylation is thus one dynamic process by which environmental exposure can get under our skin, and help shape us epigenetically. Changes in DNA methylation have been associated with exposures to a variety of factors, especially psychosocial stress (Bowers & Yehuda, 2015; Franklin et al., 2010; Heijmans et al., 2008; McGowan et al., 2009; Mueller & Bale, 2008; Mulligan, Derrico, Stees, & Hughes, 2012; Murgatroyd et al., 2009; Palma-Gudiel, Córdova-Palomera, Leza, & Fañanás, 2015; Radtke et al., 2011) and experiences in the context of early caregiving (McGowan et al., 2009; Murgatroyd & Spengler, 2011; Roth, Lubin, Funk, & Sweatt, 2009; Weaver et al., 2004). Additional factors such as exercise (Nitert et al., 2012; Rönn et al., 2013) and diet (Hardy & Tollefsbol, 2011) also readily interact with the genome (for review, see Kanherkar, Bhatia-Dey, & Csoka, 2014), indicating our epigenome is dynamically regulated and shaped by environmental exposures and experiences throughout our lives.
Resilience has been defined as the capacity of a dynamic system to adapt successfully to disturbances that threaten system function (Masten, 2013). Like epigenetic regulation, resilience is a process that is thought to be on a continuum and dependent on context (Pietrzak & Southwick, 2011; for review see Southwick, Bonanno, Masten, Panter-Brick, & Yehuda, 2014). Thus, the study of resilience, in the context of epigenetics, further aids in the understanding of how our genome is dynamically regulated in response to our environments.
The present review seeks to examine epigenetic responses to adversity throughout the lifespan in both human and animal models, and their association with behavior or disease etiology. Moreover, we highlight various intervention strategies, including diet, exercise, mindfulness meditation, talk therapy, and childhood programs that are efficacious in altering the epigenome, and improving health outcomes. Indeed, while the epigenome is affected by adverse outcomes, data from these intervention strategies provide evidence that the epigenome is malleable both within and outside of sensitive periods. While more research is required to understand the nuances of how various environmental factors affect the epigenome at specific time points, the current evidence provided in this review suggests that the epigenome should be considered a valuable asset in understanding how our experiences, positive or negative, get under our skin and shape underlying biology and behavior. Further, policy and healthcare implications are explored.
Evidence the Epigenome Is Alterable by Adversity
Shortly after Conrad Waddington penned the term epigenetics, a great famine took place in the Netherlands nearing the end of World War 2. Indeed, the Dutch Hunger Winter became a prolific event in history; nearly 4.5 million individuals were affected by the famine, with a reported 15,000 to 25,000 deaths occurring in this region (Ekamper, Bijwaard, Poppel, & Lumey, 2017). Notably, infants exposed to the famine in utero in early, as opposed to late, gestation experienced prominent increases in obesity and cardiovascular issues (Schulz, 2010; Stein et al., 2007), even after controlling for smoking and social class (Painter et al., 2006). In a twin study, Heijmans et al. (2008) provided evidence that the exposure had profound effects on the epigenome; those who had been exposed to the famine in utero had different methylation patterns compared to same-sex siblings who were not exposed to famine. More specifically, as measured in whole blood, siblings exposed to famine had significant hypomethylation of the insulin-like growth factor II (IGF2) gene differentially methylated region, compared with siblings not exposed to famine. Consequently, in addition to developing obesity, exposure to famine in utero has been associated with developing psychopathologies, including schizophrenia (Hoek, Brown, & Susser, 1998).
In addition to the Dutch Hunger Winter, other work examining Holocaust survivor offspring show differential methylation of a gene known for proper stress responsivity, the glucocorticoid receptor gene (NR3C1) (Yehuda et al., 2014; for review, see Palma-Gudiel et al., 2015). Hypermethylation of this same gene in the hippocampus has been observed in those who had a history of abuse and had committed suicide (McGowan et al., 2009). Furthermore, in work with rodents, hippocampal methylation of the glucocorticoid receptor gene has been associated with poor stress responsivity (Weaver et al., 2004), further linking this gene to behavior and psychopathology. Despite most of the human literature focusing on documenting epigenetic responses to traumatic events that have occurred over an extended period of time, there have been a handful of studies examining the effects of stress exposure over much shorter periods. For example, there have been differential methylation patterns observed in US military members pre- and postdeployment as measured in serum. Service members who developed PTSD postdeployment had significantly increased methylation of the IL18 gene, and those who did not develop PTSD had reduced methylation levels of the IL18 and H19 genes (Rusiecki et al., 2013). In addition, study participants show rapid changes in DNA methylation in both the response to and recovery from the Trier Social Stress Test, as measured in saliva and buccal cells (Edelman et al., 2012; Wiegand et al., 2018). Altogether, data highlight the responsivity of the epigenome upon exposure to adversity that is detectable across different peripheral tissue types.
While human studies provide insight into a relationship between the epigenome and the etiology of disease in those affected by trauma, it is difficult to make definitive statements regarding causality. Indeed, the vast majority of the epigenetic work in humans is centered on studies after documented traumatic events; it would be more informative if both pre- and postepigenetic profiles were established, but of course, one simply cannot predict when trauma will occur. Moreover, if we follow the definition proposed by David Moore (2015) in which epigenetics is defined as how gene expression is changed in different environmental contexts, it is nearly impossible to control for all of the different contexts an individual can experience throughout their lifetime. Animal models are a necessary and sound extension of clinical work; they allow experimental exploration into whether the epigenome responds to stress. With animal models, causality with regard to the etiology of behavioral phenomena or disease can be established, environmental exposures can be carefully controlled, various age points can be sampled, and systems can be perturbed to explore necessity and sufficiency. Moreover, actual brain tissue can be extracted, as opposed to the reliance on peripheral tissues in humans such as blood, saliva, or buccal cells; the best analog to brain tissue is still debated (Bakulski, Halladay, Hu, Mill, & Fallin, 2016).
Animal models do have some limitations. For example, given the complexity of disorders like schizophrenia or bipolar disorder, animal models typically rely on studying endophenotypes or subsets of the symptomology (Beyer & Freund, 2017; Jones, Watson, & Fone, 2011). Moreover, animal methodology often relies on lesions or genetic manipulations, and such rather extreme perturbations may affect behavioral and disease pathways differently than smaller naturally occurring ones do in humans. Additionally, there can be debate in the field about how to interpret behavioral data from commonly used paradigms, such as the forced swim test (e.g., Mul, Zheng, & Goodyear, 2016). Nonetheless, animal models are clearly valuable preclinical tools to shed light on the biological bases of behavior or disease, which are necessary to inform policy and healthcare.
Summarizing the current stage of knowledge from an ever-growing body of animal work, adversity exposure preconception, during periods of gestation, and throughout the lifespan in various animal models demonstrate the malleability of the epigenome. For example, chronic unpredictable stress approximately 2 weeks prior to mating produced marked behavioral and epigenetic differences in offspring in the frontal cortex (Zaidan, Leshem, & Gaisler-Salomon, 2013). Exposure to stress during the early (Mueller & Bale, 2008; Pankevich, Mueller, Brockel, & Bale, 2009) and later (Champagne & Meaney, 2006; Mairesse et al., 2007) stages of gestation produce divergent epigenetic responses and anxiety phenotypes. Further, F1 male offspring reared from fathers who experienced maternal separation stress display different methylation patterns in their sperm, and display anxiety- and depressive-like phenotypes (Franklin et al., 2010).
Animal studies make it clear that after birth, the epigenome remains attuned to its environment. Indeed, maternal licking and grooming, or exposure to maltreatment leave enduring epigenetic marks on genes related to brain development, plasticity, and stress responsivity in the prefrontal cortex and hippocampus (Doherty, Blaze, Keller, & Roth, 2017; Roth et al., 2009; Weaver et al., 2004). Active DNA methylation and demethylation are known to occur in the adult brain and are processes pivotal for brain function and memory (Halder et al., 2016; Lubin, Roth, & Sweatt, 2008; Miller et al., 2010) and responsiveness to psychosocial stress (LaPlant et al., 2010; Makhathini, Abboussi, Stein, Mabandla, & Daniels, 2017; Roth, Zoladz, Sweatt, & Diamond, 2011; Wright et al., 2017). Considering the epigenome is responsive to environmental influences outside of sensitive periods, it argues that we should not necessarily view early-life experiences as determinative of either our epigenetic landscapes or psychopathologies. Indeed, tapping into the potential of the responsive epigenome, via pharmacological or nonpharmacological interventions, is a promising avenue to change brain and behavior development to promote resilience.
Exploiting the Malleability of the Early-Life Epigenome to Change Outcomes
Data exist suggestive of an early-life sensitive period where the epigenome is perhaps most malleable (Curley & Champagne, 2016; Dunn et al., 2019; Faulk & Dolinoy, 2011). In humans, recent data suggest exposure to adversity between birth and 2 years of age is predicative of differentially methylated regions at age 7, as measured in cord blood or blood leukocytes (Dunn et al., 2019). Exposure to adversity early in life has been associated with various psychopathologies, including depression (LeMoult et al., 2020; Syed & Nemeroff, 2017), anxiety (Fonzo et al., 2015; Lähdepuro et al., 2019) and posttraumatic stress disorder (Yehuda et al., 2010). This is likely because this period is critical for brain growth and development (Gilmore, Knickmeyer, & Gao, 2018), with structural and functional relations already forming between neural networks (Haartsen, Jones, & Johnson, 2016).
When one synthesizes findings, it is critical that type, timing, and duration of stressors be taken into account in examining the propensity for future psychopathology (Cavigelli et al., 2018; Provenzi, Giorda, Beri, & Montirosso, 2016); if one examines a gene-by-environment by timing interaction, perhaps it would be more informative in determining how stress impacts the developing brain. Furthermore, if we accept that timing of stress matters, this also supposes that the timing of the intervention matters (Heim & Binder, 2012). Since we know that methylation is reversible (Ramchandani, Bhattacharya, Cervoni, & Szyf, 1999; Szyf, Tang, Hill, & Musci, 2016) and associated with stress exposure in early life (e.g., McGowan et al., 2009; Roth et al., 2009; Weaver et al., 2004), it provides a sound therapeutic target, and biomarker, in examining the efficacy of various early preventative measures and interventions (Szyf et al., 2016). These include interventions targeting a mother’s nutrition and programs directed at families to provide a more positive early life environment.
As we learned through the Dutch Hunger Winter, maternal diet in utero can have profound effects on offspring health (Painter et al., 2006; Schulz, 2010; Stein et al., 2007), differences in epigenetic methylation of particular genes (Heijmans et al., 2008), and increased incidences of psychopathology (Hoek et al., 1998). Indeed, maternal consumption of dietary fibers, carbohydrates, vitamins, and folic acid can all alter epigenetic mechanisms of infants in utero, making proper nutrition important for regulating infant growth, and processes including immunity and inflammation (Martínez, Cordero, Campión, & Milagro, 2012; Paparo et al., 2014). Particularly, maternal consumption of folic acid is essential in epigenetic development, being important for proper cognitive development (Irwin et al., 2016), and regulating genes known to be associated with genetic imprinting and diabetes (Irwin et al., 2019). Moreover, folate deficiency has been associated with increased cancer risk (Bistulfi, Vandette, Matsui, & Smiraglia, 2010). Taken together, these data indicate that the earliest intervention/prevention for offspring health starts with maternal diet, as the epigenome’s malleability to exposures in utero can alter disease trajectory.
The Bucharest Early Intervention Project (BEIP) began in 2000 and examines the effects of institutionalization and early life deprivation on brain growth and development, with high quality foster care as a potential intervention. Institutionalization is often characterized as having low quality of care, with often insufficient environments for proper developmentally required stimulation, and parental caregiving. Consequently, children in institutionalization often have deficiencies in attachment (Zeanah, Smyke, Koga, & Carlson, 2005), lower IQ’s (Almas, Degnan, Nelson, Zeanah, & Fox, 2016), as well as smaller cortical gray volume compared with children not institutionalized (Sheridan, Fox, Zeanah, Mclaughlin, & Nelson, 2012). However, if these children are placed in high quality caregiving before 2 years of age, there are significant improvements in cognitive outcomes later in life, further supporting this time point as a sensitive period in humans (Dunn et al., 2019; Nelson et al., 2007). Furthermore, there was a negative correlation found between methylation, and time spent in institutional care at specific cytosine sites of the serotonin transporter gene (SLC6A4), as measured in buccal cells at 12.5 years of age (Non et al., 2016).
Family Centered Development Care (FCDC) is a program viewing the family as an essential contributor to developmentally supportive care of their baby, and has the goal of improving parent/baby interactions, especially babies necessitating the neonatal intensive care unit (NICU). It utilizes a team-based approach, in which care workers and families develop relationships to facilitate the development of proper infant-caregiver relationships throughout development (Craig et al., 2015). This intervention program has been a proposed method to regulate the epigenome of preterm infants requiring the NICU, especially considering brain development normally taking place in utero occurs postnatally in NICU cases, and this population is consequently at risk for neurodevelopmental disorders (Ment & Vohr, 2008; Samra, Mcgrath, Wehbe, & Clapper, 2012). Other family-centered intervention programs have been successful in ameliorating epigenetic profiles of 20-year-old adults exposed to harsh parenting and parental depression, if the children were entered into the program by age 11. More specifically, children exposed to parental depression had increased epigenetic aging of peripheral blood mononuclear cells, and a family-based intervention program administered at age 11 ameliorated this epigenetic response to a harsh early life environment, and was associated with lower emotional distress (Brody, Yu, Chen, Beach, & Miller, 2015). Taken together, these data provide empirical support that family-based intervention programs, both with preterm and early adolescents, can ameliorate the effects of early-life stress and associated epigenetic changes to promote positive behavioral change.
The Nurse-Family Partnership (NFP) program is another early intervention program, in which nurses visit homes of at-risk families and instruct mothers to identify developing health issues. The program has three goals: improvement of a mother’s behaviors that are thought to mediate pregnancy outcomes, facilitate integration of the mother into other relationships to build a support network, and provide an avenue for mothers to access other needed health resources. Consequently, mothers enrolled in this program have better dietary management and engage in less incidences of child abuse (Olds, Hill, Obrien, Racine, & Moritz, 2003), both factors known to impact the epigenome (Doherty et al., 2017; Heijmans et al., 2008; Irwin et al., 2019; Roth et al., 2009) and lead to increased psychopathology, including suicidality (McGowan et al., 2009). Moreover, not only do mothers report positive experiences in this program (Landy, Jack, Wahoush, Sheehan, & Macmillan, 2012), but there are less incidences of childhood maltreatment, youth substance abuse, and infant death (Miller, 2015). In a 27-year follow-up study of youth originally engaged in this program, investigators found differentially methylated regions in whole blood in response to those engaged in the program and those who did not. In a principal component analysis, those who were exposed to childhood adversity or the NFP had significant DNA methylation variability at 27 years of age (O’Donnell et al., 2018). Moreover, individuals exposed to child abuse/neglect had enrichment of variably methylated CpG sites (vCpGs) within genes regulated by hormone receptors, including the glucocorticoid receptor gene (NR3C1). While this work is in its infancy and directionality of methylation in various peripheral tissues needs to be considered, these data suggest that the NFP is, in part, efficacious in reshaping our epigenome. Taken together, these data demonstrate that the NFP, like the FCDC, is a sound intervention strategy that can exploit the malleability and resiliency of the epigenome.
Finally, the Attachment and Biobehavioral Catch-up (ABC) intervention is another intervention targeting at-risk children, focusing on parent-child relationships that are essential in early, and throughout, development (Dozier & Bernard, 2017). The ABC intervention is a 10-session home visitation program, in which a parent coach helps to train parents to provide adequate caregiving, often focusing on proper social dynamics between the infant and parent. The parent coach will teach parents how to engage in nurturing ways, including appropriate response strategies aimed at regulating a child’s psychological reactions by following their lead, in addition to reduce behaviors such as yelling or screaming. During this process, the parent coach can provide active feedback to the caregiver, to facilitate learning and making adjustments to improve the parent-infant dynamic, ultimately leading to more organized attachments and proper child regulatory skills (Dozier & Bernard, 2017). Using this protocol, children have demonstrated more normalized cortisol diurnal rhythms, (Bernard, Hostinar, & Dozier, 2015), higher vocabulary scores (Bernard, Lee, & Dozier, 2017), and higher rates of organized attachment (Bernard et al., 2012), making this program efficacious in improving infant outcomes. In a preliminary study, ABC intervention in children aged 6–21 months promoted differential methylation in gene pathways associated with neuronal differentiation, neuronal development, and cell signaling as measured in saliva (Hoye et al., 2019).
While the epigenetic measurements of such work are in early stages, current data posit that the epigenome can be utilized as both a biomarker and a target to promote healthy development. Pharmacological interventions aimed at altering DNA methylation, including valproic acid (VPA) and 5-Azacytidine are in clinical trials for epigenetic drug therapies for tumor suppression (Egger, Liang, Aparicio, & Jones, 2004; Szyf, 2009; Ganesan, Arimondo, Rots, Jeronimo, & Berdasco, 2019), and one day these drugs or others may be worthy intervention avenues to help promote resilience. While current epigenetic pharmacological therapeutics proposes challenges of gene target specificity (Hyman, 2012), work with these agents in animal models are useful to test the notion that if one could potentially prevent aberrant epigenetic activity, and if the epigenetic activity is causally related to behavioral outcome, then it should be possible to block maladaptive behavioral development from occurring altogether. Histone deacetylase inhibitors (HDACi) such as VPA, sodium butyrate (NaB), and Trichostatin A (TSA) have been shown to decrease DNA methylation (Sarkar et al., 2011; Weaver et al., 2004), and have been efficacious in the treatment of depressive- (Covington et al., 2009; Fuchikami et al., 2016; Schmauss, 2015), schizophrenic- (Revenga et al., 2018), and anxiety-like (Weaver et al., 2004) phenotypes. Likewise, DNA methyltransferase inhibitors (DNMTi), such as zebularine, have been shown to reverse epigenetic marks and behavior associated with exposure to early adversity (Keller, Doherty, & Roth, 2019; Roth et al., 2009).
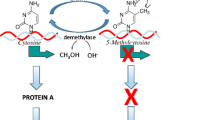
As described elsewhere (Walker et al., 2017), the scarcity adversity paradigm is one way to experimentally expose infant rats to adversity in the context of caregiving. Based upon conditions created by the experimenter, a dam spends significantly less time nurturing and significantly more time displaying aversive caregiving behaviors towards pups (Fig. 1a; e.g., Blaze, Scheuing, & Roth, 2013; Roth et al., 2009). Though the experimental conditions do not appear to render elevations in corticosterone (Fig. 1b), they do create aberrant brain methylation and a host of behavioral abnormalities (Fig. 1a; Blaze et al., 2013; Blaze, Asok, & Roth, 2015; Blaze & Roth, 2017; Doherty et al., 2017; Doherty, Chajes, Reich, Duffy, & Roth, 2019; Keller, Doherty, & Roth, 2018; Keller et al., 2019; Roth et al., 2009; Roth, Matt, Chen, & Blaze, 2014). This model has proven useful to test whether epigenetic therapeutics, administered early, can alter the epigenome. To date, the HDACi sodium butyrate (Doherty et al., 2019) and the DNMTi 5-azacytidine-2′-deoxycytidine (Fig. 2) have proven efficacious in lowering maltreatment-induced aberrant DNA methylation in the prefrontal cortex. Taken together, these data further demonstrate the malleability of the epigenome. Future research underway is exploring whether these strategies are sufficient to alter the development of behavior, including the perpetuation of phenotype to progeny. Of course, it is also important to utilize animal models to explore the capacity of behavioral interventions to promote resilience. Indeed, cross-fostering to provide a more nurturing caregiving environment has been shown to promote different epigenetic and behavioral outcomes (Weaver et al., 2004) and ameliorate some of the epigenetic changes associated with early life adversity (Roth et al., 2009). Further, environmental enrichment prevents the perpetuation of the epigenetic effects of early adversity in the form of maternal separation (Gapp et al., 2016).
a Because of an unfamiliar environment and insufficient nesting material dams spends significantly less time nurturing and significantly more time displaying aversive caregiving behaviors towards pups. These behaviors elicit vocalizations from the pups, and later aberrant brain methylation and behavioral abnormalities. Bdnf = Brain-derived neurotrophic factor; PFC = prefrontal cortex; mPFC = medial prefrontal cortex; FST = forced swim test; NOR = novel object recognition. b Depicts the concentration of plasma corticosterone in 8-day-old pups across treatment conditions from the scarcity adversity paradigm. No significance was found between treatment groups. n = 5–6 pups/group, error bars represent SEM. Normal and cross-foster = exposure to only nurturing care from a dam. Maltreatment = exposure to brief and repeated bouts of maltreatment from a dam
Methyl-specific real-time PCR results for Bdnf DNA methylation (IX) in 8-day-old pups showing higher methylation associated with maltreatment, which was prevented if the higher dose of 5-aza was delivered concurrent with exposure to maltreatment. Error bars represent SEM. n = 19–23/per group. Bdnf = Brain-derived neurotrophic factor; NUR = nurturing (exposure to only nurturing care from a dam) and MAL = maltreatment (exposure to brief and repeated bouts of maltreatment from a dam); SAL = saline. **p < 0.01 MAL vs. NUR main effect of infant condition; #p < 0.05 MAL/SAL vs. NUR/SAL and MAL 1.0 mg
Taken together, data in both humans and rodents indicate that early-life sensitive periods provide an ideal time point for intervention; both neural structures and the epigenome are sensitive to environmental inputs, and can shift developmental trajectories depending upon context. This context is not, however, exclusive to early life, as will be explored in the next section.
Exploiting the Malleability of the Later Life Epigenome to Promote Change
While the epigenome and brain display developmental periods more responsive to environmental inputs, the epigenome remains attuned to the environment throughout the lifespan (for review, see Kanherkar et al., 2014). Indeed, in clinical studies, environmental factors including exercise, diet, psychotherapy, and meditation all prove efficacious in improving disease etiology with associated epigenomic changes; the epigenome again shows that it has the capacity to change depending upon context. Indeed, not everyone exposed to stress in early life develops psychopathology, and data in both human and animal models suggests that few incidences of early life stress may even promote later stress resiliency (Gapp et al., 2014; Parker, Buckmaster, Hyde, Schatzberg, & Lyons, 2019; Santarelli et al., 2017). To navigate these findings and determine how development impacts the propensity for disease or resiliency, it is thus critical to consider how the epigenome dynamically responds to contexts outside of developmentally sensitive periods.
Exercise outside of the sensitive period of development improves symptomatology associated with anxiety (Moor, Beem, Stubbe, Boomsma, & Geus, 2006), depression (Bridle, Spanjers, Patel, Atherton, & Lamb, 2012), and Alzheimer’s disease (Intlekofer & Cotman, 2013). One way in which this may occur is through alterations in epigenetic mechanisms. Indeed, exercise has been shown to lead to epigenetic remodeling (Voisin, Eynon, Yan, & Bishop, 2015), including increases in Brain-derived neurotrophic factor (BDNF) gene expression (Gomez-Pinilla, Zhuang, Feng, Ying, & Fan, 2011; Sleiman et al., 2016), a gene that promotes neurogenesis and synaptic plasticity in the hippocampus (Intlekofer & Cotman, 2013), which is a brain region implicated in mediating stress responses and where decreases in volume often occur in pathology (Mcewen, Nasca, & Gray, 2016). Since this brain region is epigenetically regulated in the etiology of disease based on early environmental input (McGowan et al., 2009; Weaver et al., 2004), and we know BDNF methylation has been associated with early life adversity (Kundakovic et al., 2015; Perroud et al., 2013; Roth et al., 2009), exercise may prove to be efficacious to alter the epigenome and subsequently improve disease symptomology in later stages of development. Indeed, epigenetic alterations are found in numerous exercise intervention programs targeting adolescents or adults (Nitert et al., 2012; Rönn et al., 2013; Zeng et al., 2012) which were associated with mitigation of disease. While data suggests that any level of intensity of exercise is efficacious in regulating depression (Helgadóttir, Hallgren, Ekblom, & Forsell, 2016), other data suggests that decreases in methylation are intensity-dose dependent as measured in skeletal muscle (Barrès et al., 2012). Taken together, these data on exercise are consistent with the notion that exercise is efficacious in both regulating the epigenome and promoting mental health.
Various behavioral therapies, including but not limited to cognitive-behavioral therapy (CBT) and psychodynamics, are efficacious in improving psychopathology, including depression (Butler, Chapman, Forman, & Beck, 2006; Driessen et al., 2010) and anxiety disorders (Hoffman & Smits, 2008; Keefe, Mccarthy, Dinger, Zilcha-Mano, & Barber, 2014). Recent work suggests psychotherapy may act as an epigenetic therapeutic, through perhaps improving functional neural circuits implicated in the etiology of disease (Miller, 2017; Stahl, 2011). Indeed, one study found that in patients with borderline personality disorder, incidences of childhood trauma were associated with increases in methylation of the BDNF gene in peripheral blood leukocytes (Perroud et al., 2013). However, patients who were responders to the therapy had a decrease in BDNF methylation, and these changes in methylation were associated with decreased depression severity, hopelessness, and impulsivity (Perroud et al., 2013). BDNF is important for proper dendritic growth and plasticity during development (Cohen-Cory, Kidane, Shirkey, & Marshak, 2010), and increased methylation of this gene has been implicated in many psychopathologies (D'Addario et al., 2012; Kang et al., 2013; Keller et al., 2010; Xie et al., 2017). Pharmacological administration of citalopram, a common antidepressant medication, has also demonstrated efficacy in increasing BDNF gene expression of individuals responsive to the drug in whole blood; there was also significantly reduced histone H3 lysine 27 tri-methylation in responders, a histone marker of repression (Lopez et al., 2013).
Finally, eastern traditions, including mindfulness meditation, have been increasing in popularity. Indeed, mindfulness meditation practices are one of the fastest growing health trends and have been proposed as an integral piece of healthcare (Mars & Abbey, 2010). Current data suggests that mindfulness meditation improves many diseases, including anxiety and depression (Schreiner & Malcolm, 2008) and PTSD (King et al., 2013), including treatment-resistant depression (Deen, Sipe, & Eisendrath, 2016). Mindfulness meditation has been demonstrated to modulate brain activity in regions known to be important for attention (Kozasa et al., 2012), pain processing (Zeidan, Grant, Brown, Mchaffie, & Coghill, 2012), and the default mode network (DMN) (Berkovich-Ohana, Glicksohn, & Goldstein, 2012; King et al., 2016). The DMN is a group of brain structures functionally active at rest as opposed to attention-oriented tasks (Whitfield-Gabrieli & Ford, 2012), and has been implicated in rumination associated with depression (Zhou et al., 2020). Some research suggests that changes in functional connectivity and morphology associated with meditation are dependent on increasing levels of experience (Tomasino, Fregona, Skrap, & Fabbro, 2013). Indeed, long-term meditators, compared with controls, display epigenetic alterations in genes linked to human diseases (García-Campayo et al., 2017), as well as slower epigenetic clocks in response to aging (Chaix et al., 2017). Though these data are in their infancy, they further support the idea that our epigenome is malleable to various intervention strategies.
Studies with adult rodents likewise provide an extension to the early developmental data, demonstrating that the epigenome retains its responsivity and is a plausible mechanism for behavioral intervention. In the scarcity-adversity paradigm of maltreatment as described previously (Fig. 1), rat pups exposed to early life maltreatment display increased Bdnf methylation in the prefrontal cortex (Doherty et al., 2019; Roth et al., 2009) that persists into adulthood (Roth et al., 2009), and is associated with behavioral abnormalities (Doherty et al., 2017), including a perpetuation of maltreatment (Keller et al., 2019; Roth et al., 2009). If zebularine is administered to animals in adulthood with a history of maltreatment, there is a normalization of Bdnf methylation and a reduction in aversive caregiving behaviors (Keller et al., 2019). Furthermore, administration of zebularine in adulthood normalized aberrant forced swim behavior of maltreated females to levels comparable of controls (Keller et al., 2018), which is a common paradigm utilized to study depressive-like phenotypes. Administration of antidepressants in socially defeated depressed mice brought about transcriptional changes promoting resiliency (Bagot et al., 2017; Lorsch et al., 2019). Finally, other work looking at environmental enrichment in adult rats has shown reductions in addictive-like behaviors (Imperio et al., 2018) and memory deficits (Morse, Butler, Davis, Soller, & Lubin, 2015) mediated through changes in methylation.
Summary and Policy Implications
Epigenetics is the process by which genetic material is activated, or deactivated, in different environmental contexts (2015). Indeed, work in both humans and various animal models demonstrate the capacity of the environment to get under the skin to interact with our genome and shape our epigenetic landscapes. Work also demonstrates the epigenome has a remarkable capacity to adapt to interventions, including nutrition, exercise, parenting and behavioral intervention programs, environmental enrichment, and pharmacological therapeutics, with positive behavioral outcomes.
Altogether, this reveals some of the mechanisms driving the development of behavior and health, helps us understand our epigenome’s capacity to change because of experience, and identifies important targets for intervention work to promote resilience. Such data can also better inform public outreach and policy efforts. For example, since diet and exercise have the capacity to shape the epigenome and disease etiology throughout the entire lifespan, public outreach and policy efforts should talk about the importance of nutrition and exercise not only through the lens of cardiovascular concern for the generation at hand but also how they impact the epigenome for the next generation. Indeed, no matter the stage of development, from in utero through adulthood, diet is an integral component of DNA methylation as folate is essential in the synthesis of methyl groups that are necessary for DNA methylation, and abnormal folate metabolism is associated with disease (for review, see Zheng & Cantley, 2018).
Similarly, data indicate early-life intervention programs for children exposed to stress are not only efficacious in improving health outcomes, but also in reprogramming the epigenome after stress exposure. The biological impact of adversity should be a clear message to the public and policy makers, and these intervention programs should be made aware and available to families who need them, as the economic costs of mental health are likely to be far greater than the funding required to sustain these programs. Further, behavioral therapy and mindfulness meditation have already demonstrated utility in patient outcomes of incidences of various psychiatric disorders; their ability to affect individuals at the molecular level are now just being appreciated but need to have their place too in public outreach and policy efforts promoting health. In conclusion, further research exploring preventable and reversible epigenetic states holds great promise of helping us advance adversity and resilience science and policy.
References
Almas, A. N., Degnan, K. A., Nelson, C. A., Zeanah, C. H., & Fox, N. A. (2016). IQ at age 12 following a history of institutional care: findings from the Bucharest early intervention project. Developmental Psychology, 52(11), 1858–1866.
Bagot, R. C., Cates, H. M., Purushothaman, I., Vialou, V., Heller, E. A., Yieh, L., LaBonté, B., Peña, C. J., Shen, L., Wittenberg, G. M., & Nestler, E. J. (2017). Ketamine and imipramine reverse transcriptional signatures of susceptibility and induce resilience-specific gene expression profiles. Biological Psychiatry, 81(4), 285–295.
Bakulski, K. M., Halladay, A., Hu, V. W., Mill, J., & Fallin, M. D. (2016). Epigenetic research in neuropsychiatric disorders: the “Tissue Issue”. Current Behavioral Neuroscience Reports, 3(3), 264–274.
Barrès, R., Yan, J., Egan, B., Treebak, J. T., Rasmussen, M., Fritz, T., Caidahl, K., Krook, A., O'Gorman, D. J., & Zierath, J. R. (2012). Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metabolism, 15(3), 405–411.
Berkovich-Ohana, A., Glicksohn, J., & Goldstein, A. (2012). Mindfulness-induced changes in gamma band activity – Implications for the default mode network, self-reference and attention. Clinical Neurophysiology, 123(4), 700–710.
Bernard, K., Dozier, M., Bick, J., Lewis-Morrarty, E., Lindhiem, O., & Carlson, E. (2012). Enhancing attachment organization among maltreated children: results of a randomized clinical trial. Child Development, 83(2), 623–636.
Bernard, K., Hostinar, C. E., & Dozier, M. (2015). Intervention effects on diurnal cortisol rhythms of child protective services–referred infants in early childhood. JAMA Pediatrics, 169(2), 112–119.
Bernard, K., Lee, A. H., & Dozier, M. (2017). Effects of the ABC intervention on foster children’s receptive vocabulary: follow-up results from a randomized clinical trial. Child Maltreatment, 22(2), 174–179.
Bestor, T. H. (2000). The DNA methyltransferases of mammals. Human Molecular Genetics, 9(16), 2395–2402.
Beyer, D. K., & Freund, N. (2017). Animal models for bipolar disorder: From bedside to the cage. International Journal of Bipolar Disorders, 5(1), 35.
Bistulfi, G., Vandette, E., Matsui, S.-I., & Smiraglia, D. J. (2010). Mild folate deficiency induces genetic and epigenetic instability and phenotype changes in prostate cancer cells. BMC Biology, 8(1).
Blaze, J., Asok, A., & Roth, T. L. (2015). Long-term effects of early-life caregiving experiences on brain-derived neurotrophic factor histone acetylation in the adult mPFC. Stress, 18(6), 607–615.
Blaze, J., & Roth, T. L. (2017). Caregiver maltreatment causes altered neuronal DNA methylation in female rodents. Development and Psychopathology, 29(2), 477–489.
Blaze, J., Scheuing, L., & Roth, T. L. (2013). Differential methylation of genes in the medial prefrontal cortex of developing and adult rats following exposure to maltreatment or nurturing care during infancy. Developmental Neuroscience, 35(4), 306–316.
Bowers, M. E., & Yehuda, R. (2015). Intergenerational transmission of stress in humans. Neuropsychopharmacology, 41(1), 232–244.
Bridle, C., Spanjers, K., Patel, S., Atherton, N. M., & Lamb, S. E. (2012). Effect of exercise on depression severity in older people: systematic review and meta-analysis of randomised controlled trials. British Journal of Psychiatry, 201(3), 180–185.
Brody, G. H., Yu, T., Chen, E., Beach, S. R., & Miller, G. E. (2015). Family-centered prevention ameliorates the longitudinal association between risky family processes and epigenetic aging. Journal of Child Psychology and Psychiatry, 57(5), 566–574.
Butler, A., Chapman, J., Forman, E., & Beck, A. (2006). The empirical status of cognitive-behavioral therapy: a review of meta-analyses. Clinical Psychology Review, 26(1), 17–31.
Cavigelli, S. A., Bao, A. D., Bourne, R. A., Caruso, M. J., Caulfield, J. I., Chen, M., & Smyth, J. M. (2018). Timing matters: the interval between acute stressors within chronic mild stress modifies behavioral and physiologic stress responses in male rats. Stress, 21(5), 453–463.
Chaix, R., Alvarez-López, M. J., Fagny, M., Lemee, L., Regnault, B., Davidson, R. J., Lutz, A., & Kaliman, P. (2017). Epigenetic clock analysis in long-term meditators. Psychoneuroendocrinology, 85, 210–214.
Champagne, F. A., & Meaney, M. J. (2006). Stress during gestation alters postpartum maternal care and the development of the offspring in a rodent model. Biological Psychiatry, 59(12), 1227–1235.
Cohen-Cory, S., Kidane, A. H., Shirkey, N. J., & Marshak, S. (2010). Brain-derived neurotrophic factor and the development of structural neuronal connectivity. Developmental Neurobiology, NA.
Covington, H. E., Maze, I., Laplant, Q. C., Vialou, V. F., Ohnishi, Y. N., Berton, O., et al. (2009). Antidepressant actions of histone deacetylase inhibitors. Journal of Neuroscience, 29(37), 11451–11460.
Craig, J. W., Glick, C., Phillips, R., Hall, S. L., Smith, J., & Browne, J. (2015). Recommendations for involving the family in developmental care of the NICU baby. Journal of Perinatology, 35(S1), S5–S8.
Curley, J. P., & Champagne, F. A. (2016). Influence of maternal care on the developing brain: mechanisms, temporal dynamics and sensitive periods. Frontiers in Neuroendocrinology, 40, 52–66.
D'Addario, C., Dellosso, B., Palazzo, M. C., Benatti, B., Lietti, L., Cattaneo, E., et al. (2012). Selective DNA methylation of BDNF promoter in bipolar disorder: differences among patients with BDI and BDII. Neuropsychopharmacology, 37(7), 1647–1655.
Deen, S., Sipe, W., & Eisendrath, S. J. (2016). Mindfulness-based cognitive therapy for treatment-resistant depression. Mindfulness-Based Cognitive Therapy: Innovative applications., 133–144,. https://doi.org/10.1007/978-3-319-29866-5_12
Doherty, T. S., Blaze, J., Keller, S. M., & Roth, T. L. (2017). Phenotypic outcomes in adolescence and adulthood in the scarcity-adversity model of low nesting resources outside the home cage. Developmental Psychobiology, 59(6), 703–714.
Doherty, T. S., Chajes, J. R., Reich, L., Duffy, H. B., & Roth, T. L. (2019). Preventing epigenetic traces of caregiver maltreatment: a role for HDAC inhibition. International Journal of Developmental Neuroscience, 78(1), 178–184.
Dozier, M., & Bernard, K. (2017). Attachment and biobehavioral catch-up: addressing the needs of infants and toddlers exposed to inadequate or problematic caregiving. Current Opinion in Psychology, 15, 111–117.
Driessen, E., Cuijpers, P., Maat, S. C. D., Abbass, A. A., Jonghe, F. D., & Dekker, J. J. (2010). The efficacy of short-term psychodynamic psychotherapy for depression: a meta-analysis. Clinical Psychology Review, 30(1), 25–36.
Dunn, E. C., Soare, T. W., Zhu, Y., Simpkin, A. J., Suderman, M. J., Klengel, T., Smith, A. D. A. C., Ressler, K. J., & Relton, C. L. (2019). Sensitive periods for the effect of childhood adversity on DNA methylation: results from a prospective, longitudinal study. Biological Psychiatry, 85(10), 838–849.
Edelman, S., Shalev, I., Uzefovsky, F., Israel, S., Knafo, A., Kremer, I., Mankuta, D., Kaitz, M., & Ebstein, R. P. (2012). Epigenetic and genetic factors predict womens salivary cortisol following a threat to the social self. PLoS One, 7(11), e48597.
Egger, G., Liang, G., Aparicio, A., & Jones, P. A. (2004). Epigenetics in human disease and prospects for epigenetic therapy. Nature, 429(6990), 457–463.
Ekamper, P., Bijwaard, G., Poppel, F. V., & Lumey, L. H. (2017). War-related excess mortality in the Netherlands, 1944–45: New estimates of famine- and non-famine-related deaths from national death records. Historical Methods: A Journal of Quantitative and Interdisciplinary History, 50(2), 113–128.
Faulk, C., & Dolinoy, D. C. (2011). Timing is everything. Epigenetics, 6(7), 791–797.
Felsenfeld, G. (2014). A brief history of epigenetics. Cold Spring Harbor Perspectives in Biology, 6(1), a018200.
Fonzo, G. A., Ramsawh, H. J., Flagan, T. M., Simmons, A. N., Sullivan, S. G., Allard, C. B., Paulus, M. P., & Stein, M. B. (2015). Early life stress and the anxious brain: evidence for a neural mechanism linking childhood emotional maltreatment to anxiety in adulthood. Psychological Medicine, 46(5), 1037–1054.
Franklin, T. B., Russig, H., Weiss, I. C., Gräff, J., Linder, N., Michalon, A., Vizi, S., & Mansuy, I. M. (2010). Epigenetic transmission of the impact of early stress across generations. Biological Psychiatry, 68(5), 408–415.
Fuchikami, M., Yamamoto, S., Morinobu, S., Okada, S., Yamawaki, Y., & Yamawaki, S. (2016). The potential use of histone deacetylase inhibitors in the treatment of depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 64, 320–324.
Ganesan, A., Arimondo, P. B., Rots, M. G., Jeronimo, C., & Berdasco, M. (2019). The timeline of epigenetic drug discovery: From reality to dreams. Clinical Epigenetics, 11(1), 174.
Gapp, K., Bohacek, J., Grossmann, J., Brunner, A. M., Manuella, F., Nanni, P., & Mansuy, I. M. (2016). Potential of environmental enrichment to prevent transgenerational effects of paternal trauma. Neuropsychopharmacology, 41(11), 2749–2758.
Gapp, K., Soldado-Magraner, S., Alvarez-Sánchez, M., Bohacek, J., Vernaz, G., Shu, H., et al. (2014). Early life stress in fathers improves behavioural flexibility in their offspring. Nature Communications, 5(1), 5466–5473.
García-Campayo, J., Puebla-Guedea, M., Labarga, A., Urdánoz, A., Roldán, M., Pulido, L., et al. (2017). Epigenetic response to mindfulness in peripheral blood leukocytes involves genes linked to common human diseases. Mindfulness, 9(4), 1146–1159.
Gilmore, J. H., Knickmeyer, R. C., & Gao, W. (2018). Imaging structural and functional brain development in early childhood. Nature Reviews Neuroscience, 19(3), 123–137.
Gomez-Pinilla, F., Zhuang, Y., Feng, J., Ying, Z., & Fan, G. (2011). Exercise impacts brain-derived neurotrophic factor plasticity by engaging mechanisms of epigenetic regulation. European Journal of Neuroscience, 33(3), 383–390.
Haartsen, R., Jones, E. J., & Johnson, M. H. (2016). Human brain development over the early years. Current Opinion in Behavioral Sciences, 10, 149–154.
Halder, R., Hennion, M., Vidal, R. O., Shomroni, O., Rahman, R., Rajput, A., et al. (2016). DNA methylation changes in plasticity genes accompany the formation and maintenance of memory. Nature Neuroscience, 19(1), 102–110.
Hardy, T. M., & Tollefsbol, T. O. (2011). Epigenetic diet: impact on the epigenome and cancer. Epigenomics, 3(4), 503–518.
Heijmans, B. T., Tobi, E. W., Stein, A. D., Putter, H., Blauw, G. J., Susser, E. S., Slagboom, P. E., & Lumey, L. H. (2008). Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proceedings of the National Academy of Sciences, 105(44), 17046–17049.
Heim, C., & Binder, E. B. (2012). Current research trends in early life stress and depression: review of human studies on sensitive periods, gene–environment interactions, and epigenetics. Experimental Neurology, 233(1), 102–111.
Helgadóttir, B., Hallgren, M., Ekblom, Ö., & Forsell, Y. (2016). Training fast or slow? Exercise for depression: a randomized controlled trial. Preventive Medicine, 91, 123–131.
Hoek, H. W., Brown, A. S., & Susser, E. (1998). The Dutch famine and schizophrenia spectrum disorders. Social Psychiatry and Psychiatric Epidemiology, 33(8), 373–379.
Hoffman, S. G., & Smits, J. A. J. (2008). Cognitive-behavioral therapy for adult anxiety disorders. The Journal of Clinical Psychiatry, 69(4), 621–632.
Hoye, J. R., Cheishvili, D., Yarger, H. A., Roth, T. L., Szyf, M., & Dozier, M. (2019). Preliminary indications that the attachment and biobehavioral catch-up intervention alters DNA methylation in maltreated children. Development and Psychopathology, 1–9. https://doi.org/10.1017/s0954579419001421. Online ahead of print.
Hyman, S. E. (2012). Target practice: HDAC inhibitors for schizophrenia. Nature Neuroscience, 15(9), 1180–1181.
Imperio, C. G., Mcfalls, A. J., Hadad, N., Blanco-Berdugo, L., Masser, D. R., Colechio, E. M., et al. (2018). Exposure to environmental enrichment attenuates addiction-like behavior and alters molecular effects of heroin self-administration in rats. Neuropharmacology, 139, 26–40.
Intlekofer, K. A., & Cotman, C. W. (2013). Exercise counteracts declining hippocampal function in aging and Alzheimers disease. Neurobiology of Disease, 57, 47–55.
Irwin, R. E., Pentieva, K., Cassidy, T., Lees-Murdock, D. J., Mclaughlin, M., Prasad, G., et al. (2016). The interplay between DNA methylation, folate and neurocognitive development. Epigenomics, 8(6), 863–879.
Irwin, R. E., Thursby, S.-J., Ondičová, M., Pentieva, K., Mcnulty, H., Richmond, R. C., et al. (2019). A randomized controlled trial of folic acid intervention in pregnancy highlights a putative methylation-regulated control element at ZFP57. Clinical Epigenetics, 11(1), 31.
Jones, C., Watson, D., & Fone, K. (2011). Animal models of schizophrenia. British Journal of Pharmacology, 164(4), 1162–1194.
Kang, H.-J., Kim, J.-M., Lee, J.-Y., Kim, S.-Y., Bae, K.-Y., Kim, S.-W., Shin, I. S., Kim, H. R., Shin, M. G., & Yoon, J.-S. (2013). BDNF promoter methylation and suicidal behavior in depressive patients. Journal of Affective Disorders, 151(2), 679–685.
Kanherkar, R. R., Bhatia-Dey, N., & Csoka, A. B. (2014). Epigenetics across the human lifespan. Frontiers in Cell and Developmental Biology, 2(49), 1–19.
Keefe, J. R., Mccarthy, K. S., Dinger, U., Zilcha-Mano, S., & Barber, J. P. (2014). A meta-analytic review of psychodynamic therapies for anxiety disorders. Clinical Psychology Review, 34(4), 309–323.
Keller, S. M., Doherty, T. S., & Roth, T. L. (2018). Pharmacological manipulation of DNA methylation in adult female rats normalizes behavioral consequences of early-life maltreatment. Frontiers in Behavioral Neuroscience, 12.
Keller, S. M., Doherty, T. S., & Roth, T. L. (2019). Pharmacological manipulation of DNA methylation normalizes maternal behavior, DNA methylation, and gene expression in dams with a history of maltreatment. Scientific Reports, 9(1).
Keller, S., Sarchiapone, M., Zarrilli, F., Videtič, A., Ferraro, A., Carli, V., Sacchetti, S., Lembo, F., Angiolillo, A., Jovanovic, N., Pisanti, F., Tomaiuolo, R., Monticelli, A., Balazic, J., Roy, A., Marusic, A., Cocozza, S., Fusco, A., Bruni, C. B., Castaldo, G., & Chiariotti, L. (2010). Increased BDNF promoter methylation in the Wernicke area of suicide subjects. Archives of General Psychiatry, 67(3), 258–267.
King, A. P., Block, S. R., Sripada, R. K., Rauch, S., Giardino, N., Favorite, T., Angstadt, M., Kessler, D., Welsh, R., & Liberzon, I. (2016). Altered default mode network (Dmn) resting state functional connectivity following a mindfulness-based exposure therapy for posttraumatic stress disorder (Ptsd) in combat veterans of Afghanistan and Iraq. Depression and Anxiety, 33(4), 289–299.
King, A. P., Erickson, T. M., Giardino, N. D., Favorite, T., Rauch, S. A., Robinson, E., et al. (2013). A pilot study of group mindfulness-based cognitive therapy (Mbct) for combat veterans with posttraumatic stress disorder (Ptsd). Depression and Anxiety, 30(7), 638–645.
Kozasa, E. H., Sato, J. R., Lacerda, S. S., Barreiros, M. A., Radvany, J., Russell, T. A., et al. (2012). Meditation training increases brain efficiency in an attention task. NeuroImage, 59(1), 745–749.
Kundakovic, M., Gudsnuk, K., Herbstman, J. B., Tang, D., Perera, F. P., & Champagne, F. A. (2015). DNA methylation of BDNF as a biomarker of early-life adversity. Proceedings of the National Academy of Sciences, 112(22), 6807–6813.
Lähdepuro, A., Savolainen, K., Lahti-Pulkkinen, M., Eriksson, J. G., Lahti, J., Tuovinen, S., Kajantie, E., Pesonen, A. K., Heinonen, K., & Räikkönen, K. (2019). The impact of early life stress on anxiety symptoms in late adulthood. Scientific Reports, 9(1), 4395.
Landy, C. K., Jack, S. M., Wahoush, O., Sheehan, D., & Macmillan, H. L. (2012). Mothers’ experiences in the nurse-family partnership program: a qualitative case study. BMC Nursing, 11(1).
Laplant, Q., Vialou, V., Covington, H. E., Dumitriu, D., Feng, J., Warren, B. L., et al. (2010). Dnmt3a regulates emotional behavior and spine plasticity in the nucleus accumbens. Nature Neuroscience, 13(9), 1137–1143.
Lemoult, J., Humphreys, K. L., Tracy, A., Hoffmeister, J.-A., Ip, E., & Gotlib, I. H. (2020). Meta-analysis: exposure to early life stress and risk for depression in childhood and adolescence. Journal of the American Academy of Child & Adolescent Psychiatry, 59(7), 842–855.
Lopez, J. P., Mamdani, F., Labonte, B., Beaulieu, M.-M., Yang, J. P., Berlim, M. T., Ernst, C., & Turecki, G. (2013). Epigenetic regulation of BDNF expression according to antidepressant response. Molecular Psychiatry, 18(4), 398–399.
Lorsch, Z. S., Hamilton, P. J., Ramakrishnan, A., Parise, E. M., Salery, M., Wright, W. J., Lepack, A. E., Mews, P., Issler, O., McKenzie, A., Zhou, X., Parise, L. F., Pirpinias, S. T., Ortiz Torres, I., Kronman, H. G., Montgomery, S. E., Loh, Y. H. E., Labonté, B., Conkey, A., Symonds, A. E., Neve, R. L., Turecki, G., Maze, I., Dong, Y., Zhang, B., Shen, L., Bagot, R. C., & Nestler, E. J. (2019). Stress resilience is promoted by a Zfp189-driven transcriptional network in prefrontal cortex. Nature Neuroscience, 22(9), 1413–1423.
Lubin, F. D., Roth, T. L., & Sweatt, J. D. (2008). Epigenetic regulation of BDNF gene transcription in the consolidation of fear memory. Journal of Neuroscience, 28(42), 10576–10586.
Mairesse, J., Lesage, J., Breton, C., Bréant, B., Hahn, T., Darnaudéry, M., Dickson, S. L., Seckl, J., Blondeau, B., Vieau, D., Maccari, S., & Viltart, O. (2007). Maternal stress alters endocrine function of the feto-placental unit in rats. American Journal of Physiology-Endocrinology and Metabolism, 292(6), E1526–E1533.
Makhathini, K. B., Abboussi, O., Stein, D. J., Mabandla, M. V., & Daniels, W. M. (2017). Repetitive stress leads to impaired cognitive function that is associated with DNA hypomethylation, reduced BDNF and a dysregulated HPA axis. International Journal of Developmental Neuroscience, 60(1), 63–69.
Mars, T. S., & Abbey, H. (2010). Mindfulness meditation practise as a healthcare intervention: A systematic review. International Journal of Osteopathic Medicine, 13(2), 56–66.
Martínez, J. A., Cordero, P., Campión, J., & Milagro, F. I. (2012). Interplay of early-life nutritional programming on obesity, inflammation and epigenetic outcomes. Proceedings of the Nutrition Society, 71(2), 276–283.
Masten, A. S. (2013). Global perspectives on resilience in children and youth. Child Development, 85(1), 6–20.
Mcewen, B. S., Nasca, C., & Gray, J. D. (2016). Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology, 41(1), 3–23.
Mcgowan, P. O., Sasaki, A., Dalessio, A. C., Dymov, S., Labonté, B., Szyf, M., et al. (2009). Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature Neuroscience, 12(3), 342–348.
Ment, L. R., & Vohr, B. R. (2008). Preterm birth and the developing brain. The Lancet Neurology, 7(5), 378–379.
Miller, T. R. (2015). Projected outcomes of nurse-family partnership home visitation during 1996–2013, USA. Prevention Science, 16(6), 765–777.
Miller, C. W. T. (2017). Epigenetic and neural circuitry landscape of psychotherapeutic interventions. Psychiatry Journal, 2017, 1–38.
Miller, C. A., Gavin, C. F., White, J. A., Parrish, R. R., Honasoge, A., Yancey, C. R., Rivera, I. M., Rubio, M. D., Rumbaugh, G., & Sweatt, J. D. (2010). Cortical DNA methylation maintains remote memory. Nature Neuroscience, 13(6), 664–666.
Moor, M. D., Beem, A., Stubbe, J., Boomsma, D., & Geus, E. D. (2006). Regular exercise, anxiety, depression and personality: a population-based study. Preventive Medicine, 42(4), 273–279.
Moore, D. S. (2015). The developing genome: an introduction to behavioral epigenetics. New York: Oxford University Press.
Morse, S., Butler, A., Davis, R., Soller, I., & Lubin, F. (2015). Environmental enrichment reverses histone methylation changes in the aged hippocampus and restores age-related memory deficits. Biology, 4(2), 298–313.
Mueller, B. R., & Bale, T. L. (2008). Sex-specific programming of offspring emotionality after stress early in pregnancy. Journal of Neuroscience, 28(36), 9055–9065.
Mul, J. D., Zheng, J., & Goodyear, L. J. (2016). Validity assessment of 5 day repeated forced-swim stress to model human depression in young-adult C57BL/6J and BALB/cJ mice. Eneuro, 3(6), ENEURO.0201–ENEU16.2016.
Mulligan, C., Derrico, N., Stees, J., & Hughes, D. (2012). Methylation changes atNR3C1 in newborns associate with maternal prenatal stress exposure and newborn birth weight. Epigenetics, 7(8), 853–857.
Murgatroyd, C., Patchev, A. V., Wu, Y., Micale, V., Bockmühl, Y., Fischer, D., Holsboer, F., Wotjak, C. T., Almeida, O. F. X., & Spengler, D. (2009). Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nature Neuroscience, 12(12), 1559–1566.
Murgatroyd, C., & Spengler, D. (2011). Epigenetics of early child development. Frontiers in Psychiatry, 2(16), 1–15.
Nan, X., Ng, H.-H., Johnson, C. A., Laherty, C. D., Turner, B. M., Eisenman, R. N., & Bird, A. (1998). Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature, 393(6683), 386–389.
Nelson, C. A., Zeanah, C. H., Fox, N. A., Marshall, P. J., Smyke, A. T., & Guthrie, D. (2007). Cognitive recovery in socially deprived young children: the Bucharest early intervention project. Science, 318(5858), 1937–1940.
Nitert, M. D., Dayeh, T., Volkov, P., Elgzyri, T., Hall, E., Nilsson, E., Yang, B. T., Lang, S., Parikh, H., Wessman, Y., Weishaupt, H., Attema, J., Abels, M., Wierup, N., Almgren, P., Jansson, P. A., Ronn, T., Hansson, O., Eriksson, K. F., Groop, L., & Ling, C. (2012). Impact of an exercise intervention on DNA methylation in skeletal muscle from first-degree relatives of patients with type 2 diabetes. Diabetes, 61(12), 3322–3332.
Non, A. L., Hollister, B. M., Humphreys, K. L., Childebayeva, A., Esteves, K., Zeanah, C. H., Fox, N. A., Nelson, C. A., & Drury, S. S. (2016). DNA methylation at stress-related genes is associated with exposure to early life institutionalization. American Journal of Physical Anthropology, 161(1), 84–93.
O’Donnell, K. J., Chen, L., Macisaac, J. L., Mcewen, L. M., Nguyen, T., Beckmann, K., et al. (2018). DNA methylome variation in a perinatal nurse-visitation program that reduces child maltreatment: a 27-year follow-up. Translational Psychiatry, 8(1), 15.
Olds, D. L., Hill, P. L., Obrien, R., Racine, D., & Moritz, P. (2003). Taking preventive intervention to scale: the nurse-family partnership. Cognitive and Behavioral Practice, 10(4), 278–290.
Painter, R. C., Rooij, S. R. D., Bossuyt, P. M., Simmers, T. A., Osmond, C., Barker, D. J., et al. (2006). Early onset of coronary artery disease after prenatal exposure to the Dutch famine. The American Journal of Clinical Nutrition, 84(2), 322–327.
Palma-Gudiel, H., Córdova-Palomera, A., Leza, J. C., & Fañanás, L. (2015). Glucocorticoid receptor gene (NR3C1) methylation processes as mediators of early adversity in stress-related disorders causality: a critical review. Neuroscience & Biobehavioral Reviews, 55, 520–535.
Pankevich, D. E., Mueller, B. R., Brockel, B., & Bale, T. L. (2009). Prenatal stress programming of offspring feeding behavior and energy balance begins early in pregnancy. Physiology & Behavior, 98(1–2), 94–102.
Paparo, L., Costanzo, M. D., Scala, C. D., Cosenza, L., Leone, L., Nocerino, R., & Canani, R. (2014). The influence of early life nutrition on epigenetic regulatory mechanisms of the immune system. Nutrients, 6(11), 4706–4719.
Parker, K. J., Buckmaster, C. L., Hyde, S. A., Schatzberg, A. F., & Lyons, D. M. (2019). Nonlinear relationship between early life stress exposure and subsequent resilience in monkeys. Scientific Reports, 9(1), 16232.
Perroud, N., Salzmann, A., Prada, P., Nicastro, R., Hoeppli, M.-E., Furrer, S., Ardu, S., Krejci, I., Karege, F., & Malafosse, A. (2013). Response to psychotherapy in borderline personality disorder and methylation status of the BDNF gene. Translational Psychiatry, 3(1), e207.
Pietrzak, R. H., & Southwick, S. M. (2011). Psychological resilience in OEF–OIF veterans: application of a novel classification approach and examination of demographic and psychosocial correlates. Journal of Affective Disorders, 133(3), 560–568.
Provenzi, L., Giorda, R., Beri, S., & Montirosso, R. (2016). SLC6A4 methylation as an epigenetic marker of life adversity exposures in humans: A systematic review of literature. Neuroscience & Biobehavioral Reviews, 71, 7–20.
Radtke, K. M., Ruf, M., Gunter, H. M., Dohrmann, K., Schauer, M., Meyer, A., & Elbert, T. (2011). Transgenerational impact of intimate partner violence on methylation in the promoter of the glucocorticoid receptor. Translational Psychiatry, 1(7), e21.
Ramchandani, S., Bhattacharya, S. K., Cervoni, N., & Szyf, M. (1999). DNA methylation is a reversible biological signal. Proceedings of the National Academy of Sciences, 96(11), 6107–6112.
Revenga, M. D. L. F., Ibi, D., Saunders, J. M., Cuddy, T., Ijaz, M. K., Toneatti, R., et al. (2018). HDAC2-dependent antipsychotic-like effects of chronic treatment with the HDAC inhibitor SAHA in mice. Neuroscience, 388, 102–117.
Rönn, T., Volkov, P., Davegårdh, C., Dayeh, T., Hall, E., Olsson, A. H., Nilsson, E., Tornberg, Å., Dekker Nitert, M., Eriksson, K. F., Jones, H. A., Groop, L., & Ling, C. (2013). A six months exercise intervention influences the genome-wide DNA methylation pattern in human adipose tissue. PLoS Genetics, 9(6), e1003572.
Roth, T. L., Lubin, F. D., Funk, A. J., & Sweatt, J. D. (2009). Lasting epigenetic influence of early-life adversity on the BDNF gene. Biological Psychiatry, 65(9), 760–769.
Roth, T. L., Matt, S., Chen, K., & Blaze, J. (2014). Bdnf DNA methylation modifications in the hippocampus and amygdala of male and female rats exposed to different caregiving environments outside the homecage. Developmental Psychobiology, 56(8), 1755–1763.
Roth, T. L., Zoladz, P. R., Sweatt, J. D., & Diamond, D. M. (2011). Epigenetic modification of hippocampal BDNF DNA in adult rats in an animal model of post-traumatic stress disorder. Journal of Psychiatric Research, 45(7), 919–926.
Rusiecki, J. A., Byrne, C., Galdzicki, Z., Srikantan, V., Chen, L., Poulin, M., et al. (2013). PTSD and DNA methylation in select immune function gene promoter regions: a repeated measures case-control study of U.S. military service members. Frontiers in Psychiatry, 4(56), 1–12.
Samra, H. A., Mcgrath, J. M., Wehbe, M., & Clapper, J. (2012). Epigenetics and family-centered developmental care for the preterm infant. Advances in Neonatal Care, 12(5). S2–S9.
Santarelli, S., Zimmermann, C., Kalideris, G., Lesuis, S. L., Arloth, J., Uribe, A., Dournes, C., Balsevich, G., Hartmann, J., Masana, M., Binder, E. B., Spengler, D., & Schmidt, M. V. (2017). An adverse early life environment can enhance stress resilience in adulthood. Psychoneuroendocrinology, 78, 213–221.
Sarkar, S., Abujamra, A. L., Loew, J. E., Forman, L. W., Perrine, S. P., & Faller, D. V. (2011). Histone deacetylase inhibitors reverse CpG methylation by regulating DNMT1 through ERK signaling. Anticancer Research, 31(9), 2723–2732.
Schmauss, C. (2015). An HDAC-dependent epigenetic mechanism that enhances the efficacy of the antidepressant drug fluoxetine. Scientific Reports, 5(8171), 1–8.
Schreiner, I., & Malcolm, J. P. (2008). The benefits of mindfulness meditation: changes in emotional states of depression, anxiety, and stress. Behaviour Change, 25(3), 156–168.
Schulz, L. C. (2010). The Dutch hunger winter and the developmental origins of health and disease. Proceedings of the National Academy of Sciences, 107(39), 16757–16758.
Sheridan, M. A., Fox, N. A., Zeanah, C. H., Mclaughlin, K. A., & Nelson, C. A. (2012). Variation in neural development as a result of exposure to institutionalization early in childhood. Proceedings of the National Academy of Sciences, 109(32), 12927–12932.
Sleiman, S. F., Henry, J., Al-Haddad, R., Hayek, L. E., Haidar, E. A., Stringer, T., et al. (2016). Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. 5:e15092. https://doi.org/10.7554/eLife.15092.
Smith, Z. D., & Meissner, A. (2013). DNA methylation: roles in mammalian development. Nature Reviews Genetics, 14(3), 204–220.
Southwick, S. M., Bonanno, G. A., Masten, A. S., Panter-Brick, C., & Yehuda, R. (2014). Resilience definitions, theory, and challenges: interdisciplinary perspectives. European Journal of Psychotraumatology, 5(1), 25338.
Stahl, S. M. (2011). Psychotherapy as an epigenetic ‘drug’: psychiatric therapeutics target symptoms linked to malfunctioning brain circuits with psychotherapy as well as with drugs. Journal of Clinical Pharmacy and Therapeutics, 37(3), 249–253.
Stein, A. D., Kahn, H. S., Rundle, A., Zybert, P. A., Bruin, K. V. D. P. D., & Lumey, L. (2007). Anthropometric measures in middle age after exposure to famine during gestation: evidence from the Dutch famine. The American Journal of Clinical Nutrition, 85(3), 869–876.
Syed, S. A., & Nemeroff, C. B. (2017). Early life stress, mood, and anxiety disorders. Chronic Stress, 1, 247054701769446.
Szyf, M. (2009). Epigenetics, DNA methylation, and chromatin modifying drugs. Annual Review of Pharmacology and Toxicology, 49(1), 243–263.
Szyf, M., Tang, Y.-Y., Hill, K. G., & Musci, R. (2016). The dynamic epigenome and its implications for behavioral interventions: a role for epigenetics to inform disorder prevention and health promotion. Translational Behavioral Medicine, 6(1), 55–62.
Tomasino, B., Fregona, S., Skrap, M., & Fabbro, F. (2013). Meditation-related activations are modulated by the practices needed to obtain it and by the expertise: an ALE meta-analysis study. Frontiers in Human Neuroscience, 6.
Voisin, S., Eynon, N., Yan, X., & Bishop, D. J. (2015). Exercise training and DNA methylation in humans. Acta Physiologica, 213(1), 39–59.
Waddington, C. H. (1940). Organisers and Genes. Nature, 413–413.
Waddington, C. H. (1942). The epigenotype. Endeavour, 1, 18–20.
Walker, C., Bath, K. G., Joels, M., Korosi, A., Larauche, M., Lucassen, P. J., et al. (2017). Chronic early life stress induced by limited bedding and nesting (LBN) material in rodents: critical considerations of methodology, outcomes and translational potential. Stress, 20(5), 421–448.
Weaver, I. C. G., Cervoni, N., Champagne, F. A., Dalessio, A. C., Sharma, S., Seckl, J. R., et al. (2004). Epigenetic programming by maternal behavior. Nature Neuroscience, 7(8), 847–854.
Whitfield-Gabrieli, S., & Ford, J. M. (2012). Default mode network activity and connectivity in psychopathology. Annual Review of Clinical Psychology, 8(1), 49–76.
Wiegand, C., Heusser, P., Klinger, C., Cysarz, D., Büssing, A., Ostermann, T., & Savelsbergh, A. (2018). Stress-associated changes in salivary microRNAs can be detected in response to the trier social stress test: an exploratory study. Scientific Reports, 8(1), 7112.
Wright, E. C., Johnson, S. A., Hao, R., Kowalczyk, A. S., Greenberg, G. D., Sanchez, E. O., et al. (2017). Exposure to extrinsic stressors, social defeat or bisphenol A, eliminates sex differences in DNA methyltransferase expression in the amygdala. Journal of Neuroendocrinology, 29(6).
Xie, B., Xu, Y., Liu, Z., Liu, W., Jiang, L., Zhang, R., Cui, D., Zhang, Q., & Xu, S. (2017). Elevation of peripheral BDNF promoter methylation predicts conversion from amnestic mild cognitive impairment to Alzheimer’s disease: A 5-year longitudinal study. Journal of Alzheimers Disease, 56(1), 391–401.
Yehuda, R., Daskalakis, N. P., Lehrner, A., Desarnaud, F., Bader, H. N., Makotkine, I., Flory, J. D., Bierer, L. M., & Meaney, M. J. (2014). Influences of maternal and paternal PTSD on epigenetic regulation of the glucocorticoid receptor gene in holocaust survivor offspring. American Journal of Psychiatry, 171(8), 872–880.
Yehuda, R., Flory, J. D., Pratchett, L. C., Buxbaum, J., Ising, M., & Holsboer, F. (2010). Putative biological mechanisms for the association between early life adversity and the subsequent development of PTSD. Psychopharmacology, 212(3), 405–417.
Zaidan, H., Leshem, M., & Gaisler-Salomon, I. (2013). Prereproductive stress to female rats alters corticotropin releasing factor type 1 expression in ova and behavior and brain corticotropin releasing factor type 1 expression in offspring. Biological Psychiatry, 74(9), 680–687.
Zeanah, C. H., Smyke, A. T., Koga, S. F., & Carlson, E. (2005). Attachment in institutionalized and community children in Romania. Child Development, 76(5), 1015–1028.
Zeidan, F., Grant, J., Brown, C., Mchaffie, J., & Coghill, R. (2012). Mindfulness meditation-related pain relief: Evidence for unique brain mechanisms in the regulation of pain. Neuroscience Letters, 520(2), 165–173.
Zeng, H., Irwin, M. L., Lu, L., Risch, H., Mayne, S., Mu, L., Deng, Q., Scarampi, L., Mitidieri, M., Katsaros, D., & Yu, H. (2012). Physical activity and breast cancer survival: an epigenetic link through reduced methylation of a tumor suppressor gene L3MBTL1. Breast Cancer Research and Treatment, 133(1), 127–135.
Zheng, Y., & Cantley, L. C. (2018). Toward a better understanding of folate metabolism in health and disease. Journal of Experimental Medicine, 216(2), 253–266.
Zhou, H.-X., Chen, X., Shen, Y.-Q., Li, L., Chen, N.-X., Zhu, Z.-C., Castellanos, F. X., & Yan, C.-G. (2020). Rumination and the default mode network: meta-analysis of brain imaging studies and implications for depression. NeuroImage, 206, 116287.
Availability of Data and Materials
Data that support any findings reported are available from the corresponding author upon request.
Code Availability
Not applicable.
Funding
This article was supported by a grant from The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD; 1R01HD087509-01).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
For data reported, all animal procedures were performed with approval from the Institutional Animal Care and Use Committee (IACUC) following NIH established guidelines.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Collins, N., Phillips, N.L.H., Reich, L. et al. Epigenetic Consequences of Adversity and Intervention Throughout the Lifespan: Implications for Public Policy and Healthcare. ADV RES SCI 1, 205–216 (2020). https://doi.org/10.1007/s42844-020-00015-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42844-020-00015-5